Abstract
Background
Uveal melanoma arises in an immune‐privileged site and can itself add to the immunosuppressive environment. Previous studies on cutaneous melanoma have shown the presence of tolerogenic dendritic cells (DCs), which could play an important role in the progression of the tumour.
Aim
To examine the presence and functional status of DCs in a small series of uveal melanomas.
Methods
10 cases of uveal melanoma were examined for the expression of FXIIIa, CD68, human leucocyte antigen (HLA)‐DR, CD40, CD83, transforming growth factor βR1 and indolamine 2,3 dioxygenase by immunohistochemical analysis on sections embedded in paraffin wax.
Results
CD68‐positive macrophages were present in all of the tumours and were evenly distributed throughout. DCs expressing FXIIIa‐positive were seen in 7 cases, and were often found concentrated in foci within the tumour mass. These cells were dendritic and expressed high levels of HLA‐DR. The DCs did not express the maturation markers CD83 or CD40. In one case, concentration of DCs around the area of tumour necrosis was observed, and some of these cells expressed CD83.
Conclusion
Numerous tolerising antigen‐presenting cells may play a role in melanoma‐related immunosuppression in the eye, although activation of DCs may be associated with tumour necrosis.
Uveal melanoma is a potentially immunostimulatory tumour1,2,3 that is particularly interesting as it develops in the eye, an immune‐privileged site.4,5 Immune privilege in the eye is achieved by a number of mechanisms, including the secretion of immunosuppressive cytokines—particularly, two isoforms of transforming growth factor (TGF)β.6,7 TGFβ mediates a variety of effects, including the modulation of fibrosis in response to injury, suppression of the growth of melanocytes (but not malignant melanoma cells) and immunosuppression. TGFβ is also ubiquitously expressed by uveal melanoma, and the presence of TGFβ2 has been correlated with the progression of tumour.8
Despite this background of immune suppression, numerous immune cells have been found within uveal melanoma, including macrophages and T lymphocytes.9,10,11,12 In particular, tumour‐infiltrating macrophages enhance melanoma‐inhibitory activity12 and microvascularisation: a fundamental condition for melanoma dissemination.13
Dendritic cells (DCs) function as professional antigen‐presenting cells (APCs) and are critical for the initiation of primary immune responses14 and have been associated with tumour progression in cutaneous melanoma.15,16 Therefore, in this preliminary study, we have investigated the presence and maturation of DCs in uveal melanoma, including their morphology, surface antigen expression and tolerogenic function expressed by secretion of indolamine 2,3 dioxygenase (IDO)–an immunosuppressive enzyme produced mainly by APCs of myeloid origin.17,18
Materials and methods
Patients and samples
Ten uveal melanoma specimens obtained during therapeutic enucleation were investigated (table 1). The specimens were fixed for 24–48 h in 10% neutral buffered formalin and processed to paraffin wax in a vacuum processor (Shandon, DAKO Autostainer Universal Staining System, DAKO, Ely, Cambridgeshire, UK). All patients gave informed consent before the surgery. The study was conducted with multi‐centre and local research ethics committee approval.
Table 1 Data of patients included in the study.
| Sex | Age (years) | Maximal tumour diameter (mm) | Tumour type | |
|---|---|---|---|---|
| 1 | M | 56 | 19 | Mixed |
| 2 | M | 55 | 23 | Spindle |
| 3 | M | 53 | 8 | Mixed |
| 4 | F | 64 | 9 | Mixed |
| 5 | F | 55 | 13 | Mixed |
| 6 | M | 56 | 13 | Mixed |
| 7 | M | 59 | 15 | Mixed |
| 8 | M | 41 | 14 | Spindle |
| 9 | M | 25 | 20 | Epithelioid |
| 10 | M | 70 | 20 | Epithelioid |
F, female; M, male.
Immunohistochemistry
Sections, 4 μm thick were stained for FXIIIa (rabbit polyclonal antibody: Calbiochem, Notingham, UK), a marker expressed by interdigitating DCs independently of their maturity and subset,16,17 CD68 (PGM1), expressed by tissue macrophages and tissue DCs, human leucocyte antigen (HLA)‐DR (mouse monoclonal antibodies: Dako, Cambridgeshire, UK), expressed by APCs and essential for antigen presentation; CD40, expressed by activated DCs and necessary for correct interactions with T lymphocytes; CD83, expressed by mature DCs, TGFβR1 (mouse monoclonal antibodies, Novocastra, Newcastle Upon Tyne), necessary for TGFβ signal transduction; and IDO (mouse monoclonal antibody, Chemicon, Chandlers Ford, Hampshire, UK), an immunosuppressive enzyme secreted mainly by tolerogenic myeloid APCs.18 Antibody binding was detected in a three‐step procedure using a multipurpose biotinylated secondary antibody, and streptavidin‐conjugated alkaline phosphatase (Vectastain Universal ABC‐AP kit, Vector Laboratories, Peterborough, UK) or streptavidin‐conjugated horse peroxidase. To avoid confusion with melanin, a red chromogen, Fuchsin (Dako), was used. For double staining, we chose lightly pigmented sections and used DAB (Kem‐En‐Tec Diagnostic, Taastrup, Denmark) as the second chromogen.
Microscopic examination
All slides were examined by three independent investigators (SDP, IAC and MEP), and the staining assessed, (1) for the presence of positive cells at the periphery compared with the centre of the tumour; (2) random distribution through the section compared with concentration in specific areas with absence in remaining tissue and (3) forming groups compared with single‐cell appearance only.
Image analysis
Photographs scanning whole sections were taken using a digital 3CCD JVC camera (KY‐F55B, London, UK) linked to an Olympus CH microscope; one photograph representing one rectangular optical field (OF; area 0.297 mm2). Additionally, for morphology analyses, high‐power photographs of areas selected for high positive cell concentration (hot spot (HS); area 0.35 mm2) were taken. PGM1‐positive HSs were selected by choosing fields that did not contain either melanophages or FXIIIa‐positive cells. Both cell morphology and total staining area were measured using Image ProPlus 4.5 (Media Cybernetics UK, Buckinghamshire, UK) software. Cell morphology was assessed using the coefficient of shape, the dendrite number and dendritic length. The higher the value of the coefficient of shape, the more irregular or dendritic the cell.
Statistical analysis
Median values were used for comparisons between cases. Analyses of CD68, FXIIIa and HLA‐DR staining were performed using one parameter paired analysis of variance (ANOVA).
Results
Presence and distribution of macrophages and DCs within uveal melanoma
Data are shown as percentage positive staining per total tumour area, with the median area per OF and range of values in parentheses (both in μm2; table 2).
Table 2 Extent of the staining for antigen‐presenting cell markers.
| FXIIIa | CD68 | HLA‐DR | |
|---|---|---|---|
| 1 | 0.27% (576, 0–13 888) | 1.86% (3082, 0–45 361) | 0.18% (847, 0–1800) |
| 2 | 0.00% (0, 0–89) | 0.02% (508, 0–10 061) | 0.05% (1215, 0–13 367) |
| 3 | 0.00% (0, 0) | 0.19% (292, 0–2646) | 0.26% (861, 0–1690) |
| 4 | 0.00% (0, 0–123) | 0.07% (510, 0–12 867) | 0.03% (376, 0–7296) |
| 5 | 0.29% (724, 0–4576) | 0.84% (899, 0–30 857) | 0.19% (604, 0–9884) |
| 6 | 0.10% (485, 0–3716) | 0.75% (2153, 0–4578) | 0.15% (731, 0–3738) |
| 7 | 0.00% (0, 0) | 0.01% (177, 0–1130) | 0.01% (40, 0–1708) |
| 8 | 0.00% (0, 0) | 0.07% (434, 0–9988) | 0.03% (2189, 0–10 801) |
| 9 | 0.03% (0, 0–3084) | 0.09% (439, 0–4263) | 0.02% (135, 3328) |
| 10 | 0.05% (450, 0—957) | 0.32% (2589, 0–21 330) | 0.17% (622, 0–8841) |
HLA, human leucocyte antigen.
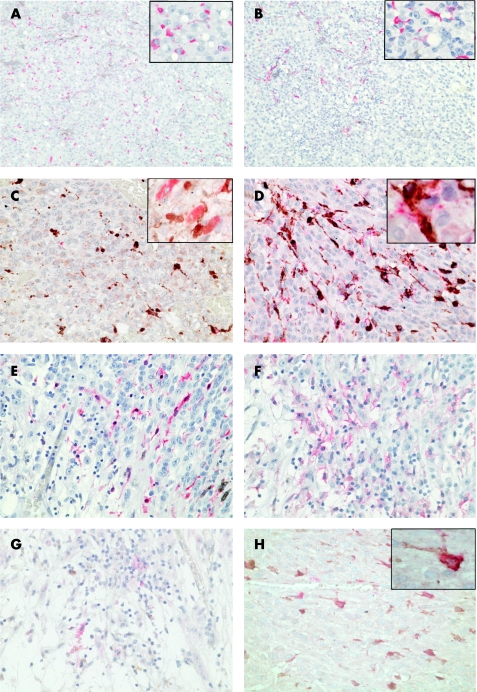
To identify macrophages, we performed immunohistochemical analysis with CD68 (PGM1) antibody. Although the tumour deposits varied in size and melanoma cell type, we identified numerous CD68‐positive cells in all cases, evenly distributed throughout the section (fig 1A). Three main cell types expressing CD68 were identified: smaller, non‐pigmented cells of either rounded or irregular shape, and very large, pigmented melanophages. An influx of melanophages was seen in all of the sections, often concentrated on the border of the tumour adjacent to normal tissue. The CD68‐positive non‐pigmented cells were abundant in all of the tumours investigated, occupying 0.01–1.86% of the tumour area (median 177.5–3082.8 μm2; table 2). They were randomly distributed throughout the section, either as single cells or in small groups (fig 1A; table 3). Areas of concentration were observed in three cases (table 3).
Figure 1 Expression of dendritic cell markers in uveal melanoma. The photographs (×200) show the typical distribution of (A) CD68‐positive cells, randomly distributed throughout the section and (B) FXIIIa‐positive cells, concentrated mainly in the left top part of the section. The insets in the right upper corner (×400) show the typical morphology of (A) rounded CD68‐positive and (B) irregular FXIIIa‐positive cells, with dendrites. (C) Double staining with CD68 (brown) and FXIIIa (red) in areas of high concentration of FXIIIa‐positive cells (×400), with a detailed picture in the inset. (D) Double staining with HLA‐DR (red) and CD68 (brown) (×400), with a detailed picture in the inset, showing that the human leucocyte antigen (HLA)‐DR antigen‐presenting molecule is expressed mainly by antigen‐presenting cells (APCs). (E) FXIIIa, (F) HLA‐DR and (G) CD83 in an area of tumour necrosis (×200). (H) Double staining with FXIIIa and transforming growth factor (TGF) βR1 (×400) shows that all FXIIIa‐positive dendritic cells express TGFβR1. A double‐positive APC is shown in detail in the inset.
Table 3 Distribution of antigen‐presenting cell markers in uveal melanoma.
| Periphery | Centre | Single cells | Concentration areas | Clusters | |
|---|---|---|---|---|---|
| FXIIIa | 7/7* | 3/7 | 6/7 | 6/7 | 3/7 |
| CD68 | 10/10 | 10/10 | 9/10 | 3/10 | 4/10 |
| HLA‐DR | 10/10 | 10/10 | 9/10 | 6/10 | 5/10 |
| TGFβ1R | 10/10 | 10/10 | 9/10 | 4/10 | 4/10 |
HLA, human keucocyte antigen; TGF, transforming growth factor.
*Number of positive cases/number of cases in category.
As both tissue macrophages and interdigitating DCs express CD68, to further discriminate DCs we used an antibody against FXIIIa (fig 1B). The marker was chosen as particularly suitable, as it visualises DCs irrespectively of their functional subset and maturation status.16,17 To confirm the identity of FXIIIa‐positive cells, their morphology was examined carefully, as it is another hallmark of DCs, independent of subset‐associated antigen expression.19 FXIIIa‐positive DCs were present in seven tumours, occupying between 0.01% and 2.9% of the tumour area (table 2). The cells were unevenly distributed, and, although usually concentrated in particular areas, they did not create clusters but were independently located, appearing to infiltrate the melanoma from the border of the tumour and from blood vessels within the tumour mass (table 3). In one case, they were so abundant that they formed strings or networks (fig 1B). However, FXIIIa‐positive cells were absent in residual normal eye tissue. FXIIIa‐positive cells were present deep within the tumour tissue, and in the areas infiltrated by melanophages, usually on the periphery of the tumour (table 2). In both situations, the majority of FXIIIa‐positive cells were of irregular shape with extensive dendrites, often surrounding adjacent cells. Figure 1C shows the coexpression of CD68 (brown) and FXIIIa (red).
In one case, we observed a large area of tumour necrosis heavily infiltrated by FXIIIa‐positive DC and large, granular, tissue macrophages (fig 1D–F). Interestingly, expression of FXIIIa was strictly limited to the necrotic area (fig 1D), whereas CD68‐positive cells, although concentrated there, were also present in the remaining tumour tissue. No melanophages were seen in the necrotic area. The DCs were irregular in shape, with numerous dendrites, and expressed HLA‐DR (fig 1E). Moreover, some of the cells expressed CD83, a marker that identifies fully mature DCs (fig 1F).
Expression of HLA‐DR
We next examined the expression of HLA‐DR as a measure of DC maturation and APC function. HLA‐DR was widely distributed on a variety of cell types throughout the tumour, including melanophages (table 3/fig 1E,G,H). HLA‐DR‐positive cells occupied between 0.01% and 0.26% of the tumour area, and the median area of staining per OF ranged from 40.5 to 1215.4 μm2. The extent of staining exceeded that of FXIIIa in seven cases (table 2); however, HS areas of HLA‐DR‐positive cells were found in six out of seven FXIIIa‐positive cases, and corresponded to the foci of FXIIIa (table 3). The distribution closely matched that of CD68, but was generally less extensive (table 3). HLA‐DR staining was generally weaker and the intensity was similar on all cell types. Figure 1G shows the coexpression of CD68 (brown) and HLA‐DR (red) in an FXIIIa‐negative case.
Morphology
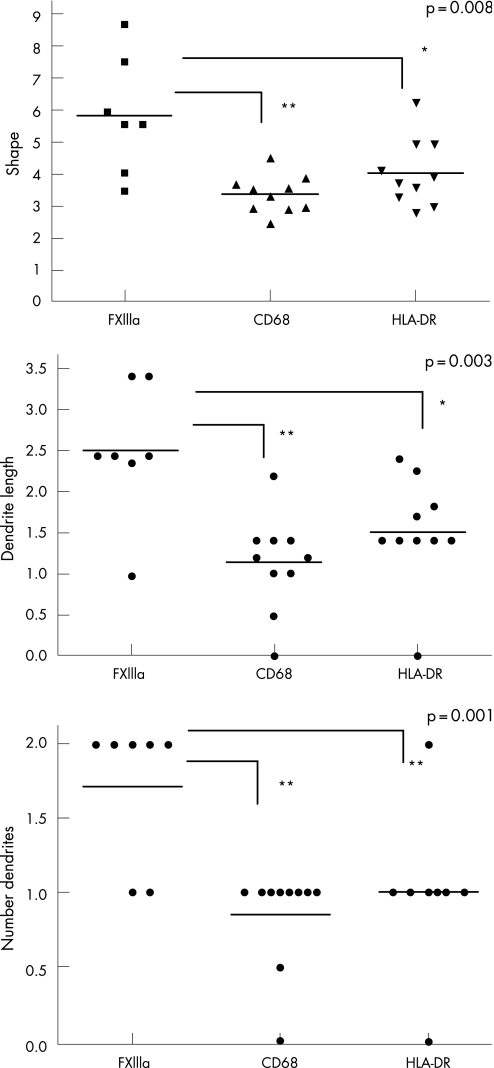
Since mature DCs are irregular in shape, with numerous, long dendrites, we analysed the shape, dendrite number and dendrite length of detected APCs by means of image analysis. FXIIIa‐positive DCs were much more irregular than CD68‐ or HLADR‐positive cells, with a median coefficient of shape of 5.50 compared with 3.43 and 3.73 for CD68 and HLA‐DR, respectively (p = 0.008, paired ANOVA). Nevertheless, in three cases containing the most significant numbers of DCs (1, 5 and 6), the case median coefficient of shape of HLADR‐positive cells was much closer to that of FXIIIa‐positive cells (data not shown). FXIIIa‐positive cells had more numerous dendrites. The median number of dendrites per cell was 2.00, compared with just 1.00 for both CD68 and HLA‐DR‐positive cells (p = 0.001, paired ANOVA). The dendrites were also longer, with median length 2.41 μm compared with 1.20 μm for CD68‐positive cells and 1.41 μm for HLA‐DR‐positive cells (p = 0.0027, paired ANOVA). Cells expressing HLA‐DR were slightly more dendritic than CD68, but far less than FXIIIa‐positive cells (fig 2). An example of the typical morphology of CD68‐ and FXIIIa‐positive cells is shown in the right upper corner of fig 1A and 1B, respectively (×400).
Figure 2 Morphology of antigen‐presenting cells in uveal melanoma. For morphology analysis, high‐power photographs of selected areas of high positive cell concentration (hot spot (HS); area: 0.35 mm2) were taken. The graphs show the coefficient of shape (A), dendrite length (B) and number of dendrites (C) on FXIIIa, CD68 and human leucocyte antigen (HLA)‐DR expressing cells. The dots represent median values for all cells in the HS area. The horizontal line represents the median value for each morphologic feature. The higher the coefficient of shape, the more dendritic the object. The asterisks show the significance of the difference using analysis of variance. *p<0.05, **p<0.01.
Expression of TGFβR1
Uveal melanoma contains large amounts of TGFβ1 and TGFβ2.8 Because this molecule has potent immunosuppressive effects, we examined the expression of one of the receptors, TGFβR1, on uveal melanoma and infiltrating APCs. Expression of TGFβR1 is essential for TGFβ signal transduction, after the growth factor is bound by TGFβRII, and a complex with TGFβR1 is created.20 TGFβR1 was widely distributed throughout the tumour, including some melanoma cells, but greater expression was seen on large numbers of small, rounded and dendritic cells. The cells intensely expressing TGFβR1 were more numerous than those expressing any other marker, indicating that this receptor can be found on non‐melanoma cells other than CD68 APC. The results of a double staining with FXIIIa and TGFβR1 antibody (fig 1H) show that FXIIIa‐positive DCs express the receptor to TGFβ.
Maturity of DCs present in uveal melanoma
To investigate the maturation of DC in uveal melanomas, we analysed the expression of CD40 and CD83. Both of these molecules are expressed by activated, mature DCs. CD40 was not seen on any DC, but was observed on pigmented melanophages in seven cases. CD83 was observed in only one case, on DC within an area of necrosis (fig 1F).
Expression of IDO
One mechanism of immunosuppression exhibited by DC is secretion of the enzyme IDO, which is associated with the progression of cutaneous melanoma.21 The expression of IDO was uncommon in uveal melanoma, and was associated only with melanophages in three cases. The positive cells were found mainly on the tumour border.
Discussion
As APCs, especially DCs, play an important role in the progression of cutaneous melanoma, we examined the presence of intratumoral APC in uveal melanoma. We confirmed that CD68‐positive cells were present in all cases examined, comprising a heterogeneous population of APCs. In normal eyes, DCs are found in corneal epithelium and stroma,22,23,24,25 and in the choroid and ciliary body.26 We observed FXIIIa‐positive DCs with dendritic morphology within the tumour mass in 70% of cases, in surprisingly high numbers in three cases. These cells coexpressed CD68 and HLA‐DR; however, unlike CD68‐positive macrophages, they were concentrated together in foci rather than randomly distributed throughout the tissue. They also had visibly more irregular shape than CD68‐positive macrophages. As the majority of tumour APCs were macrophages, this was reflected in the comparable morphology of CD68 and HLA‐DR cells. However, in cases where the percentage of FXIIIa‐positive cells was more substantial, both the shape and the localisation of HLA‐DR‐positive cells resembled those of DCs. The distribution of DCs within the tumour suggests that they penetrate the tumour from the periphery to the centre of the tumour mass, or from the vasculature and actively concentrate in necrotic tissue, probably in response to chemokines.
Both DCs residing in the eye and those migrating from the blood stream are immature peripheral DCs that after antigen uptake and appropriate secondary stimulation, may differentiate into mature DCs and initiate immune responses in peripheral lymphoid tissue.27 However, in uveal melanoma, the microenvironment is strongly immunosuppressive, largely due to TGFβ,8 which can immobilise DCs and prevent their maturation.28 TGFβ may also indirectly interfere with DC maturation, by inhibiting the production of IFNγ by natural killer cells,29 or, by way of interactions with TGFβ‐modulated regulatory T lymphocytes, convert them into the tolerogenic DC‐secreting immunosuppressive enzyme, IDO.30,31 We visualised very strong expression of TGFβR1 on CD68‐positive macrophages, as was observed in ovarian cancer,32 and on FXIIIa‐positive DC, rendering them susceptible to TGFβ‐mediated immunosuppression. The DCs did not express maturation and activation antigens—that is, CD40, CD83—and did not overexpress HLA‐DR, in contrast with their more mature, dendritic morphology. Moreover, their distribution suggests that they can actively migrate within the tissue and concentrate in specific areas. However, visualised DCs did not secrete IDO—an immunosuppressive enzyme secreted by tolerogenic DCs. We therefore conclude that, even though tumour DCs are probably affected by the immune‐privileged environment inside the eye, this prevents their maturation, and does not turn them into DCs with a specialised, tolerogenic phenotype.
Immature DCs, although capable of antigen presentation, lack immune‐activating abilities and can suppress antigen‐specific immune responses, anergise T lymphocytes and promote tumour progression.33,34 If they stay within the tumour mass, they may impair the function of any incoming T lymphocytes, and at the same time support the immune‐privileged character of the site. If they leave the eye via the bloodstream, they could potentially carry tumour antigens and present these without correct costimulatory signals in spleen and in peripheral lymph nodes.35 Such migrating antigen‐loaded permanently immature DCs would attenuate systemic anti‐tumour immune responses and may assist tumour survival and metastasis. They may also play a physiological role as important promoters of anterior chamber‐associated immune deviation, a mechanism of peripheral tolerance to eye‐derived antigens.36
It is perhaps of note that more mature DCs were seen in an area of necrosis, suggesting that, given the correct stimuli, they may undergo final maturation. It agrees with a finding by others, that the tissue injury may lead to antitumour immunity.37 Recent studies on mice showed that additional stimulation of DCs with CD40 ligand caused successful eradication of tumour.38 It is possible, therefore, that DCs in uveal melanoma might be responsive to external adjuvant treatment, ultimately inducing a local anti‐tumour immune response.
The presence of DCs in uveal melanoma is likely to affect the immunobiology of the tumour and its metastases, and may therefore be relevant to disease management. However, further investigation involving a significantly larger number of subjects is required to evaluate the exact role, mechanisms of action and correlation of these observations with the process of metastasis.
Acknowledgements
We thank Cancer Research UK (grant no C7498/A5113) and the Wessex Cancer Trust (grant no 128) for their support.
Abbreviations
ANOVA - analysis of variance
APC - antigen‐presenting cell
DC - dendritic cell
HLA - human leucocyte antigen
HS - hot spot
IDO - indolamine 2,3 dioxygenase
OF - optical field
TGF - transforming growth factor
Footnotes
Competing interests: None declared.
References
- 1.Carlring J, Shaif‐Muthana M, Sisley K.et al Apoptotic cell death in conjunction with CD80 costimulation confers uveal melanoma cells with the ability to induce immune responses. Immunology 200310941–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaif‐Muthana M, McIntyre C, Sisley K.et al Dead or alive: immunogenicity of human melanoma cells when presented by dendritic cells. Cancer Res 2000606441–6447. [PubMed] [Google Scholar]
- 3.Kan‐Mitchell J, Liggett P E, Harel W.et al Lymphocytes cytotoxic to uveal and skin melanoma cells from peripheral blood of ocular melanoma patients. Cancer Immunol Immunother 199133333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallermalm K, De Geer A, Kiessling R.et al Autocrine secretion of Fas ligand shields tumor cells from Fas‐mediated killing by cytotoxic lymphocytes. Cancer Res 2004646775–6782. [DOI] [PubMed] [Google Scholar]
- 5.Ijland S A, Jager M J, Heijdra B M.et al Expression of angiogenic and immunosuppressive factors by uveal melanoma cell lines. Melanoma Res 19999445–450. [DOI] [PubMed] [Google Scholar]
- 6.Schlotzer‐Schrehardt U, Dorfler S. Immunolocalization of growth factors in the human ciliary body epithelium. Curr Eye Res 199312893–905. [DOI] [PubMed] [Google Scholar]
- 7.Pasquale L R, Dorman‐Pease M E, Lutty G A.et al Immunolocalization of TGF‐beta 1, TGF‐beta 2, and TGF‐beta 3 in the anterior segment of the human eye. Invest Ophthalmol Vis Sci 19933423–30. [PubMed] [Google Scholar]
- 8.Esser P, Grisanti S, Bartz‐Schmidt K. TGF‐beta in uveal melanoma. Microsc Res Tech 200152396–400. [DOI] [PubMed] [Google Scholar]
- 9.Woodward J, Sisley K, Reeves G.et al Evidence of macrophage and lymphocyte, but not dendritic cell, infiltration in posterior uveal melanomas, whilst cultured uveal melanomas demonstrate pluripotency by expressing CD68 and CD163. Int J Exp Pathol 20048535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toivonen P, Makitie T, Kujala E.et al Microcirculation and tumor‐infiltrating macrophages in choroidal and ciliary body melanoma and corresponding metastases. Invest Ophthalmol Vis Sci 2004451–6. [DOI] [PubMed] [Google Scholar]
- 11.de Waard‐Siebinga I, Hilders C G, Hansen B E.et al HLA expression and tumor‐infiltrating immune cells in uveal melanoma. Graefe's Arch Clin Exp Ophthalmol 199623434–42. [DOI] [PubMed] [Google Scholar]
- 12.Callejo S A, Marshall J C, Cools‐Lartigue J.et al Macrophage‐derived soluble factor enhances melanoma inhibitory activity expression by uveal melanoma cells in vitro. Melanoma Res 20041491–95. [DOI] [PubMed] [Google Scholar]
- 13.Makitie T, Summanen P, Tarkkanen A.et al Tumor‐infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest Ophthalmol Vis Sci 2001421414–1421. [PubMed] [Google Scholar]
- 14.Hart D N. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 1997903245–3287. [PubMed] [Google Scholar]
- 15.Lana A M, Wen D R, Cochran A J. The morphology, immunophenotype and distribution of paracortical dendritic leucocytes in lymph nodes regional to cutaneous melanoma. Melanoma Res 200111401–410. [DOI] [PubMed] [Google Scholar]
- 16.Polak M E, Johnson P, Di Palma S.et al Presence and maturity of dendritic cells in melanoma lymph node metastases. J Pathol 200520783–90. [DOI] [PubMed] [Google Scholar]
- 17.Cerio R, Griffiths C E, Cooper K D.et al Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol 1989121421–431. [DOI] [PubMed] [Google Scholar]
- 18.Mellor A L, Munn D H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 20044762–774. [DOI] [PubMed] [Google Scholar]
- 19.Steinman R M, Witmer‐Pack M, Inaba K. Dendritic cells: antigen presentation, accessory function and clinical relevance. Adv Exp Med Biol 19933291–9. [DOI] [PubMed] [Google Scholar]
- 20.Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol 200552329–337. [PubMed] [Google Scholar]
- 21.Polak M E, Borthwick N J, Gabriel F G.et al Mechanisms of local immunotolerance in cutaneous melanoma. Br J Cancer. In press [DOI] [PMC free article] [PubMed]
- 22.Yamagami S, Yokoo S, Usui T.et al Distinct populations of dendritic cells in the normal human donor corneal epithelium. Invest Ophthalmol Vis Sci 2005464489–4494. [DOI] [PubMed] [Google Scholar]
- 23.McMenamin P G. The distribution of immune cells in the uveal tract of the normal eye. Eye 199711(Pt 2)183–193. [DOI] [PubMed] [Google Scholar]
- 24.Catry L, Van den Oord J, Foets B.et al Morphologic and immunophenotypic heterogeneity of corneal dendritic cells. Graefe's Arch Clin Exp Ophthalmol 1991229182–185. [DOI] [PubMed] [Google Scholar]
- 25.Hamrah P, Liu Y, Zhang Q.et al The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci 200344581–589. [DOI] [PubMed] [Google Scholar]
- 26.Forrester J V, Lumsden L, Duncan L.et al Choroidal dendritic cells require activation to present antigen and resident choroidal macrophages potentiate this response. Br J Ophthalmol 200589369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieira P L, de Jong E C, Wierenga E A.et al Development of Th1‐inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol 20001644507–4512. [DOI] [PubMed] [Google Scholar]
- 28.Halliday G M, Le S. Transforming growth factor‐beta produced by progressor tumors inhibits, while IL‐10 produced by regressor tumors enhances, Langerhans cell migration from skin. Int Immunol 2001131147–1154. [DOI] [PubMed] [Google Scholar]
- 29.Meadows S K, Eriksson M, Barber A.et al Human NK cell IFN‐gamma production is regulated by endogenous TGF‐beta. Int Immunopharmacol 200661020–1028. [DOI] [PubMed] [Google Scholar]
- 30.Huber S, Schramm C, Lehr H A.et al Cutting edge: TGF‐beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol 20041736526–6531. [DOI] [PubMed] [Google Scholar]
- 31.Manavalan J S, Kim‐Schulze S, Scotto L.et al Alloantigen specific CD8+CD28‐ FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol 2004161055–1068. [DOI] [PubMed] [Google Scholar]
- 32.Tamura M, Fukaya T, Enomoto A.et al Transforming growth factor‐beta isoforms and receptors in endometriotic cysts of the human ovary. Am J Reprod Immunol 199942160–167. [DOI] [PubMed] [Google Scholar]
- 33.Lutz M B, Schuler G. Immature, semi‐mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 200223445–449. [DOI] [PubMed] [Google Scholar]
- 34.Suciu‐Foca N, Manavalan J S, Scotto L.et al Molecular characterization of allospecific T suppressor and tolerogenic dendritic cells: review. Int Immunopharmacol 200557–11. [DOI] [PubMed] [Google Scholar]
- 35.Boonman Z F, van Mierlo G J, Fransen M F.et al Intraocular tumor antigen drains specifically to submandibular lymph nodes, resulting in an abortive cytotoxic T cell reaction. J Immunol 20041721567–1574. [DOI] [PubMed] [Google Scholar]
- 36.Stein‐Streilein J, Streilein J W. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol 200221123–152. [DOI] [PubMed] [Google Scholar]
- 37.Boonman Z F, van Mierlo G J, Fransen M F.et al Maintenance of immune tolerance depends on normal tissue homeostasis. J Immunol 20051754247–4254. [DOI] [PubMed] [Google Scholar]
- 38.van Mierlo G J, Boonman Z F, Dumortier H M.et al Activation of dendritic cells that cross‐present tumor‐derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol 20041736753–6759. [DOI] [PubMed] [Google Scholar]