Abstract
Background
Although ocular tonography measures a pulsatile component of the ocular perfusion, the retinal and/or choroidal components of this pulsatile flow remain undefined.
Aim
To compare ocular tonography with the assessment of flow velocities in arteries supplying the retina, choroid and entire orbit.
Methods
22 normal eyes from 11 subjects were studied. Pulsatile ocular blood flow (POBF) was measured using the ocular blood flow tonograph, and flow velocities in the ophthalmic, central retinal (CRA) and temporal short posterior ciliary arteries (TSPCA) using colour Doppler imaging. The correlation between POBF and retrobulbar flow velocities was determined.
Results
POBF correlated significantly with peak systolic velocity (PSV) of the CRA (r = 0.56, p = 0.007) and the TSPCA (r = 0.48, p = 0.02), and with the resistive index of the TSPCA (r = 0.45, p = 0.04). Additionally, pulse amplitude (PSV−end diastolic velocity) in the CRA and the TSPCA correlated significantly with POBF measurements (each p<0.05). However, POBF did not correlate with any flow velocity indices in the ophthalmic artery.
Conclusion
POBF is associated with systolic and pulsatile components of blood flow velocities in both the CRA and the TSPCA. This result suggests that POBF determinations are influenced by the pulsatile components of both choroidal and retinal perfusion.
Ischaemia may play a role in neuronal apoptosis and necrosis.1 There is growing evidence that patients with glaucoma, age‐related macular degeneration and arteritic ischaemic optic neuropathy may also suffer from inadequate blood flow.2,3,4 Although there are several methods to assess ocular perfusion in humans, each technique examines a different facet of the ocular circulation, and no single method provides a complete description of the haemodynamic state of the eye.
Arterial blood flow to the eye varies with the cardiac cycle; correspondingly, blood volume and intraocular pressure (IOP) peak during systole and dip during diastole. The pulsatile ocular blood flow (POBF) analyser (Paradigm Medical Industries, Salt Lake City, Utah, USA) is a modified pneumotonometer interfaced with a microcomputer. POBF, determined from the IOP pulse, has been widely used because it is rapid, easy to use and relatively inexpensive.5,6,7 POBF measures pulsatile IOP waves during the cardiac cycle, acquiring approximately 200 measurements per second. The rhythmic pulse wave exhibits a nearly sinusoidal pattern.8 POBF calculates the real‐time change in ocular volume on the basis of real‐time measurement of IOP.
POBF is influenced by refractive error, axial length of the eye, central cornea thickness, and by age, sex and ethnic origin.9,10,11,12,13,14 Despite wide interindividual variation, the technique is characterised by excellent reproducibility.15,16,17 In healthy adults, mild systemic hypoxia did not alter POBF, suggesting that global pulsatile choroidal blood flow was not vulnerable to mild transient hypoxia.18 It has been reported that POBF readings are not altered by the Valsalva manoeuvre (unlike IOP) in healthy subjects.19
The majority of the blood flow in the eye is within the choroidal circulation; therefore, it is presumed that POBF primarily measures the pulsatile component of choroidal perfusion, independent of the retinal or retrobulbar circulation.20 To our knowledge, this presumption has never been tested.
This study was designed to determine whether POBF is strictly an index of pulsatile choroidal blood flow. We compared POBF with colour Doppler imaging (CDI) (Siemens Quantum 2000, Siemens Medical System, Issaquah, Washington, USA; 7.5 MHz linear phase transducer) recordings of blood flow velocities in the central retinal, posterior ciliary and ophthalmic arteries in healthy adults. The vessels being examined by CDI supply the retina, choroid and entire globe, which allows us to evaluate any association between POBF and the three separate areas of ocular perfusion.
Methods
For this prospective study, 22 normal eyes from 3 men and 8 women of age ranging from 19 to 60 years (mean 33 years) were recruited at the Indiana University School of Medicine, Department of Ophthalmology, Indianapolis, Indiana, USA. Volunteer subjects were healthy, and had no history of ocular or systemic diseases. None of the subjects were using topical or systemic medication at the time of the study. Before participation in the study, subjects signed informed consent to procedures reviewed and approved by an institutional review board at the Indiana University School of Medicine. All experimental procedures conformed to the tenets of the Declaration of Helsinki. In this exploratory pilot study, a sample size of 22 was hypothesised to provide sufficient statistical power to prospectively investigate the correlations between CDI and POBF.
All subjects underwent separate IOP measurements using the Goldmann applanation tonometer (GAT) and the POBF tonograph. After instillation of a topical anaesthesia drop, the pneumotonometer was applanated on the central cornea for five sinusoidal cycles lasting 5–10 s. Patients were studied while seated after resting for 10 min.
CDI was performed on the ophthalmic, central retinal (CRA) and temporal short posterior ciliary arteries (TSPCAs), while subjects comfortably reclined at a 60° angle. Peak systolic velocity (PSV) and end diastolic velocity (EDV) were recorded from each vessel. Additionally, resistive index ((PSV−EDV)/PSV) was calculated for each vessel. To examine the ophthalmic artery, the sample volume was oriented nasally and superior to the optic nerve, just lateral to and abutting the visible hyporeflective stripe representing the nerve. The CRA was anterior to the optic nerve: the sample volume was placed about 3 mm behind the surface of the optic disc. The TSPCA was temporal to the optic nerve shadow. As the posterior ciliary arteries are smaller than 200 μm, it is not always possible to resolve individual vessels. Nevertheless, the presence of coloured pixels in this region and the characteristic Doppler spectrum obtained from them confirmed the presence of posterior ciliary artery flow. All POBF and CDI measurements for the entire study population were performed by a single operator, to ensure consistency in measurement. The time between POBF and CDI measurements was ⩽15 min.
Results
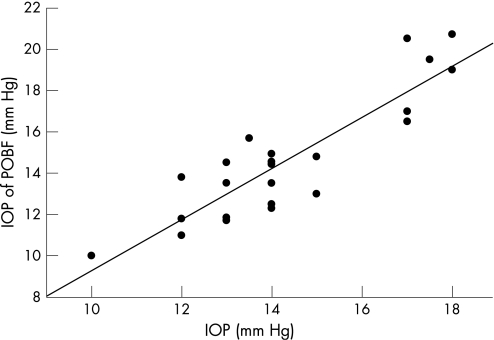
IOP measured with the POBF correlated closely with that measured by GAT (r = 0.901, p<0.001) (fig 1).
Figure 1 Intraocular pressure (IOP) measured by the ocular blood flow (OBF) tonograph correlated highly with that measured by Goldmann applanation tonometry (GAT; r = 0.901, p<0.001). The mean (SD) IOP was 14.9 (3.1) mm Hg using the OBF tonograph, and 14.4 (2.2) mm Hg using GAT.
The mean (SD) IOP was 14.9 (3.1) mm Hg using the POBF and 14.4 (2.2) mm Hg using GAT. The mean (SD) pulse amplitude was 2.2 (0.7) mm Hg (range 1.3–4.4), and POBF 744 (219) μl/min (range 401–1175).
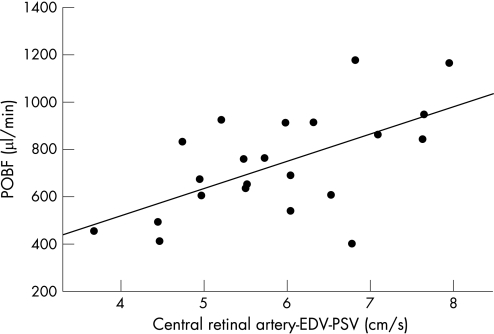
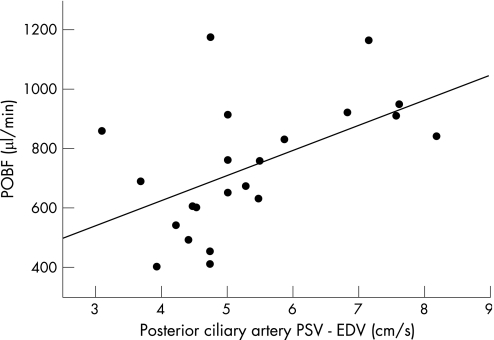
POBF correlated significantly with PSV of the CRA (r = 0.56, p = 0.007) and the TSPCA (r = 0.48, p = 0.023) (table 1). POBF also correlated with the resistance index of the TSPCA (r = 0.45, p = 0.04; table 1). There was non‐significant correlation of POBF with the resistance index within the CRA (r = 0.41, p = 0.057; table 1). CDI pulse amplitude (PSV−EDV) within the CRAs and TSPCAs correlated significantly with POBF (r = 0.60, p = 0.003 for CRA and r = 0.54, p = 0.01 for the short posterior ciliary artery; table 1; figs 2 and 3). Neither PSV nor pulse amplitude within the TSPCAs and CRAs correlated with each other.
Table 1 Correlation between pulsatile ocular blood flow and colour Doppler imaging measures.
| Retrobulbar | PSV | EDV | RI | PSV‐EDV (pulse amplitude) | ||||
|---|---|---|---|---|---|---|---|---|
| arteries | r | p | r | p | r | p | r | p |
| OA | 0.002 | 0.995 | 0.151 | 0.502 | 0.102 | 0.652 | 0.042 | 0.853 |
| CRA | 0.556 | 0.007* | 0.016 | 0.943 | 0.412 | 0.057 | 0.601 | 0.003* |
| TSPCA | 0.483 | 0.023* | 0.042 | 0.853 | 0.445 | 0.038* | 0.537 | 0.010* |
CRA, central retinal artery; EDV, end diastolic velocity (cm/s); OA, ophthalmic artery; PSV, peak systolic velocity (cm/s); r, correlation coefficient; RI, Resistance index = ((PSV−EDV)/PSV); TSPCA, temporal short posterior ciliary artery
*p<0.05.
Figure 2 Pulse amplitude (peak systolic velocity–end systolic velocity (EDV)) of the central retinal artery (CRA), determined using colour Doppler imaging, correlated significantly with pulsatile ocular blood flow (POBF) measured using the ocular blood flow tonograph (r = 0.60, p = 0.003).
Figure 3 Pulse amplitude of the SPCA using CDI correlated significantly with POBF (r = 0.54, p = 0.01).
Pulsatile ocular blood flow (POBF) did not correlate with end diastolic velocities in either the posterior ciliary or the central retinal arteries (table 1). Flow velocities and the resistance index, as measured in the ophthalmic artery, failed to correlate with POBF (table 1).
Discussion
POBF measurements are obtained by monitoring IOP pulsations, then converting beat‐by‐beat pressure changes into volume changes on the basis of established relationships between the eye volume and the IOP.16
POBF measurements face a number of interpretive uncertainties—primarily, the relationship of the pulsatile flow measurement to the circulation within specific ocular regions.20 More than 80% of ocular flow is choroidal; hence, it has been assumed that the variation in the pulsatile component of the choroidal blood flow primarily generates the variation in the ocular pulse.16 Our results confirm that variation among individuals in the ocular pulse correlate with the amplitude of the choroidal flow velocity pulse, as estimated using CDI analysis of the TSPCA.
POBF is based on a direct determination of pressure, while CDI measures flow velocities, not actual bulk perfusion. However, the correlation of POBF with the amplitude of the posterior ciliary arterial pulse amplitude (PSV–EDV) suggests that the same physiological signal, the pulsatile component of choroidal inflow, influences both of these measurements.
Retinal blood flow is volumetrically much smaller than the choroidal perfusion; past authors have presumed that the pulsatile component of blood flow into the retina may have no measurable impact on the ocular pulse. Nonetheless, in this study, we found that both PSV and the CRA pulse amplitude correlated with POBF. In the face of the much larger choroidal perfusion, it could be argued that this correlation is spurious, arising from an association between the CRA and posterior ciliary arterial flow velocities. However, we found no correlation between these two vessels in either PSV or the flow velocity pulse amplitude. This result suggests, yet does not prove, that the pulsatile component of the retinal perfusion contributes a measurable portion of the POBF signal.
The ophthalmic artery, at its point of measurement with CDI, nourishes the entire orbit, including the eye itself. It is thus not surprising that variations among individuals in ocular pulsation do not correlate with any aspect of flow velocity measured in the ophthalmic artery. This finding provides further evidence that the choroidal and retinal circulations are the primary contributors to the ocular pulse.
Past studies have shown that the pneumatic POBF probe provides a high‐fidelity measure of both IOP and its time variation.16 We confirmed that OBF‐based IOP measurements correlated closely with IOP determined using the GAT (r = 0.90).
In conclusion, measurements of flow velocities in vessels supplying the choroid and retina find associations of these flow velocities with the ocular pulse, suggesting that each of these circulations contributes to the pulsatile ocular blood flow calculated at the corneal surface.
Acknowledgements
This study was supported in part by NIH Grant EY10801 (to AH), and by an unrestricted grant from Research to Prevent Blindness. AH is the recipient of the William and Mary Greve International Research Scholar Award (1995).
Abbreviations
CDI - colour Doppler imaging
CRA - central retinal artery
EDV - end diastolic velocity
GAT - Goldmann applanation tonometer
IOP - intraocular pressure
OBF - ocular blood flow
POBF - pulsatile ocular blood flow
PSV - peak systolic velocity
TSPCA - temporal short posterior ciliary artery
Footnotes
Competing interests: None declared.
References
- 1.Cummins D, Kitano S, Caprioli J. The effects of excitatory amino acids and ischemia on cultured rat retinal ganglion cells. Invest Ophthalmol Vis Sci 1992331031–1035. [Google Scholar]
- 2.Hayreh S S. Retinal and optic nerve head ischemic disorders and atherosclerosis: role of serotonin. Prog Ret Eye Res 199918191–221. [DOI] [PubMed] [Google Scholar]
- 3.Friedman E, Krupsky S, Lane A.et al Ocular blood flow velocity in age‐related macular degeneration. Ophthalmology 1995102640–646. [DOI] [PubMed] [Google Scholar]
- 4.O'Brart D P S, de Souza Lima M, Bartsch D ‐ U.et al Indocyanine green angiography of the peripapillary region in glaucomatous eyes by confocal scanning laser ophthalmoscopy. Am J Ophthalmol 1997123657–666. [DOI] [PubMed] [Google Scholar]
- 5.Friedenwald J S. Contribution to the theory and practice of tonometry. Am J Ophthalmol 193720985–1024. [Google Scholar]
- 6.Silver D M, Farrell R A, Langham M E.et al Estimation of pulsatile ocular blood flow from intraocular pressure. Acta Ophthalmol 1989191(Suppl)25–29. [DOI] [PubMed] [Google Scholar]
- 7.Walker R E, Litovitz T L, Langham M E. Pneumatic applanation tonometer studies. II. Rabbit corneal data. Exp Eye Res 197213187–193. [DOI] [PubMed] [Google Scholar]
- 8.Harris A, Kagemann L, Cioffi G A. Assessment of human ocular hemodynamics. Surv Ophthalmol 42 6509–533. [DOI] [PubMed] [Google Scholar]
- 9.James C B, Trew D R, Clark K.et al Factors influencing the ocular pulse‐‐axial length. Graefe's Arch Clin Exp Ophthalmol 1991229341–344. [DOI] [PubMed] [Google Scholar]
- 10.Lam A K, Chan S T, Chan B.et al The effect of axial length on ocular blood flow assessment in anisometropes. Ophthalmic Physiol Opt 200323315–320. [DOI] [PubMed] [Google Scholar]
- 11.Geyer O, Silver D M, Mathalon N.et al Gender and age effects on pulsatile ocular blood flow. Ophthalmic Res 200335247–250. [DOI] [PubMed] [Google Scholar]
- 12.Gunvant P, Baskaran M, Vijaya L.et al Effect of corneal parameters on measurements using the pulsatile ocular blood flow tonograph and goldmann applanation tonometer. Br J Ophthalmol 200488518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunvant P, Baskaran M, Vijaya L.et al Comparison of pulsatile ocular blood flow in Indians and Europeans. Eye 2005191163–1168. [DOI] [PubMed] [Google Scholar]
- 14.Lam A K, Chan S T, Chan H.et al The effect of age on ocular blood supply determined by pulsatile ocular blood flow and color Doppler ultrasonography. Optom Vis Sci 200380305–311. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y C, Hulbert M F G, Batterbury M.et al Pulsatile ocular blood flow measurements in healthy eyes: reproducibility and reference values. J Glaucoma 19976175–179. [PubMed] [Google Scholar]
- 16.Silver D M, Farrell R A. Validity of pulsatile ocular blood flow measurements. Surv Ophthalmol 199438(Suppl)S72–S80. [DOI] [PubMed] [Google Scholar]
- 17.Gunvant P, Watkins R J, Broadway D C.et al Repeatability and effects of sequential measurements with POBF tonograph. Optom Vis Sci 200481794–799. [DOI] [PubMed] [Google Scholar]
- 18.Kergoat H, Marinier J A, Lovasik J V. Effects of transient mild systemic hypoxia on the pulsatile choroidal blood flow in healthy young human adults. Curr Eye Res 2005465–470. [DOI] [PubMed]
- 19.Lam A K, Lam C H. Effects of breath––holding on pulsatile ocular blood flow measurement in normal subjects. Optom Vis Sci 200481597–600. [DOI] [PubMed] [Google Scholar]
- 20.Hitchings R. The ocular pulse. Br J Ophthalmol 19917565. [DOI] [PMC free article] [PubMed] [Google Scholar]