Abstract
Background
Ocular surface temperature (OST) could berelated to retrobulbar haemodynamics in patients with glaucoma.
Aims
To compare OST measurements in patients with glaucoma and healthy controls, and to investigate the correlation between OST, intraocular pressure (IOP) and retrobulbar haemodynamics in patients with glaucoma.
Methods
32 patients with primary open‐angle glaucoma (POAG) and 40 controls were included in the study. The parameters considered both in patients with POAG and in controls were IOP and OST values measured by infrared ocular thermography. Colour Doppler imaging was used to determine haemodynamic parameters in ophthalmic artery (OA), central retinal artery (CRA) and short posterior ciliary arteries (SPCAs) in patients with POAG.
Results
OST values were significantly lower in patients with POAG than in controls (p<0.001). OST was negatively related with resistivity index of OA (p<0.001), CRA (p = 0.001) and SPCAs (p<0.001), and positively related with end‐diastolic velocity of OA (p = 0.02) and SPCAs (p = 0.05).
Conclusion
This study suggested that OST could be a marker of impaired retrobulbar haemodynamics in patients with glaucoma.
Glaucoma pathogenesis is multifactorial and not yet well established. Ocular hypertension is the main risk factor for the development and progression of the disease.1,2,3,4 Vascular factors have assumed an increasing relevance in the past years. A reduction or an impaired autoregulation of blood supply to the optic nerve head (ONH), at least in some types of glaucoma, has been demonstrated.5,6,7,8,9,10 Colour Doppler imaging (CDI) gives important information about the retrobulbar circulation. In patients with glaucoma, changes in retrobulbar haemodynamics are correlated with a progression in functional damage.11,12,13,14,15,16
A vascular imaging method widely used to study the abnormalities in blood flow is the infrared thermography. This technique measures the radiated heat from the body surface, which is related to the local blood flow. A recent review displayed the clinical applications of infrared thermography.17 The characteristics of the ocular thermographic profiles have been clarified by previous investigations.18,19,20,21,22 The real‐time measurements of ocular surface temperature (OST) by infrared thermo‐cameras proved their utility in ophthalmology. Changes in OST have been described in several pathological states of the adnexa and in the anterior segment of the eye, as well as in the cataract and refractive surgery.23,24,25,26,27 Some studies showed a correlation between OST and ocular blood flow. An increase of intraocular pressure (IOP) was found to be related to a contemporary decrease of ocular perfusion pressure and ocular temperature in monkeys.28 In normal human subjects, a correlation between ocular temperature and finger temperature has been displayed.29 In carotid artery stenosis, the eye on the affected side showed a reduction in corneal temperature and impaired retrobulbar haemodynamics.18,30 Thermography could be suitable to provide information about the role of vascular factors in the physiopathology of glaucomatous optic neuropathy. Changes in OST would be expected in affected eyes, presumably correlated to the impairment of retrobulbar haemodynamics. Gugleta et al31 demonstrated a relationship between corneal temperature and retrobulbar haemodynamics.
In the present study, OST measurements were evaluated in a group of patients with primary open‐angle glaucoma (POAG) and in a group of healthy controls. The correlation between OST, IOP and retrobulbar haemodynamics was investigated using CDI in patients with glaucoma.
Materials and methods
A total of 32 patients with POAG and 40 controls were evaluated. Written informed consent was obtained from each subject. The procedures conformed with those of the Declaration of Helsinki and of the local committee of the University of Florence, Florence, Italy. All the subjects underwent the following ophthalmic examinations: best‐corrected visual acuity measurement with Snellen chart; Goldmann applanation tonometry; slit‐lamp examination of the anterior and posterior segments; Schirmer's test; break‐up time test; and, only in patients with POAG, Humphrey 24–2 full‐threshold visual field test. The following exclusion criteria were applied: myopia or hyperopia ⩾4 diopters; astigmatism ⩾1.5 diopters; contact lens wear; any inflammation of the adnexa, the anterior or posterior segments of eye; abnormal Schirmer's test (<10 mm/5 min); reduced break‐up time (<10 s); any cardiovascular pathologies; systemic or topical therapy with drugs acting on cardiovascular system; and body temperature <36.4°C or >36.7°C. In each subject one eye was evaluated. All the study eyes of patients with POAG were treated with prostaglandins and showed similar visual field defects. The study eye of the controls was chosen randomly. Table 1 lists the data of patients with POAG and controls.
Table 1 Characteristics of the enrolled subjects.
| Controls | Patients with POAG | p Value | |
|---|---|---|---|
| n | 40 | 32 | |
| Mean (SD) age (years) | 65.10 (11.81) | 64.75 (11.21) | 0.899 |
| Sex (M:F) | 20:20 | 15:17 | 0.796 |
| Mean (SD) IOP (mm Hg) | 12.90 (1.53) | 19.12 (3.98) | <0.001 |
| Mean (SD) deviation | Not done | 6.77 (1.04) | |
| Mean (SD) corrected pattern | Not done | 3.56 (0.83) | |
| Hypotensive therapy | None | Yes (prostaglandins) |
F, female; POAG, primary open‐angle glaucoma; IOP, intraocular pressure; M, male.
Thermography was performed in all the subjects using an infrared detector (Agema Thermovision 800 LWB, AGEMA Infrared Systems 1991 AB, Donderyd, Sweden). OST was always measured between 9:00 and 10:00, to avoid bias due to an increase in OST throughout the day.17 Before each examination, room temperature, humidity and air flow were recorded, to make sure to have relatively constant environmental parameters. At each examination, the subject was requested to keep the eyes closed for 3–5 s, then to open both eyes wide. OST measurements lasted for 20 s, and the data were registered every second, but only the thermograms taken at the eye opening and at the 20th second after opening (frames 0 and 109, respectively) were evaluated in the statistical analysis. The temperature of five anatomical points across a line running horizontally through the centre of the cornea was recorded: the internal canthus (point 1), half‐way from the internal canthus and nasal limbus (point 2), the centre of the cornea (point 3), half‐way from the temporal limbus and external canthus (point 4) and the external canthus (point 5).
Patients with POAG underwent a retrobulbar haemodynamic evaluation by Colour Doppler Dynaview TM II SSD‐1700 (Aloka, Tokyo, Japan), using a 6 MHz probe. The peak‐systolic velocity (PSV), the end‐diastolic velocity (EDV) and the resistivity index (RI) were recorded in ophthalmic artery (OA), central retinal artery (CRA) and short posterior ciliary arteries (SPCAs).
Student's t test for unpaired data was used to compare the values obtained from patients with POAG and controls. Linear regression was applied to analyse the correlation between OST, IOP and CDI parameters. A p⩽0.05 was regarded as significant.
Results
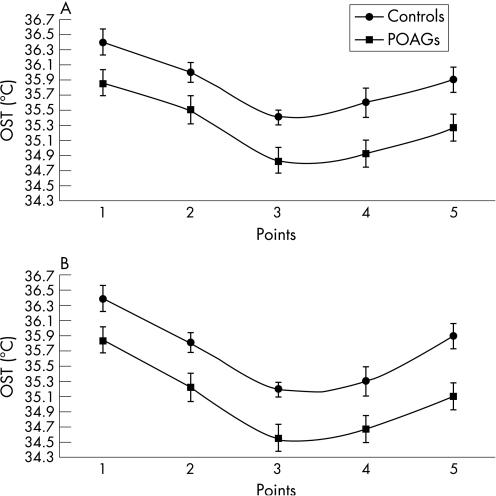
Tables 2 and 3 outline the mean (SD) values of OST, expressed in degrees Celsius (°C), in patients with POAG and in controls.
Table 2 Ocular surface temperature values at frame 0.
| Mean (SD) OST | Controls | Patients with POAG | p Value |
|---|---|---|---|
| Point 1 | 36.40 (0.10) | 35.86 (0.41) | <0.001 |
| Point 2 | 36.00 (0.18) | 35.50 (0.60) | <0.001 |
| Point 3 | 35.40 (0.15) | 34.83 (0.52) | <0.001 |
| Point 4 | 35.60 (0.16) | 34.92 (0.49) | <0.001 |
| Point 5 | 35.90 (0.12) | 35.27 (0.49) | <0.001 |
OST, ocular surface temperature; POAG, primary open‐angle glaucoma.
Table 3 Ocular surface temperature values at frame 109.
| Mean (SD) OST | Controls | Patients with POAG | p Value |
|---|---|---|---|
| Point 1 | 36.39 (0.17) | 35.84 (0.54) | <0.001 |
| Point 2 | 35.81 (0.13) | 35.22 (0.66) | <0.001 |
| Point 3 | 35.20 (0.10) | 34.55 (0.68) | <0.001 |
| Point 4 | 35.30 (0.20) | 34.67 (0.50) | <0.001 |
| Point 5 | 35.88 (0.17) | 35.10 (0.51) | <0.001 |
OST, ocular surface temperature; POAG, primary open‐angle glaucoma.
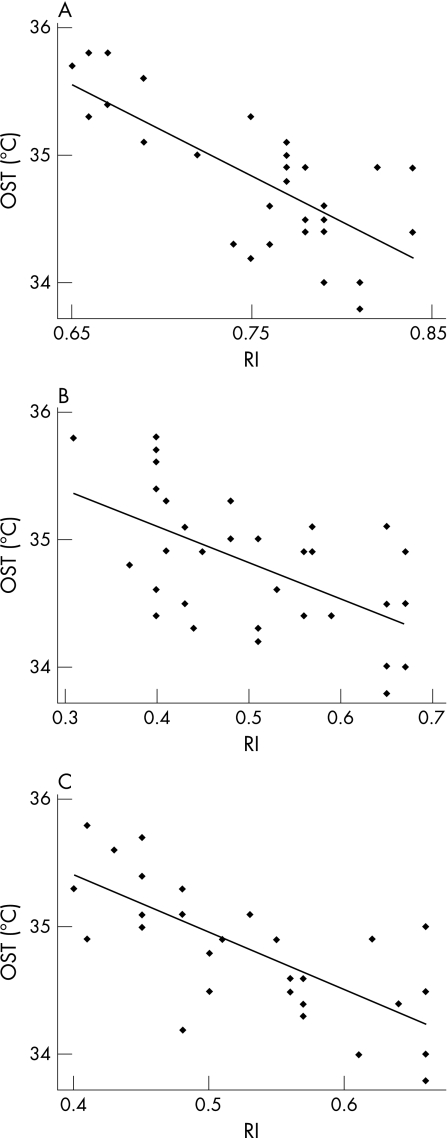
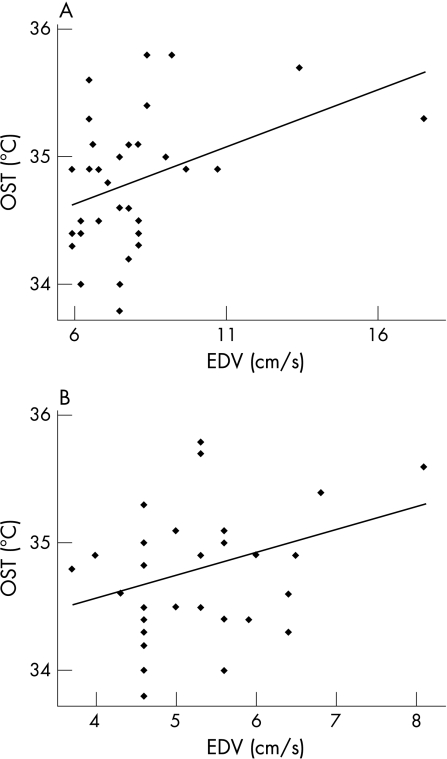
OST values are significantly lower in patients with POAG than in controls at every point in the two frames (p<0.001; fig 1). The measurements at point 3 were considered the most reliable values of OST, because the avascularity of the cornea should minimise the influence of the conjunctival vascular network on thermographic analysis.20 The data recorded immediately after eye opening (frame 0) are less influenced by environmental conditions and tear‐film evaporation, so they were used to perform the subsequent statistical analyses in patients with glaucoma—that is, the correlation between OST, IOP and CDI parameters.22 OST did not correlate with IOP values (R2 = 0.008, p = 0.62). A negative correlation between OST and RI was found in OA (R2 = 0.542, p<0.001), CRA (R2 = 0.321, p = 0.001) and SPCAs (R2 = 0.525, p<0.001; fig 2). A positive correlation between OST and EDV was reported in OA (R2 = 0.156, p = 0.02) and in SPCAs (R2 = 0.115, p = 0.05), not in CRA (R2 = 0.009, p = 0.60; fig 3). Regarding PSV, it was not related to OST in OA (R2 = 0.037, p = 0.29), whereas in CRA and SPCAs, there was a negative correlation (R2 = 0.320, p = 0.001 and R2 = 0.144, p = 0.03, respectively).
Figure 1 Comparison between ocular surface temperature (OST) values in patients with glaucoma and in controls (p<0.001 in both frames). (A) Analysis at frame 0. (B) Analysis at frame 109. POAG, patients with primary open‐angle glaucoma. The numerical data for the (A) and (B) are listed in tables 2 and 3, respectively.
Figure 2 Linear regression between ocular surface temperature (OST) and resistivity index (RI). Correlation in (A) ophthalmic artery (p<0.001), (B) central retinal artery (p = 0.001) and (C) short posterior ciliary arteries (p<0.001). Numerical data for fig 2 are listed in table 4.
Figure 3 Linear regression between ocular surface temperature (OST) and end‐diastolic velocity (EDV). Correlation in (A) ophthalmic artery (p = 0.02) and (B) short posterior ciliary arteries (p = 0.05). Numerical data for fig 3 are listed in table 5.
Table 4 Numerical data for ocular surface temperature and resistivity index.
| Eye | OST | RI OA | RI CRA | RI SPCA |
|---|---|---|---|---|
| 1 | 35.3 | 0.75 | 0.48 | 0.48 |
| 2 | 35.1 | 0.77 | 0.57 | 0.48 |
| 3 | 34.8 | 0.74 | 0.37 | 0.50 |
| 4 | 34.5 | 0.79 | 0.65 | 0.66 |
| 5 | 34.9 | 0.78 | 0.57 | 0.51 |
| 6 | 34.9 | 0.77 | 0.45 | 0.62 |
| 7 | 34.9 | 0.84 | 0.67 | 0.55 |
| 8 | 34.9 | 0.82 | 0.56 | 0.62 |
| 9 | 34.5 | 0.78 | 0.43 | 0.50 |
| 10 | 34.6 | 0.79 | 0.40 | 0.56 |
| 11 | 34.2 | 0.75 | 0.51 | 0.48 |
| 12 | 34.4 | 0.79 | 0.40 | 0.64 |
| 13 | 33.8 | 0.81 | 0.65 | 0.66 |
| 14 | 34 | 0.81 | 0.65 | 0.66 |
| 15 | 34.5 | 0.79 | 0.67 | 0.56 |
| 16 | 34.3 | 0.79 | 0.67 | 0.61 |
| 17 | 34.3 | 0.76 | 0.51 | 0.57 |
| 18 | 34.4 | 0.84 | 0.59 | 0.64 |
| 19 | 34.3 | 0.74 | 0.44 | 0.57 |
| 20 | 34.4 | 0.78 | 0.56 | 0.57 |
| 21 | 34.9 | 0.78 | 0.41 | 0.41 |
| 22 | 34.6 | 0.76 | 0.53 | 0.57 |
| 23 | 35 | 0.77 | 0.51 | 0.66 |
| 24 | 35 | 0.72 | 0.48 | 0.45 |
| 25 | 35.3 | 0.66 | 0.41 | 0.40 |
| 26 | 35.1 | 0.69 | 0.43 | 0.45 |
| 27 | 35.5 | 0.69 | 0.65 | 0.53 |
| 28 | 35.4 | 0.67 | 0.40 | 0.45 |
| 29 | 35.6 | 0.69 | 0.40 | 0.43 |
| 30 | 35.8 | 0.66 | 0.31 | 0.41 |
| 31 | 35.8 | 0.67 | 0.40 | 0.41 |
| 32 | 35.7 | 0.65 | 0.40 | 0.45 |
CRA, central retinal artery; OA, ophthalmic artery; OST, ocular surface temperature; RI, resistivity index; SPCA, short posterior ciliary artery.
Table 5 Numerical data for ocular surface temperature and end‐diastolic velocity.
| Eye | OST | EDV OA | EDV SPCA |
|---|---|---|---|
| 1 | 35.3 | 6.5 | 4.6 |
| 2 | 35.1 | 7.5 | 5.0 |
| 3 | 34.8 | 7.1 | 3.7 |
| 4 | 34.5 | 6.2 | 4.6 |
| 5 | 34.9 | 6.5 | 5.3 |
| 6 | 34.9 | 9.7 | 6.0 |
| 7 | 34.9 | 5.9 | 5.3 |
| 8 | 34.9 | 10.7 | 4.0 |
| 9 | 34.5 | 8.1 | 5.0 |
| 10 | 34.6 | 7.8 | 4.3 |
| 11 | 34.2 | 7.8 | 4.6 |
| 12 | 34.4 | 8.1 | 5.6 |
| 13 | 33.8 | 7.5 | 4.6 |
| 14 | 34 | 7.5 | 4.6 |
| 15 | 34.5 | 6.8 | 5.3 |
| 16 | 34.3 | 6.2 | 5.6 |
| 17 | 34.3 | 8.1 | 6.4 |
| 18 | 34.4 | 5.9 | 4.6 |
| 19 | 34.3 | 5.9 | 4.6 |
| 20 | 34.4 | 6.2 | 5.9 |
| 21 | 34.9 | 6.8 | 6.5 |
| 22 | 34.6 | 7.5 | 4.6 |
| 23 | 35 | 7.5 | 4.6 |
| 24 | 35 | 9.0 | 5.6 |
| 25 | 35.3 | 17.5 | 8.1 |
| 26 | 35.1 | 7.8 | 5.6 |
| 27 | 35.5 | 8.1 | 5.6 |
| 28 | 35.4 | 8.4 | 6.8 |
| 29 | 35.6 | 6.5 | 8.1 |
| 30 | 35.8 | 9.2 | 5.3 |
| 31 | 35.8 | 8.4 | 5.3 |
| 32 | 35.7 | 13.4 | 5.3 |
EDV, end‐diastolic velocity; OA, ophthalmic artery; OST, ocular surface temperature; SPCA, short posterior ciliary artery.
Discussion
In the present study, OST measurements in a group of patients with POAG and a group of controls were compared. Our results display a significant difference between the two groups, with lower OST values in patients with POAG. This finding may suggest an altered perfusion of the ONH in patients with POAG. Interestingly, the measurements at point 3, considered the most reliable, were lower than the values regarded as normal for corneal temperature (35.9°C (0.7°C)) in patients with POAG, whereas they were in the normal range in controls.20 To clarify this finding, the relationship between OST, IOP and CDI parameters was investigated in patients with POAG. OST does not correlate with IOP values, although it does with some CDI parameters. In fact, lower OST measurements are significantly associated with higher RI values in all the retrobulbar vessels and with lower EDV values in OA and SPCAs.
Among the semiologic methods used to evaluate the vascular component in glaucoma, CDI seems to be the most advisable, because of its non‐invasivity and reproducibility.32,33,34 It probably provides the most useful data, allowing to study the vessels that are more involved in supplying blood to the ONH—that is, OA and SPCAs. The most indicative parameters of ONH perfusion are EDV and RI. EDV, reflecting the average blood flow during the longest phase of the cardiac cycle, seems to be more suitable than PSV, which represents an instantaneous variation of blood flow. RI, the most reproducible and reliable CDI parameter, is a measure of peripheral vascular resistance, whose higher values indicate a greater impedence in the territory supplied by the examined vessel. The changes in PSV, EDV and RI in the OA and SPCAs could lead to a reduction in blood supply to ONH.11,12,13,14,15,16 The aim of our study was not to confirm the well‐known haemodynamic abnormalities in glaucoma. Therefore, CDI was performed only in patients with POAG, which showed a significant reduction of OST, when compared with normal values. Lower OST measurements are possibly related to a poorer ocular circulation. Eyes with a reduced blood supply, as in significant carotid artery stenosis, have appeared cooler than normal controls.18,30 On the contrary, the increase in local blood flow, in case of conjunctivitis and anterior uveitis, has been associated with an increase in OST.18,23,24,25 Gugleta et al31 proved a relationship between corneal temperature and retrobulbar haemodynamics comparable in patients with glaucoma and in controls. In the present analysis, a significant correlation between OST values and the most reliable CDI parameters has been demonstrated, supporting the hypothesis that retrobulbar haemodynamics could influence OST.31
Our study suggests that thermography could be useful in anterior eye diseases and also in glaucomatous optic neuropathy. OST, measured by this non‐invasive and reliable technique, might be an indirect marker of impaired ONH perfusion. In our opinion, glaucoma would be a very interesting field of study for thermographic evaluations. Hence, further investigations are needed to obtain clinically useful information about the physiopathology of the diseases determined, at least partly, by impaired ocular perfusion.
Acknowledgements
We thank Gianluca Gervasi, Department of Mechanics and Industrial Technology, University of Florence, Florence, Italy, for his aid in thermographic and statistical analysis.
Abbreviations
CDI - colour Doppler imaging
CRA - central retinal artery
EDV - end‐diastolic velocity
IOP - intraocular pressure
OA - ophthalmic artery
ONH - optic nerve head
OST - ocular surface temperature
POAG - primary open‐angle glaucoma
PSV - peak‐systolic velocity
RI - resistivity index
SPCA - short posterior ciliary artery
Footnotes
Competing interests: None.
References
- 1.Gordon M O, Beiser J A, Brandt J D.et al The Ocular Hypertension Treatment Study. Baseline factors that predict the onset of primary open‐angle glaucoma. Arch Ophthalmol 2002120714–720. [DOI] [PubMed] [Google Scholar]
- 2.Heijl A, Leske M C, Bengtsson B, for the EMGT group Reduction of intraocular pressure and glaucoma progression. Results of the Early Manifest Glaucoma Trial. Arch Ophthalmol 20021201268–1279. [DOI] [PubMed] [Google Scholar]
- 3.The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS). VII: the relationship between control of IOP and visual field deterioration, Am J Ophthalmol 2000130429–440. [DOI] [PubMed] [Google Scholar]
- 4.Feiner L, Piltz‐Seymour J R. Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol 200314106–111. [DOI] [PubMed] [Google Scholar]
- 5.Flammer J. To what extent are vascular factors involved in the pathogenesis of glaucoma? In: Kaiser HJ, Flammer J, Hendrickson PH, eds. Ocular blood flow. New insights into the pathogenesis of ocular disease Basel: Karger, 199612–39.
- 6.Broadway D C, Drance S M. Glaucoma and vasospasm. Br J Ophthalmol 199882862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flammer J, Haefliger I O, Orgül S.et al Vascular dysregulation: a principal risk factor for glaucomatous damage? J Glaucoma 19998212–219. [PubMed] [Google Scholar]
- 8.Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res 200120319–349. [DOI] [PubMed] [Google Scholar]
- 9.Emre M, Orgül S, Gugleta K.et al Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol 200488662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieshaber C G, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol 20051679–83. [DOI] [PubMed] [Google Scholar]
- 11.Harris A, Sergott R C, Smith M.et al Color Doppler analysis of ocular vessel blood velocity in normal‐tension glaucoma. Am J Ophthalmol 1994118642–649. [DOI] [PubMed] [Google Scholar]
- 12.Galassi F, Sodi A, Rossi M G.et al Ocular haemodynamics in some subgroups of normal pressure glaucoma. Acta Ophthalmol Scand Suppl 199722435–36. [DOI] [PubMed] [Google Scholar]
- 13.Galassi F, Sodi A, Rossi M G.et al Results of color Doppler imaging in various types of glaucoma. In: Pillunat LE, Harris A, Anderson DR, Greve EL, eds. Current concepts on ocular blood flow in glaucoma. The Hague, The Netherlands: Kugler Publication, 1999119–127.
- 14.Galassi F, Sodi A, Ucci F.et al Ocular haemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch Ophthalmol 20031211711–1715. [DOI] [PubMed] [Google Scholar]
- 15.Satilmis M, Orgül S, Doubler B.et al Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure. Am J Ophthalmol 2003135664–669. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A, Sanchez M. Predictive value of colour Doppler imaging in a prospecive study of visual field progression in primary open‐angle glaucoma. Acta Ophthalmol Scand 200583716–722. [DOI] [PubMed] [Google Scholar]
- 17.Purslow C, Wolffsohn J S. Ocular surface temperature. A review. Eye Contact Lens 200531117–123. [DOI] [PubMed] [Google Scholar]
- 18.Mapstone R. Determinants of corneal temperature. Br J Ophthalmol 196852729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman R D, Fatt I. Environment influences on ocular temperature. Invest Ophthalmol 197312596–602. [PubMed] [Google Scholar]
- 20.Morgan P B, Soh M P, Efron N. Potential application of ocular thermography. Optom Vis Sci 199370568–576. [DOI] [PubMed] [Google Scholar]
- 21.Koçak I, Orgül S, Flammer J. Variability in the measurement of corneal temperature using a non‐contact infrared thermometer. Ophthalmologica 1999213345–349. [DOI] [PubMed] [Google Scholar]
- 22.Craig J P, Singh I, Tomlinson A.et al The role of tear physiology in ocular surface temperature. Eye 200014635–641. [DOI] [PubMed] [Google Scholar]
- 23.Efron N, Brennan N, Hore J.et al Temperature of the hyperaemic bulbar conjunctiva. Curr Eye Res 19887615–618. [DOI] [PubMed] [Google Scholar]
- 24.Fujishima H, Toda I, Yamada M.et al Corneal temperature in patients with dry eye evaluated by infrared thermometer. Br J Ophthalmol 19968029–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mapstone R. Corneal thermal patterns in anterior uveitis. Br J Ophthalmol 196852917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corvi A, Innocenti B, Mencucci R. Thermography used for analysis and comparison of different cataract surgery procedures based on phacoemulsification. Physiol Meas 200627371–384. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado‐Codina C, Morgan P B, Efron N. Thermal consequences of photorefractive keratectomy. Cornea 200120509–515. [DOI] [PubMed] [Google Scholar]
- 28.Auker C R, Parver L M, Doyle T.et al Choroidal blood flow. I. Ocular tissue temperature as a measure of flow. Arch Ophthalmol 19821001323–1326. [DOI] [PubMed] [Google Scholar]
- 29.Girardin F, Orgül S, Erb C.et al Relationship between corneal temperature and finger temperature. Arch Ophthalmol 1999117166–169. [DOI] [PubMed] [Google Scholar]
- 30.Morgan P B, Smyth J V, Tullo A B.et al Ocular temperature in carotid artery stenosis. Optom Vis Sci 199976850–854. [DOI] [PubMed] [Google Scholar]
- 31.Gugleta K, Orgül S, Flammer J. Is corneal temperature correlated with blood‐flow velocity in the ophthalmic artery? Curr Eye Res 199919496–501. [DOI] [PubMed] [Google Scholar]
- 32.Harris A, Williamson T H, Martin B.et al Test/retest reproducibility of color Doppler imaging assessment of blood flow velocity in orbital vessels. J Glaucoma 19954281–286. [PubMed] [Google Scholar]
- 33.Quaranta L, Harris A, Donato F.et al Color Doppler imaging of ophthalmic artery blood flow velocity. A study of repeatibility and agreement. Am J Ophthalmol 1997104653–658. [DOI] [PubMed] [Google Scholar]
- 34.Matthiessen E T, Zeitz O, Richard G.et al Reproducibility of blood flow velocity measurements using colour decoded Doppler imaging. Eye 200418400–405. [DOI] [PubMed] [Google Scholar]