Abstract
Background
By using dyes, it is easier to identify the extent of an epiretinal membrane (ERM) or the inner limiting membrane (ILM) during surgery. Trypan blue (TB) stains ERM and ILM weakly, but with less apparent toxicity than other intraocular dyes. Its main drawback in vitreoretinal surgery is the requirement of an air–fluid exchange (AFX) before its use.
Aim
To propose a modified form of TB denser than water, thus obviating the need for an AFX.
Design
A prospective, consecutive trial with heavy trypan blue in vitreoretinal surgery.
Methods
A consecutive group of patients with ERMs was recruited prospectively. Patients were operated on using conventional methods. Heavy TB was prepared by mixing glucose 10% with Membrane blue (Dorc, Zuidland, The Netherlands) isovolumetrically. Patients were preoperatively and postoperatively assessed at 3 and 6 months (vision and ocular coherence tomography (OCT)). Ease of surgery was also assessed.
Results
29 eyes were included in the study. Reapplication of dye was necessary in 25% of the cases, leading to improved contrast further facilitating the peeling process. In no case was an AFX necessary to obtain sufficient staining. All patients with ERM had an improvement in vision (from median 0.30 to 0.55) and macular volume and foveal thickness (from median 450 to 238 mm) on OCT. No retinal detachment or other complications developed as a result of surgery.
Conclusion
Heavy TB can be delivered efficiently to the retinal surface without an AFX. Staining was sufficient to allow a safe and efficient peeling of ERM. Repeat applications were easily performed. Its use was associated with vision improvement and decreased in foveal thickness, and the absence of adverse events in this small case series.
Removal of the internal limiting membranes (ILMs) and of idiopathic epiretinal membranes (ERMs) requires skill and experience. Non‐closure of macular holes and reproliferation of macular puckers have both been related to inadequate removal of membranes. To improve the surgical outcome and to facilitate the conduct of surgery, means of enhancing membrane visibility have been sought. These have included indirect means such as the use of slit‐beam illumination, triamcinolone and stains such as indocyanine green (ICG) and trypan blue (TB).1,2 Owing to a superior safety profile as compared with ICG, trypan blue has been used by a number of European surgeons despite its less obvious staining characteristics.3,4
The commercially available version of TB (Membrane blue, Dorc, Zuidland, The Netherlands) used in vitreoretinal surgery is diluted in phosphate‐buffered saline, and requires an air–fluid exchange (AFX) before injecting the dye, as it otherwise diffuses through the vitreous cavity and does not stain membranes sufficiently. To eliminate the need for an AFX, we modified the diluents used with TB to render it denser than saline, balanced salt solution and other vitreoretinal infusates. This solution requires the addition of a fixed volume of concentrated glucose solution to achieve the necessary density.
Using this modified “heavy” TB solution, we conducted a prospective, consecutive study of 30 patients with idiopathic ERMs, to assess the staining characteristics, ease of use and safety of the heavy TB in vitreoretinal surgery.
Methods
Patients with either idiopathic ERM or secondary ERM due to retinal detachment surgery or trauma were recruited prospectively and consecutively into this study. Appropriate approval from the hospital medical ethics committee was obtained as dictated by Dutch law for this type of medical investigation. Appropriate consent was obtained. Preoperative assessment included age, sex, best‐corrected visual acuity (BCVA) and full ocular examination. Ocular coherence tomography (OCT) was performed using the OCT3 (Zeiss, Jena, Germany) to measure macular thickness and volume changes caused by the ERM (fast macular thickness protocol). Postoperatively, BCVA and OCT were repeated at 3 months.
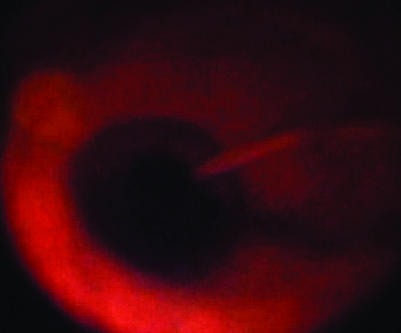
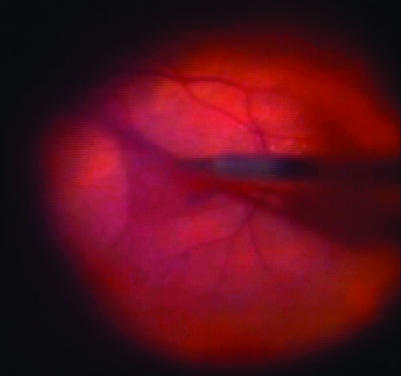
Patients were operated on conventional methods using a three‐port, 20‐gauge vitrectomy. After full vitrectomy, the infusion line was turned off and heavy by TB was injected over the macular area and left for 2 min (fig 1). Heavy TB was prepared by mixing equal volumes of glucose 10% with commercially available TB 0.15% (Membrane blue, Dorc). This provides a final glucose concentration of 5% and a final TB concentration of 0.075%. The osmolality of this solution is 320 mOsm and the pH is neutral. After the 2 min incubation, the infusion was once again opened and the excess dye was removed using a silicone‐tipped Charles flute (fig 2). Membrane peeling was achieved using a combination of a pic needle or a bent machinery vapour recompressor blade and Eckhardt microforceps (Dorc, fig 2). The staining procedure was repeated in some patients in whom staining was initially inadequate, to find a membrane edge, or where a doubt persisted regarding the complete removal of the membrane. After the membrane was fully removed, an internal search was performed and the sclerostomies and conjunctiva were closed.
Figure 1 Heavy membrane blue being injected over the macular area.
Figure 2 Epiretinal membrane removal after staining with heavy membrane blue for 2 min.
Results
A total of 29 eyes from 29 patients were included in this study. Mean (range) age was 67 (37–83) years. In all, 13 patients were women and 18 were men; 16 patients were phakic and 15 were pseudophakic. Of the ERMs, 14 were idiopathic, 3 with a lamellar hole, 3 were post‐trabeculectomy, 6 were post‐retinal detachment, 1 post‐endophthalmitis, 1 post‐trauma, 1 high myope with a staphyloma and 2 due to diabetes. Preoperative BCVA ranged from 0.01 to 0.63 (mean 0.31, median 0.32). On OCT, the preoperative foveal thickness ranged between 309 and 680 (mean 435, median 439) mm and the macular volume ranged between 7.3 and –13.5 (mean 9.5, median 9.1) mm3. Postoperative BCVA varied from 0.12 to 1.0 (mean 0.55, median 0.50). Postoperative foveal thickness improved to a mean of 238 (median 330) mm, and macular volume improved to a mean of 7.49 (range 8.03) mm3.
BCVA was improved or maintained in all patients. In two patients, vision did not improve, but they noted an improvement in metamorphopsia. In all, 19 (64%) patients improved ⩾2 lines of vision. All patients were followed up for a minimum of 3 months. During follow‐up, none of the patients had retinal detachment, macular pigment alteration or other complications.
Discussion
Removal of ERMs can be a challenge in vitreoretinal surgery. Using dyes to stain ERMs can facilitate a more complete removal. The staining method must be safe and easy to apply and use.
ICG has been used for both ERM and macular hole surgery. It intensely stains both ERM and ILM. However, there have been reports of adverse effects on outcome and toxicity for the retinal pigment epithelium.5,6 This toxicity could affect visual recovery after surgery.7
TB was initially used to stain the lens capsule (0.06% TB in Vision blue)8 and then to stain pre‐retinal structures in 0.15% concentration in Membrane blue (Dorc). Feron et al9 initially described the use of TB for removing membranes in proliferative vitreoretinopathy, after which it was described for ERM peeling and ILM peeling in concentrations varying from 0.06% to 0.2%. These concentrations were not found to be toxic to rabbit retina if removed promptly,10 and non‐toxic to cultured retinal pigment epithelial cells in concentrations of 0.06–0.3% for 5 min. None of these concentrations were reported to be toxic in clinical practice. Haritoglou et al12 found vision after ERM removal at 6 months to be similar between eyes operated with and without TB. This suggests that TB does not have a negative influence on visual outcome.11,12 Balayre et al13 performed multifocal electroretinography on patients operated for ERM using 0.15% TB, and found no decrease in macular responses and an increase 4 months after surgery.
The main disadvantage of TB, as formulated in TB (Dorc) is that an AFX is necessary to achieve adequate staining. AFX in itself increases the risk for surgical complications including retinal tears. To eliminate the need for an AFX, we proposed a heavy form of TB that can be applied into a fluid‐filled eye without dispersing.
Staining characteristics using this approach are sufficient to allow an efficient peeling of the membrane following an appropriate flush of the remaining dye. The mixture of TB with 10% glucose facilitates its use during surgery. By eliminating the need for an AFX, repeat application of the dye can also be easily and rapidly carried out. This leads to a more complete removal of the membrane without having a negative effect on vision. All patients in our study had an improvement of retinal thickness on OCT and no patients had a decrease in vision.
We conclude that TB rendered “heavy” by mixing it isovolumetrically with glucose 10% is a safe way of staining ERMs, while also eliminating the need for an AFX. None of our patients lost vision and 64% improved >2 lines at 3 months. All patients had a decrease in macular thickness and volume.
Abbreviations
AFX - air–fluid exchange
BCVA - best‐corrected visual acuity
ERM - epiretinal membrane
ICG - indocyanine green
ILM - inner limiting membrane
OCT - ocular coherence tomography
TB - trypan blue
Footnotes
Competing interests: None.
References
- 1.Kwok A K H, Li W W Y, Pang C P.et al Indocyanine green staining and removal of internal limiting membrane in macular hole surgery: histology and outcome. Am J Ophthalmol 2001132178–183. [DOI] [PubMed] [Google Scholar]
- 2.Furino C, Ferrari T M, Boscia F.et al Triamcinolone‐assisted pars plana vitrectomy for proliferative vitreoretinopathy. Retina 200323771–776. [DOI] [PubMed] [Google Scholar]
- 3.Aguilera Teba F, Mohr A, Eckardt C.et al Trypan blue staining in vitreoretinal surgery. Ophthalmol 20031102409–2412. [DOI] [PubMed] [Google Scholar]
- 4.Balayre S, Boissonnot M, Curutchet L.et al Interet du blue trypan dans la chirurgie des membranes epiretiniennes. J Fr Ophtalmol 200528290–297. [DOI] [PubMed] [Google Scholar]
- 5.Kodjikian L, Richter T, Halberstadt M.et al Toxic effects of indocyanine green, infracyanine green, and trypan blue on the human retinal pigmented epithelium. Graefe's Arch Clin Exp Ophthalmol 2005243917–925. [DOI] [PubMed] [Google Scholar]
- 6.Gale J S, Proulx A A, GOnder J R.et al Comparison of the in vitro toxicity of indocyanine green to that of trypan blue in human retinal pigment epithelium cell cultures. Am J Ophthalmol 200413864–69. [DOI] [PubMed] [Google Scholar]
- 7.Lee K L, Dean S, Guest S. A comparison of outcomes after indocyanine green and trypan blue assisted internal limiting membrane peeling during macular hole surgery. Br J Ophthalmol 200589420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melles G R L, de Waard P W, Pameyer J H.et al Trypan blue capsule staining to visualise capsulorrhexis in cataract surgery. J Cataract Refract Surg 1999257–9. [DOI] [PubMed] [Google Scholar]
- 9.Feron E J, Veckeneer M, Parys‐van Ginderdeuren R.et al Trypan blue staining of epiretinal membranes in proliferative vitreoretinopathy. Arch Ophthalmol 2002120141–144. [DOI] [PubMed] [Google Scholar]
- 10.Veckeneer M, van Overdam K A, Monzer J.et al Ocular toxicity study of trypan blue injected into the vitreous cavity of rabbit eyes. Graefe's Arch Clin Exp Ophthalmol 2001239698–704. [DOI] [PubMed] [Google Scholar]
- 11.Stalmans P, van Aken E H, Melles G R L.et al Trypan blue non toxic for retinal pigment epithelium in vitro. Am J Ophthalmol 2003135234–236. [DOI] [PubMed] [Google Scholar]
- 12.Haritoglou C, Eibl K, Schaumberger M.et al Functional outcome after trypan blue assisted vitrectomy for macular pucker: a prospective, randomised, comparative trial. Am J Ophthalmol 20041381–5. [DOI] [PubMed] [Google Scholar]
- 13.Balayre S, Boissonnot M, Paquereau J.et al Evaluation de la toxicite du bleu trypan dans la chirurgie des membranes epiretiniennes idiopathiques en testant la fonction maculaire par l'electroretinogramme multifocal. J Fr Ophtalmol 200528169–176. [DOI] [PubMed] [Google Scholar]