Abstract
Background
The pterygium is a growth onto the cornea of fibrovascular tissue that is continuous with the conjunctiva, whereas the mechanisms of cell proliferation in pterygium epithelium are unknown.
Aim
To analyse the histopathology and the expression of cell cycle‐related molecules in pterygium tissues.
Methods
Seven pterygia were surgically removed using the bare‐sclera procedure, and three normal bulbar conjunctivas were also obtained. Formalin‐fixed, paraffin‐wax‐embedded tissues were analysed by immunohistochemistry with anti‐p27(KIP1), cyclin D1 and Ki‐67 antibodies.
Results
Conjunctival epithelium consisted of several layers of round cells with a few goblet cells. Nuclear immunoreactivity for p27(KIP1) was noted in many normal epithelial cells, where cyclin D1 and Ki‐67‐positive nuclei were intermingled. A variety of goblet cells were located in the superficial layer of the pterygium head as well as those of the body epithelia. Several pterygium epithelial cells were p27(KIP1) positive, whereas nuclear immunoreactivity for cyclin D1 and Ki‐67 was detected in many epithelial cells. By contrast, immunoreactivity for p27(KIP1), cyclin D1 and Ki‐67 was hardly detected in the pterygium stroma.
Conclusion
It is suggested that pterygium growth and development are associated with the proliferation of epithelium, which is possibly involved in the expression of cell cycle‐related molecules.
The pterygium is a growth onto the cornea of fibrovascular tissue that is continuous with the conjunctiva. Epidemiological studies have shown that pterygia are particularly prevalent in individuals heavily exposed to the sun, which links this disease to excessive ultraviolet (UV) radiation.1 Cytological examination showed that pterygium was a condition of the ocular surface characterised by squamous metaplasia and goblet cell hyperplasia.2 Thus, morphology indicates that pterygium epithelium and the stroma invade the cornea. Recently, it has been shown that fibroblasts infiltrating the stroma play a role in the pathogenesis and development of pterygium. Modulation of fibroangiogenic growth factors and matrix‐degrading enzymes in pterygium fibroblasts contributed to the development and progression to corneal invasion.3 We recently reported that KL6, a mucinous glycoprotein, was characteristically expressed in pterygium epithelia, which is correlated with the proliferation of fibroblasts and epithelial–mesenchymal interactions.4
Cell cycle progression is controlled by a series of kinase complexes that are composed of cyclins and cyclin‐dependent kinases (CDKs).5 The control of mammalian cell proliferation by extracellular signals occurs largely during the G1 phase of the cell cycle. Cyclin D1 is one of the G1 cyclins and is rapidly induced upon xposure of cells to mitogens.6 By contrast, p27(KIP1), a CDK inhibitor, is eliminated during the late G1 phase. The pathological condition showed an alteration in the cell cycle state, in which p27(KIP1) expressed in a normal state disappeared after proliferative stimuli,7,8 whereas cyclin D1 was induced in the proliferating cells of the retina and lens.9,10,11,12,13 It was also reported that squamous cell carcinomas can arise from the pterygium,14 whereas the mechanisms of cell proliferation in pterygia are still unknown.
In this study, we examined the expression of cyclin D1 and p27(KIP1) in normal conjunctiva and pterygium tissues. In addition, Ki‐67, a cell proliferation marker,15 was analysed using immunohistochemistry.
Materials and methods
Operative specimens
Six patients (seven eyes) with primary pterygia who underwent the bare‐sclera procedure were enrolled in this study. The pterygium head and body were confirmed, and excised tissue was placed on the sterilising paper filter simultaneously. Normal bulbar conjunctival tissues were obtained from a patient during cataract surgery and from two patients during pterygium surgery. The tissues were then fixed using 4% paraformaldehyde. Slides prepared from the pterygia and normal conjunctiva were washed in phosphate‐buffered saline, and processed for paraffin sectioning. Informed consent was obtained according to the Declaration of Helsinki.
Immunohistochemistry
Dewaxed paraffin sections were immunostained using the streptavidin–biotin peroxidase complex method. Formalin‐fixed, paraffin‐wax‐embedded serial tissue sections were cut at 4 μm thickness, and endogenous peroxidase activity was inhibited by immersing the slides in 0.3% hydrogen peroxide in methanol for 30 min. As a pretreatment, microwave‐based antigen retrieval was performed in 10 mM citrate buffer (pH 6.0). Then, non‐specific binding of the primary antibody was blocked by incubating the slides in blocking serum for 20 min. The slides were serially incubated with anti‐p27(KIP1) monoclonal antibody (1:50, Novocastra, Newcastle, UK), anti‐cyclin D1 monoclonal antibody (1:1000; Zymed, South San Francisco, California, USA) or anti‐Ki‐67 monoclonal antibody (1:1000; Zymed) overnight at 4°C, followed by the secondary antibody and biotin–streptavidin complex for 30 min each, at room temperature. Immunoreactions were visualised with diaminobenzidine, and the sections were counterstained with haematoxylin. For the positive control of p27(KIP1) and Ki‐67, we immunohistochemically examined the expression in human retina and pterygia, respectively, and then confirmed positive signals, as described recently.4,8 Specimens of mantle cell lymphoma served as a positive control for cyclin D1. To examine the specificity of immunostaining, the primary antibody was replaced with normal mouse IgG or Tris‐buffered saline. Control slides were invariably negative for immunostaining. We counted at least 100 pterygum epithelial cells in one or two microscopic fields from pterygium tissues. Positive‐staining cells were noted by their labelling index as a percentage in each specimen, and the measurements were averaged.
Results
Normal bulbar conjunctiva
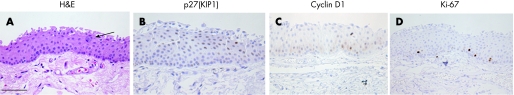
Conjunctival epithelium consisted of several layers of round cells without keratinisation, where a few goblet cells were intermingled (fig 1A, arrow). Nuclear immunoreactivity for p27(KIP1) was noted in a variety of wing and superficial cells of the conjunctiva (fig 1B). Cyclin D1‐positive nuclei were detected in several epithelial cells (fig 1C). A few basal and suprabasal cells of the epithelia expressed Ki‐67 in the nuclei (fig 1D). By contrast, p27(KIP1), cyclin D1 and Ki‐67 were not expressed in goblet cells. Table 1 summarises the number of immunopositive cells.
Figure 1 H&E staining (A), and immunoreactivity for p27(KIP1) (B), cyclin D1 (C) and Ki‐67 (D) in human normal bulbar conjunctiva. Conjunctival epithelium consists of several layers of round cells without keratinisation, where goblet cells are intermingled (A, arrow). Nuclear immunoreactivity for p27(KIP1) is noted in a variety of wing and superficial cells of the conjunctiva (B). Cyclin D1‐positive nuclei are detected in several epithelial cells (C). A few basal and suprabasal cells of the epithelia express Ki‐67 in the nuclei (D). Bar indicates 50 μm.
Table 1 Number of immunopositive cells (%) for p27(KIP1), cyclin D1 and Ki‐67 in the epithelia of human conjunctiva and pterygium.
| p27(KIP1) | Cyclin D1 | Ki‐67 | |
|---|---|---|---|
| Normal conjunctiva | 43.4 | 5.1 | 2.9 |
| Pterygium head | 11.2 | 43.8 | 11.0 |
| Pterygium body | 22.5 | 39.9 | 7.5 |
Pterygium head
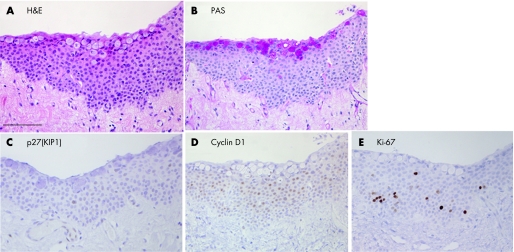
Histopathological findings showed that epithelial cells consisted of several layers without nuclear atypia (fig 2A), and that the thickness of the epithelial cell layer was highest in the pterygium head. A variety of goblet cells were located in the superficial layer of pterygium epithelium (fig 2A). The specific reddish reaction was noted in the cytoplasm of goblet cells by periodic acid Shiff staining (fig 2B). In the stroma, a variety of microvessels and fibroblasts with elastic degeneration were observed. Only a few pterygium epithelial cells were p27(KIP1) positive (fig 2C). Nuclear immunoreactivity for cyclin D1 (fig 2D) and Ki‐67 (fig 2E) was detected in many epithelial cells compared with those in the normal conjunctiva (table 1). By contrast, specific reactivity was not detected in stroma and goblet cells.
Figure 2 H&E staining (A), periodic acid Shiff (PAS) staining (B) and immunoreactivity for p27(KIP1) (C), cyclin D1 (D), and Ki‐67 (E) in the pterygium head. Pterygium epithelial cells consist of several layers without nuclear atypia (A). A variety of goblet cells are located in the superficial layer of pterygium epithelium (A). The specific reddish reaction is noted in the cytoplasm of goblet cells by PAS staining (B). Only a few pterygium epithelial cells are p27(KIP1) positive (C). Nuclear immunoreactivity for cyclin D1 (D) and Ki‐67 (E) is detected in many epithelial cells where goblet cells show no immunoreactivity. Bar indicates 50 μm.
Pterygium body
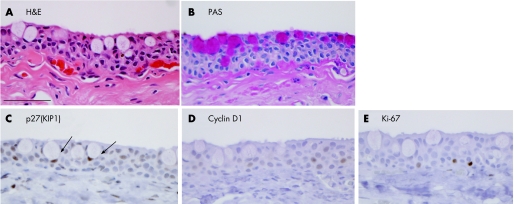
In the pterygium body, the epithelium was slightly thin compared with that of the pterygium head. Histopathologically, epithelia consisted of several round cells with several goblet cells in a superficial layer (fig 3A). Periodic acid Shiff staining detected specific reactions (fig 3B) in the body epithelium. A few p27(KIP1)‐positive cells were detected in epithelium, including goblet cells (fig 3C, arrows). Several cyclin D1 (fig 3D) and Ki‐67 (fig 3E)‐immunopositive nuclei were noted in the epithelia, but not in the goblet cells.
Figure 3 H&E staining (A), periodic acid Shiff (PAS) staining (B) and immunoreactivity for p27(KIP1) (C), cyclin D1 (D) and Ki‐67 (E) in the pterygium body. Epithelia are relatively thin and are constituted by several round cells with goblet cells (A). PAS staining shows specific reddish reaction (B). Nuclear immunoreactivity for p27(KIP1) is noted in epithelial cells, including goblet cells (C, arrows). Several cyclin D1 (D) and Ki‐67 (E) immnopositive cells are noted in the epithelia. Bar indicates 50 μm.
Discussion
We clearly demonstrated that the pterygium included a variety of goblet cells in the superficial layer compared with the normal conjunctiva. This suggests that the histopathology of the pterygium is consistent with goblet cell hyperplasia, as previously shown by cytology.2 In this study, the number of Ki‐67‐immunopositive cells was higher in the pterygium head and body epithelia than in the normal conjunctiva. Taken together, it is indicated that goblet cells are major components of the pterygium, where epithelial cells show a relatively high proliferative activity.
Nuclear immunoreactivity for p27(KIP1) was noted in a variety of epithelial cells of the normal conjunctiva, whereas the number of p27(KIP1)‐positive cells was low in pterygium tissues. By contrast, cyclin D1‐positive nuclei were prominent in head and body pterygium epithelia compared with those in the normal conjunctiva. These results suggest that p27(KIP1), expressed in a normal state, was down regulated, and that cyclin D1 was induced in the pterygium epithelia, implying that the cell cycle state varied with the pathological state of the conjunctiva. Taken together, pterygium epithelial cells show cellular transition from quiescence to the proliferative state by modulation of p27(KIP1) and cyclin D1, which play pivotal roles in cell proliferation.5,6
Attenuation of cyclin proteins correlates with the regulation of cell cycle progression,16 indicating that analyses of protein expression may contribute to a new therapeutic molecular targeting method for pterygium proliferation. We recently reported that cyclin D1 was upregulated in lens epithelial cells during development, in which the expression was possibly regulated by transcription factors.17 Di Girolamo et al18 demonstrated that in pterygium tissues, c‐fos and c‐jun proteins were expressed and that c‐fos mRNA was induced in cultured pterygium epithelial cells after UV treatment. Screening of the cyclin D1 promotor revealed that it contains an activator protein 1 (c‐fos/c‐jun)‐binding site sequence.19 These results suggest that cyclin D1 is induced through activator protein‐1 transactivation in pterygium tissues, chronically exposed to UV radiation.
The cascade of extracellular and intracellular signalling events that result in the down regulation of p27(KIP1) in human pterygium epithelium remains unknown. Recently, it has been suggested that p27(KIP1) can be degraded through the phosphorylation of extracellular signal‐regulated kinase (ERK) 1/2 (phospho‐ERK) in some cell types.20 We have reported that phospho‐ERK is correlated with the degradation of p27(KIP1) in murine models for posterior capsule opacity10 and retinal detachment.11 It has also been demonstrated that ERK is expressed in human pterygium tissues.18 Although the phosphorylation of ERK is presumed to result in the down regulation of p27(KIP1) in the epithelium found here, further analyses on the expression and regulation of CDK inhibitors are needed.
In the present study, we noticed that the expression of cyclin D1 and Ki‐67 was more characteristic in epithelial cells than in stromal fibroblasts. Thus, corneal invasion of the pterygium might be associated with proliferation of the epithelium, although it is clear that fibroblasts play important roles in epithelial–mesenchymal interactions.4 To prevent growth onto the cornea of pterygium tissue, it is necessary to understand the mechanisms involved in the regulation of cell cycle‐related molecules in epithelial cells.
Abbreviations
CDKs - cyclin‐dependent kinases
ERK - extracellular signal‐regulated kinase
UV - ultraviolet
Footnotes
Competing interests: None declared.
References
- 1.Threlfall T J, English D R. Sun exposure and pterygium of the eye: a dose‐response curve. Am J Ophthalmol 1999128280–287. [DOI] [PubMed] [Google Scholar]
- 2.Chan C M, Liu Y P, Tan D T. Ocular surface changes in pterygium. Cornea 20022138–42. [DOI] [PubMed] [Google Scholar]
- 3.Solomon A, Grueterich M, Li D Q.et al Overexpression of insulin‐like growth factor‐binding protein‐2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci 200344573–580. [DOI] [PubMed] [Google Scholar]
- 4.Kase S, Kitaichi N, Furudate N.et al Increased expression of mucinous glycoprotein KL‐6 in human pterygium. Br J Ophthalmol 2006901208–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin‐dependent kinases. Genes Dev 199591149–1163. [DOI] [PubMed] [Google Scholar]
- 6.Sherr C J. G1 phase progression: cycling on cue. Cell 199479551–555. [DOI] [PubMed] [Google Scholar]
- 7.Kase S, Yoshida K, Nakayama K I.et al Phosphorylation of p27(KIP1) in the developing retina and retinoblastoma. Int J Mol Med 200516257–262. [PubMed] [Google Scholar]
- 8.Kase S, Yoshida K, Ohgami K.et al Expression of p27(KIP1) and cell proliferation in human retina and retinoblastoma. Anticancer Res 2005253843–3846. [PubMed] [Google Scholar]
- 9.Yoshida K, Kase S, Nakayama K.et al Distribution of p27(KIP1), cyclin D1, and proliferating cell nuclear antigen after retinal detachment. Graefes Arch Clin Exp Ophthalmol 2004242437–441. [DOI] [PubMed] [Google Scholar]
- 10.Kase S, Yoshida K, Ikeda H.et al Disappearance of p27(KIP1) and increase in proliferation of the lens cells after extraction of most of the fiber cells of the lens. Curr Eye Res 200530437–442. [DOI] [PubMed] [Google Scholar]
- 11.Kase S, Yoshida K, Harada T.et al Phosphorylation of extracellular signal‐regulated kinase and p27(KIP1) after retinal detachment. Graefes Arch Clin Exp Ophthalmol 2006244352–358. [DOI] [PubMed] [Google Scholar]
- 12.Kase S, Saito W, Yokoi M.et al Expression of glutamine synthetase and cell proliferation in human idiopathic epiretinal membrane. Br J Ophthalmol 20069096–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida K, Nakayama K, Kase S.et al Involvement of p27(KIP1) in proliferation of the retinal pigment epithelium and ciliary body. Anat Embryol (Berl) 2004208145–150. [DOI] [PubMed] [Google Scholar]
- 14.Lee G A, Hirst L W. Ocular surface squamous neoplasia. Surv Ophthalmol 199539429–450. [DOI] [PubMed] [Google Scholar]
- 15.Gerdes J, Lemke H, Baisch H.et al Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J Immunol 19841331710–1715. [PubMed] [Google Scholar]
- 16.Kase S, Osaki M, Honjo S.et al A selective cyclooxygenase‐2 inhibitor, NS398, inhibits cell growth and induces cell cycle arrest in the G2/M phase in human esophageal squamous cell carcinoma cells. J Exp Clin Cancer Res 200423301–307. [PubMed] [Google Scholar]
- 17.Kase S, Yoshida K, Sakai M.et al Immunolocalization of cyclin D1 in the developing lens of c‐maf ‐/‐ mice. Acta Histochem 2006107469–472. [DOI] [PubMed] [Google Scholar]
- 18.Di Girolamo N, Coroneo M, Wakefield D. Epidermal growth factor receptor signaling is partially responsible for the increased matrix metalloproteinase‐1 expression in ocular epithelial cells after UVB radiation. Am J Pathol 2005167489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiozawa T, Miyamoto T, Kashima H.et al Estrogen‐induced proliferation of normal endometrial glandular cells is initiated by transcriptional activation of cyclin D1 via binding of c‐Jun to an AP‐1 sequence. Oncogene 2004238603–8610. [DOI] [PubMed] [Google Scholar]
- 20.Foster J S, Fernando I R, Ishida N.et al Estrogens down‐regulate p27Kip1 in breast cancer cells through SKP2, and through nuclear export mediated by the extracellular signal‐regulated kinase (Erk) pathway. J Biol Chem 20031741355–41366. [DOI] [PubMed] [Google Scholar]