Abstract
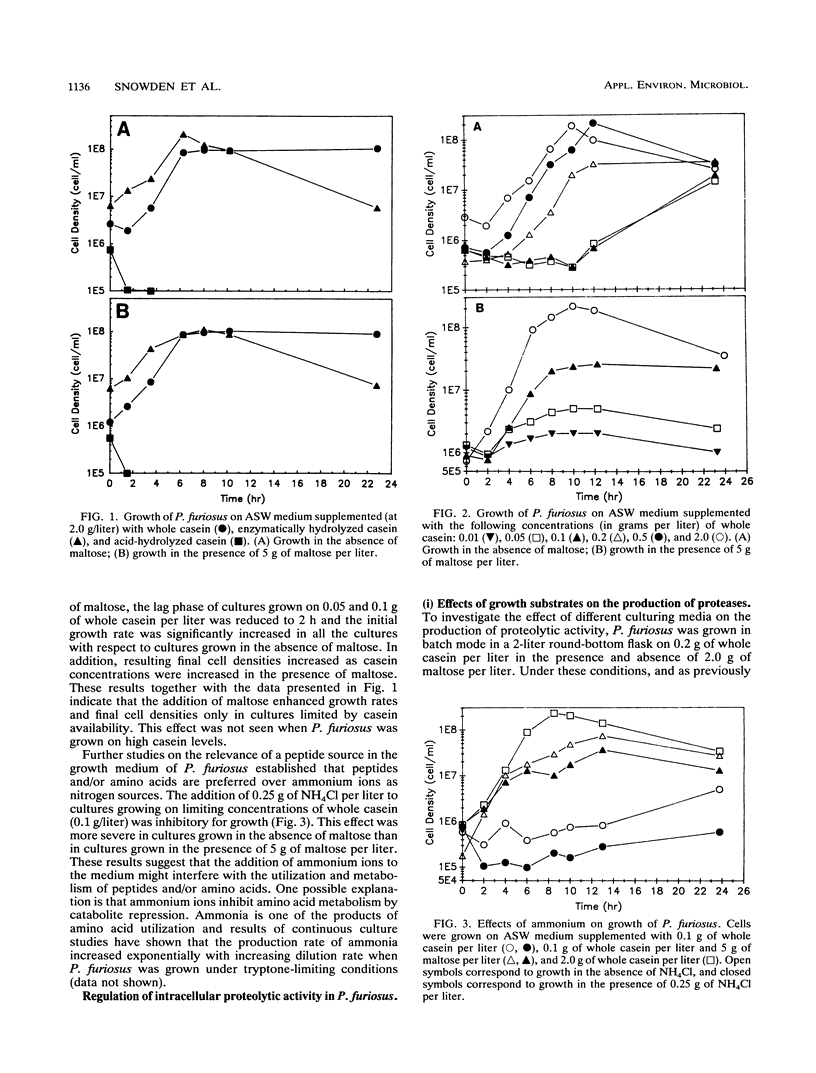
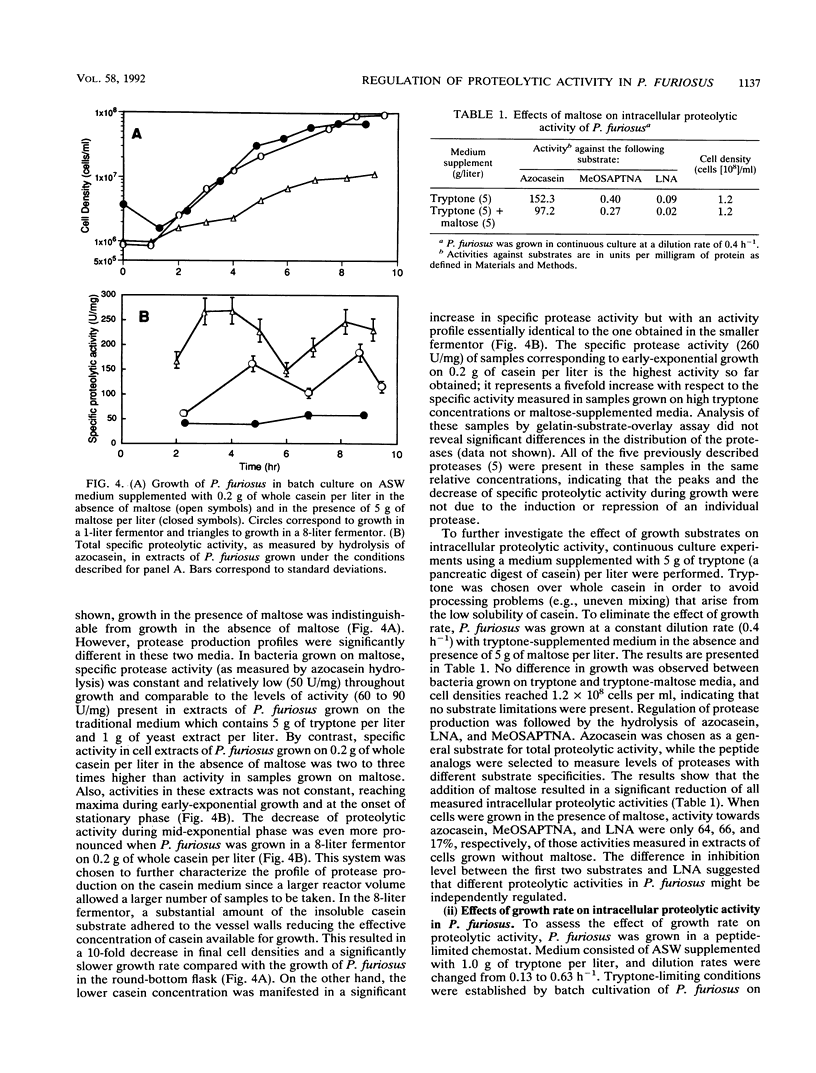
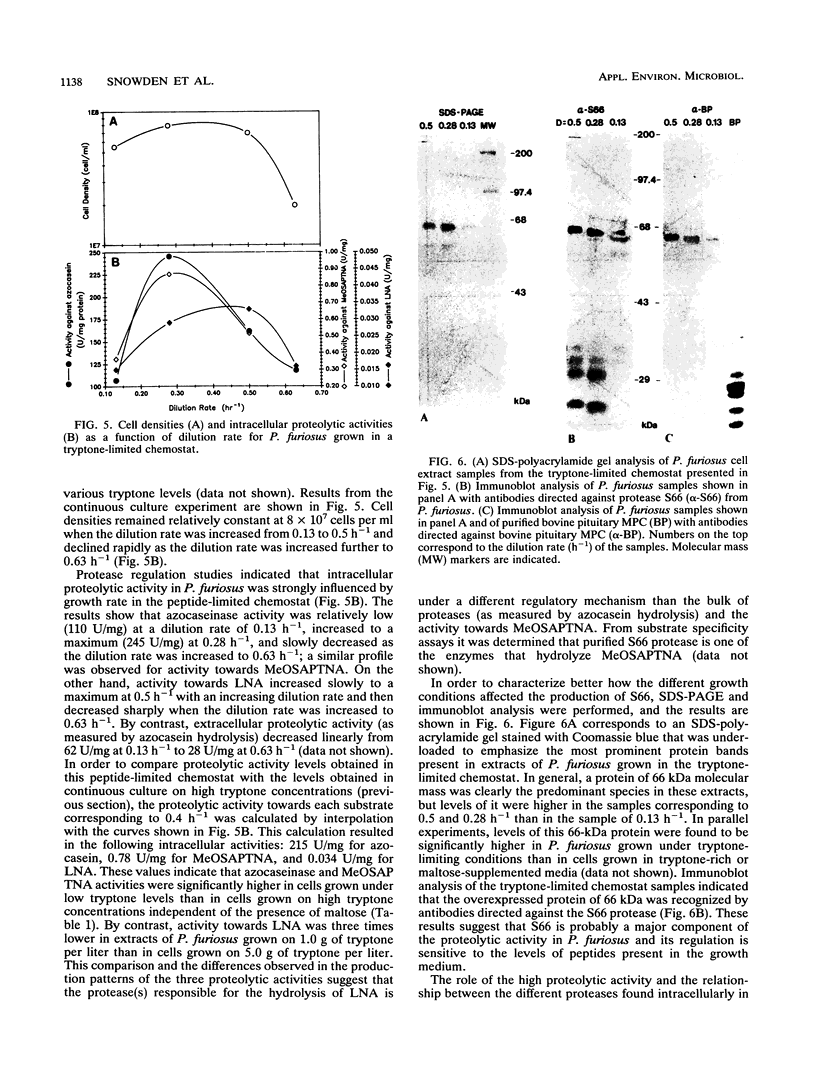
Pyrococcus furiosus was shown to grow on casein or peptides as the sole carbon, energy, and nitrogen sources, while maltose could be used as a carbon and energy source only if peptides were present in the medium. A mixture of all 20 single amino acids could not replace the peptide requirement. Specific intracellular proteolytic activity was induced under low casein or tryptone levels and was decreased by the addition of maltose to both peptide-limiting and peptide-rich media in batch and continuous cultures. In a peptide-limited chemostat, activity towards azocasein and MeO-Suc-Arg-Pro-Tyr-p-nitroanilide reached a maximum at a dilution rate of 0.28 h-1, while activity toward l-lysine-p-nitroanilide reached a maximum at 0.50 h-1. Under peptide-limiting conditions, levels of the 66-kDa protease (S66) were enhanced relative to those of other cell proteins. Preliminary evidence suggests that this protease is immunologically related to the eukaryotic multicatalytic proteinase complex (proteosome).
Full text
PDF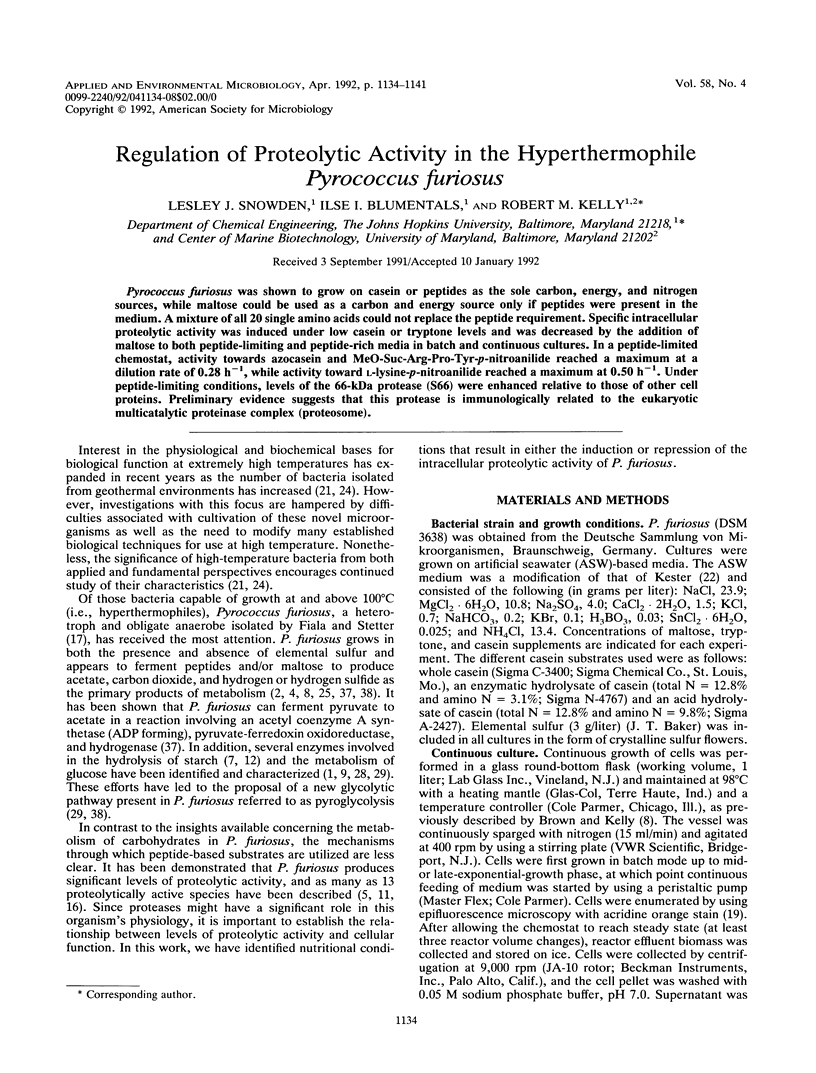




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono S., Bryant F. O., Adams M. W. A novel and remarkably thermostable ferredoxin from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Bacteriol. 1989 Jun;171(6):3433–3439. doi: 10.1128/jb.171.6.3433-3439.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Brown S. H., Schicho R. N., Skaja A. K., Costantino H. R., Kelly R. M. The hyperthermophilic archaebacterium, Pyrococcus furiosus. Development of culturing protocols, perspectives on scaleup, and potential applications. Ann N Y Acad Sci. 1990;589:301–314. doi: 10.1111/j.1749-6632.1990.tb24254.x. [DOI] [PubMed] [Google Scholar]
- Blumentals I. I., Itoh M., Olson G. J., Kelly R. M. Role of Polysulfides in Reduction of Elemental Sulfur by the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 May;56(5):1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Robinson A. S., Kelly R. M. Characterization of sodium dodecyl sulfate-resistant proteolytic activity in the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1992–1998. doi: 10.1128/aem.56.7.1992-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. H., Costantino H. R., Kelly R. M. Characterization of Amylolytic Enzyme Activities Associated with the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1985–1991. doi: 10.1128/aem.56.7.1985-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. H., Kelly R. M. Cultivation Techniques for Hyperthermophilic Archaebacteria: Continuous Culture of Pyrococcus furiosus at Temperatures near 100 degrees C. Appl Environ Microbiol. 1989 Aug;55(8):2086–2088. doi: 10.1128/aem.55.8.2086-2088.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- Costantino H. R., Brown S. H., Kelly R. M. Purification and characterization of an alpha-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115 degrees C. J Bacteriol. 1990 Jul;172(7):3654–3660. doi: 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmann B., Kopp F., Kuehn L., Niedel B., Pfeifer G., Hegerl R., Baumeister W. The multicatalytic proteinase (prosome) is ubiquitous from eukaryotes to archaebacteria. FEBS Lett. 1989 Jul 17;251(1-2):125–131. doi: 10.1016/0014-5793(89)81441-3. [DOI] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W., Wirsen C. O., Molyneaux S. J., Langworthy T. A. Extremely thermophilic fermentative archaebacteria of the genus desulfurococcus from deep-sea hydrothermal vents. Appl Environ Microbiol. 1988 May;54(5):1203–1209. doi: 10.1128/aem.54.5.1203-1209.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. M., Clark D. S. Engineering considerations for the application of extremophiles in biotechnology. Crit Rev Biotechnol. 1991;10(4):321–345. doi: 10.3109/07388559109038214. [DOI] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Mukund S., Adams M. W. Characterization of a tungsten-iron-sulfur protein exhibiting novel spectroscopic and redox properties from the hyperthermophilic archaebacterium Pyrococcus furiosus. J Biol Chem. 1990 Jul 15;265(20):11508–11516. [PubMed] [Google Scholar]
- Mukund S., Adams M. W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J Biol Chem. 1991 Aug 5;266(22):14208–14216. [PubMed] [Google Scholar]
- Nyström T., Flärdh K., Kjelleberg S. Responses to multiple-nutrient starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1990 Dec;172(12):7085–7097. doi: 10.1128/jb.172.12.7085-7097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M. The multicatalytic proteinase complex, a major extralysosomal proteolytic system. Biochemistry. 1990 Nov 13;29(45):10289–10297. doi: 10.1021/bi00497a001. [DOI] [PubMed] [Google Scholar]
- Payne J. W. Peptides and micro-organisms. Adv Microb Physiol. 1976;13:55–113. doi: 10.1016/s0065-2911(08)60038-7. [DOI] [PubMed] [Google Scholar]
- Pittman K. A., Lakshmanan S., Bryant M. P. Oligopeptide uptake by Bacteroides ruminicola. J Bacteriol. 1967 May;93(5):1499–1508. doi: 10.1128/jb.93.5.1499-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Smith M. R. Polypeptide nature of growth requirement in yeast extract for Thermoplasma acidophilum. J Bacteriol. 1975 Nov;124(2):884–892. doi: 10.1128/jb.124.2.884-892.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Ichihara A. Proteasomes (multicatalytic proteinase complexes) in eukaryotic cells. Cell Struct Funct. 1990 Jun;15(3):127–132. doi: 10.1247/csf.15.127. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersma M., Harder W. A continuous culture study of the regulation of extracellular protease production in Vibrio SA1. Antonie Van Leeuwenhoek. 1978;44(2):141–155. doi: 10.1007/BF00643217. [DOI] [PubMed] [Google Scholar]
- Wilk S., Orlowski M. Evidence that pituitary cation-sensitive neutral endopeptidase is a multicatalytic protease complex. J Neurochem. 1983 Mar;40(3):842–849. doi: 10.1111/j.1471-4159.1983.tb08056.x. [DOI] [PubMed] [Google Scholar]
- Zillig W., Holz I., Janekovic D., Klenk H. P., Imsel E., Trent J., Wunderl S., Forjaz V. H., Coutinho R., Ferreira T. Hyperthermus butylicus, a hyperthermophilic sulfur-reducing archaebacterium that ferments peptides. J Bacteriol. 1990 Jul;172(7):3959–3965. doi: 10.1128/jb.172.7.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwickl P., Lottspeich F., Dahlmann B., Baumeister W. Cloning and sequencing of the gene encoding the large (alpha-) subunit of the proteasome from Thermoplasma acidophilum. FEBS Lett. 1991 Jan 28;278(2):217–221. doi: 10.1016/0014-5793(91)80120-r. [DOI] [PubMed] [Google Scholar]
- de Sa C. M., Rollet E., de Sa M. F., Tanguay R. M., Best-Belpomme M., Scherrer K. Prosomes and heat shock complexes in Drosophila melanogaster cells. Mol Cell Biol. 1989 Jun;9(6):2672–2681. doi: 10.1128/mcb.9.6.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]



