Abstract
We have hypothesized that nicotine has two effects on reinforcement; it increases the probability of responses resulting in nicotine delivery (primary reinforcement) and enhances the apparent reward value of non-nicotine reinforcers (reinforcement enhancing effect). The present studies investigated two predictions generated by this hypothesis: 1) that the reinforcement enhancing effect will depend on apparent stimulus reward value, and 2) that the temporal profile of this effect would depend on the pharmacological profile of nicotine. In Experiment 1, rats were trained to lever press for one of two audio-visual stimuli that differed in their intrinsic reinforcing value and then the effect of pre-session nicotine (0.4 mg/kg base) or saline injections was tested. The stimulus that supported very low rates of operant responding displayed smaller increases in responding after pre-session injections of nicotine. In Experiment 2 the effect of nicotine injected 5 min before the session was compared to the effect of nicotine injected 1 h after the session using the more reinforcing stimulus condition from the first experiment. A control group received only vehicle injections. In contrast to nicotine injected just prior to the session, post-session injections of nicotine had no detectable effect on responding for the more reinforcing stimulus. These results indicate that the reinforcement enhancing action of nicotine depends on the intensity of the primary reinforcer and that enhanced reinforcement by nicotine depends on coincident access to a stimulus with reinforcing properties.
1.0 Introduction
Tobacco dependence is a syndrome characterized by subjective drug liking, perseverative drug taking (difficulty quitting), a persistent profile of relapse, and physiological symptoms of tolerance and withdrawal (American Psychiatric Association 2000). Although tobacco dependence has been attributed almost exclusively to the pharmacological action of nicotine (USDHHS 1988), many investigators have recently suggested “nicotine is not enough” (Rose 2006; see also Caggiula et al. 2001). This statement gains credence when the defining features of nicotine dependence are weighed against those of other drug dependence syndromes. For example, the difficulty quitting and rate of relapse associated with smoking is comparable to many other drugs of abuse (e.g., alcohol, opiates, and other psychomotor stimulants; Henningfield et al. 1990; Nil 1991; Russell 1977) but the subjective ratings of liking and physical symptoms of abstinence tend to be milder for nicotine and tobacco than for other drugs (Kono et al. 2001; Nil 1991). In rats, nicotine has been described as a weak primary reinforcer (Caggiula et al. 2001; Chaudhri et al. 2006a); reinforcing doses of nicotine support much lower response-rates in rats (Corrigall et al. 1994) and progressive-ratio break points in beagle dogs (Risner & Goldberg 1983) than cocaine under identical conditions; and in choice tests rats prefer a cocaine-associated lever relative to a nicotine-associated lever (Manzardo et al. 2002).
The mild primary reinforcement engendered by nicotine juxtaposed with the tenacious tobacco habit has prompted many investigators to examine other pharmacological (Belluzzi et al. 2005, Guillem et al. 2005) and nonpharmacological (Caggiula et al. 2002; Rose 2006) contributors to nicotine and tobacco dependence. We have recently demonstrated that nicotine can potently enhance responding for non-nicotine reinforcers (Donny et al. 2003; Palmatier et al. 2006a) and have argued that this effect may be an important nonpharmacological contributor to tobacco dependence (Chaudhri et al. 2006a). This enhancing effect of nicotine on sensory reinforcement was originally demonstrated in a series of studies that investigated the role of non-pharmacological stimuli associated with drug delivery in a self-administration paradigm (Caggiula et al. 2002; Donny et al. 2003). Briefly, responding for a moderately reinforcing visual stimulus (VS) was potently enhanced by nicotine that was self-administered (Palmatier et al. 2006a, b), administered non-contingently by yoking infusions to self-administering groups (Chaudhri et al. 2006b; Donny et al. 2003), continuously infused across a 1-hour session (Donny et al. 2003) or delivered according to a predetermined schedule (Liu et al. submitted). In contrast, self- or experimenter-administered presentations of VS did not influence responding for nicotine (Caggiula et al. 2002; Palmatier et al. 2006a, b), suggesting that the pharmacological effect of nicotine potentiates responding for the VS. In addition, acute pharmacological treatments that decrease the effect of nicotine in the central nervous system (i.e., replacing nicotine with saline infusions or blocking nicotinic acetylcholine receptors with mecamylamine) abolish the reinforcement enhancing effect (Palmatier et al. 2006b; for descriptions of additional parametric manipulations and empirical tests of this phenomenon, see Chaudhri et al. 2006a).
Based on our recent findings, we have hypothesized that the pharmacological effect of nicotine inflates the reward value of other reinforcers (see Chaudhri et al. 2006a) and that the appreciation of stimulus value is limited to the initial pharmacological effect of nicotine, rather than long-term neuroplastic change (Palmatier et al. 2006b). This hypothesis generates several predictions about the reinforcement enhancing effect of nicotine and the circumstances under which it is manifest. For example, if nicotine influences reward value then stimuli with little or no value should not be affected by nicotine. In contrast, the enhancing effect of nicotine should have greater impact on stimuli with more value (i.e., stimuli that are more reinforcing). We also hypothesized that the enhancing effect of nicotine should not depend on long-term pharmacodynamic or neuroplastic changes associated with repeated nicotine exposure. Rather, initial pharmacological action of nicotine would have to overlap with access to the stimulus. Therefore, the present studies had 2 main goals: 1) to characterize the reinforcing value of two qualitatively different stimuli and subsequently determine the effects of nicotine on responding for each stimulus and 2) to determine whether the reinforcement enhancing effects of nicotine were the result of initial nicotine action or whether these effects emerged after repeated nicotine exposure that was separated from access to the stimulus. These studies extended our previous work in the following ways: 1) directly testing predictions of a hypothesis that was generated by the previous studies, 2) investigating two qualitatively different stimuli with two different predicted values, and 3) investigating repeated exposure to nicotine as a potential source of the drug’s enhancing effect.
2.0 Method
2.1 General Method
2.1.1 Subjects
Sixty-three male Sprague-Dawley rats (Harlan Farms, IN) were housed individually in hanging wire mesh cages. Rats weighed 172–200 g on arrival and were housed in a temperature- and humidity-controlled colony room on a reverse 12:12 h light:dark cycle throughout the studies. Rats had free access to food for 3 days and were subsequently maintained on a restricted diet of 20 g per day throughout the remainder of the study. This diet allows limited weight gain (approximately 20 g/week, Donny et al. 1995); access to water was unrestricted throughout the study. Rats were fed immediately after experimental manipulations were complete each day, the specific time of day depended on the Experiment (approximately 14:00 for Experiment 1 and 16:00 for Experiment 2).
2.1.2 Apparatus
All experimental sessions were conducted in 26 operant conditioning chambers (BRS/LVE Model RTC-020, Laurel, MD) measuring 25 × 31 × 28 (w × l × h) cm and housed in sound attenuating cubicles (Med Associates Model ENV-018M, Georgia, VT). The chambers were equipped with two retractable levers, a 2.9 kHz tone (adjusted to 83 dB), white stimulus lights located 5.5 cm above the levers and a house-light located 2 cm below the chamber ceiling. The house-light was equipped with a hood deflector that provided ambient light throughout the chamber.
2.1.3 Stimuli
The two stimuli used in the present studies, stimulus light on (STIM-ON) and house-light off (HL-OFF) were chosen based on previous studies (Chaudhri et al. 2006b; Liu et al. 2005) in which they were both determined to be reinforcers (i.e., supported more responding than groups receiving no programmed reinforcement) but supported different operant rates. Experiment 1 was designed to determine whether the two stimuli supported different rates of behavior. For HL-OFF (Chaudhri et al. 2006b), the chamber was equipped with a white house-light [28 volt, 0.07 amp, 1.96 watt, 1 MSCP (mean spherical candlepower)] that was deflected toward the ceiling of the chamber with an aluminum hood. Meeting the schedule requirement on the active lever resulted in 5-s extinction of the house-light accompanied by 5-s presentation of an 83 dB tone. For STIM-ON (Liu et al. 2005), the chamber was equipped with the same house light and deflector, but the bulb was covered with a red polymer sleeve and remained illuminated throughout the session. Meeting the active-lever schedule requirement resulted in 5-s illumination of the white stimulus light located directly above the active lever [28 volt, 0.07 amp, 1.96 watt, 1 MSCP, covered by a white plastic shade] accompanied by 5-s presentation of the 83 dB tone.
2.1.4 Drugs
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% saline; solution pH was adjusted to 7.0 (±0.2) with a dilute NaOH. Nicotine (0.4 mg/kg, free base) was injected subcutaneously (sc) at 1 mg/ml; this dose had maximal effects on responding for a different visual reinforcer in a pilot study investigating the dose-response curve for the enhancing effects of SC nicotine. All nicotine injections occurred 5 min before or 1 h after experimental sessions (see later).
2.1.5 Reinforcement Schedule & Stability Criterion
After lever training in each study (see Procedure), stimulus presentations were earned by making one response on the active lever (fixed ratio 1, or FR1). This requirement was increased to FR2 and subsequently to FR5 when rats met a stability criterion under each schedule (≤20% variability in active lever responses for 5 consecutive sessions). A 1-min time-out (stimulus temporarily unavailable) was imposed after each reinforcer delivery (Chaudhri et al. 2006b; Liu et al. 2005). Therefore, reinforcer delivery occurred on a tandem FR/FI 1-min schedule. Saline injections (0.9% w/v, 1 ml/kg sc) were given to all rats 5-min before the last 5 sessions of the FR5 schedule for habituation to the injection protocol (see later). During the nicotine/saline treatment phases, rats remained on an FR5/TO 1-min schedule of reinforcement. Any subsequent changes in injection protocol (Experiment 2) were subject to the same stability criterion as the schedule manipulation. The present study was concerned with reinforcement efficacy; however PR schedules were not used. Although these schedules are often necessary to examine quantitative differences in drug reinforcement, they are not always necessary to establish quantitative differences in reinforcement by non-drug stimuli (see Sclafani & Ackroff 2003). In the present studies, the reinforcers of interest (sensory stimuli) and schedule (FR5/TO 1-min) are sufficient to address quantitative differences in reinforcement.
2.1.6 Data Analyses
Data are presented as average number of responses or reinforcers earned in a session. Mixed factors analyses of variance (ANOVAs) were used to determine statistical reliability of the stimulus and/or drug treatment effects on response rates and the number of reinforcers earned. Significant interactions were followed up with t-tests using Bonferroni’s correction for multiple comparisons. Statistical significance was declared at the p≤0.05 alpha, multiple p values are reported together as ‘ps’.
2.1.7 Lever Training
All rats were initially trained to respond on the right lever for sucrose pellets in a series of 3–4 sessions. In the first session (habituation), rats were placed in the darkened chamber for 45 min, levers were retracted and no stimuli presented. In the second 45-min session, also with retracted levers (magazine training) unsignaled sucrose pellets (45 mg, Test Diet, Richmond, IN) were delivered on a random time 1-min schedule (RT 1 min). In the next session, levers were extended and rats were hand-shaped to press a lever for sucrose pellets; shaping criterion was set at 75 pellets earned by a response recorded on the right (henceforth ‘active’) lever. Rats not meeting this criterion we re-shaped in a second session. All rats met the criterion after two shaping (4-total) sessions.
2.2 Specific Experiments
A schematic representation of group assignments, stimulus conditions, and drug treatments from both experiments is presented in Table 1.
Table 1.
Schematic representation of group, stimulus conditions, and drug treatments for Experiments 1 & 2.
| Treatment Phase | ||||||||
|---|---|---|---|---|---|---|---|---|
| NIC Treatment I | NIC Treatment II | Withdrawal | ||||||
| Group | Stimulus | PRE | POST | PRE | POST | PRE | POST | |
| Experiment 1 | HL-OFF-NIC (n=8) | 5-s house-light off + 5-s tone | SAL | ----- | ||||
| HL-OFF-SAL (n=7) | NIC | ----- | ||||||
|
|
||||||||
| STIM-ON-NIC (n=8) | 5-s stimulus-light on + 5-s tone (red house light) | SAL | ----- | |||||
| STIM-ON-SAL (n=8) | NIC | ----- | ||||||
|
| ||||||||
| Experiment 2 | NIC/SAL (n=8) | 5-s house-light off + 5-s tone | NIC | SAL | NIC | SAL | SAL | SAL |
| SAL/NIC Control (n=8) | SAL | NIC | SAL | NIC | SAL | SAL | ||
| SAL/NIC Switched (n=7) | SAL | NIC | NIC | SAL | SAL | SAL | ||
| SAL/SAL (n=8) | SAL | SAL | SAL | SAL | SAL | SAL | ||
PRE: injection occurs 5-min before test session; POST: injection occurs 1-h after test session; NIC: 0.4 mg/kg (free base) nicotine injection; SAL: 0.9% saline injection. Shaded cells represent control group treatments (pre-test injection was always saline). Houselights were white during test sessions unless otherwise specified.
2.2.1 Experiment 1: Stimulus Comparison and the Effect of Nicotine Treatment
2.2.1.1 Stimulus Comparison
After lever training, all 31 rats were allowed to respond for sucrose pellets on an FR1 schedule (1-pellet per response) for one 30 min test session. Rats were then randomly assigned to one of two groups: stimulus-light on (STIM-ON) or house-light off (HL-OFF), with the constraint that sucrose-seeking and response-rate for the test were matched across groups. For both groups, stimulus sessions lasted 1-h. When the session began, levers were extended and the house-light illuminated. When the session ended, levers were retracted and the house-light extinguished. The FR1 schedule was in place on sessions 1–9, the FR2 schedule on sessions 10–24, and the FR5 on sessions 25–30.
2.2.1.2 Effect of Nicotine Treatment
After meeting the FR5 criterion, rats in each of the two stimulus groups were randomly assigned to one of two drug conditions (NIC or SAL) with the constraint that response-rate and reinforcers earned during FR5 testing did not differ across conditions. Rats assigned to the NIC condition received sc injections of 0.4 mg/kg nicotine 5-min before each of 6 tests (sessions 31–36); rats in the SAL condition received 0.9% saline injections 5-min before these sessions.
2.2.2 Experiment 2: Effects of Pre-Session vs. Post-Session Nicotine Administration
2.2.2.1 HL-OFF Testing
After lever training using sucrose reinforcement, 31 rats were given access to the HL-OFF stimulus for 30 sessions. Unlike Experiment 1, additional CRF tests with sucrose reinforcement were not conducted because all rats were assigned to the same stimulus condition. During HL-OFF testing, FR schedules were adjusted according to the previously established criterion. After rats met the stability criterion on the FR5 schedule, they were randomly assigned to one of three groups [NIC/SAL (n=8), SAL/NIC (n=15), or SAL/SAL (n=8)] with the constraint that responding in all three groups was statistically similar during FR5 sessions. Vehicle injections were administered 5-min before and 1-h after sessions 26–30 in order to habituate rats to the drug-injection protocol (see later).
2.2.2.2 Pre- vs. Post-Session Nicotine
The experiment was designed to determine whether the enhanced responding for the HL-OFF stimulus observed in Experiment 1 depended on the immediate pharmacological effect of nicotine given before each test session or, alternately, repeated exposure to nicotine over the course of testing. To do so, all rats received two sc injections per session; one 5-min before each session, the other 1-h after each session. The solution administered for each injection varied with group assignment. Rats in the NIC/SAL group were injected with nicotine 5-min before each session and 0.9% saline 1-h after each session. Rats in the SAL/NIC group received 0.9% saline 5-min before each session and nicotine 1-h after. Rats in the SAL/SAL group received two saline injections on the same schedule; these animals were never exposed to nicotine and served as a benchmark for HL-OFF reinforcement. These sessions continued until all three groups met the 5-session stability criterion (sessions 31–39).
2.2.2.3 Acquisition of Reinforcement Enhancing Effects
The findings from both studies suggested that the reinforcement enhancing effects of nicotine emerged over time. This may have been the result of increasing effect of nicotine over time (i.e., sensitization, Bevins & Palmatier 2003) or a change in HL-OFF reinforcement based on new learning about stimulus value. To investigate these hypotheses rats in the SAL/NIC group were randomly assigned to one of two subgroups [SAL/NIC Control (n=8) or SAL/NIC Switched (n=7)]. The SAL/NIC Control, NIC/SAL and SAL/SAL groups continued to receive injections as previously described. The SAL/NIC Switched group dissociated the sensitization and new learning hypotheses by switching nicotine administration from 1-h after the session to 5-min before the session. If increased responding for the stimulus depends on new learning, then the reinforcement enhancing effect should emerge gradually over sessions in this group (cf. NIC/SAL group). However, if increased responding for the stimulus depends on increased sensitivity to nicotine, then prior exposure in this group from post-session injections should immediately engender maximal responding for the stimulus. Testing sessions continued until rats met the 5-session criterion for stable performance (sessions 40–44), one additional session was added so that rats had 15 nicotine exposures.
2.2.2.4 Control for Non-Specific Effects of Nicotine
Response-rates in the SAL/NIC Control condition may have been influenced by a decrease in the impact of rewarding stimuli (i.e., anhedonia) brought about by nicotine abstinence (e.g., Epping-Jordan et al., 1998). In addition, nicotine withdrawal produces a characteristic behavioral profile (somatic signs such as wet dog shakes, teeth chews, etc.) that could potentially interfere with operant responding. For these rats, testing sessions were conducted during the 23rd hour after nicotine exposure, which approximates the time at which somatic markers of nicotine withdrawal reach peak frequency and can influence a ‘sensory reinforcement’ paradigm (Besheer & Bevins, 2003). Although there is currently no evidence for somatic signs of nicotine withdrawal induced by the treatment regimen employed in the present studies, a sub-threshold state of withdrawal/anhedonia could have potentially offset the reinforcement enhancing effects of nicotine in the SAL/NIC group (i.e., reduced response rate). To investigate whether any non-specific or uncontrolled-for effects of the SAL/NIC treatment influenced the experimental findings, all four groups received saline injections before and after subsequent sessions until the non-specific effects and/or withdrawal symptoms (e.g., Besheer & Bevins, 2003) were expected to be absent and the 5-day stability criterion was met.
3.0 Results
3.1 Experiment 1
3.1.1 Stimulus Comparison
The HL-OFF stimulus maintained a higher level of responding and more stimulus presentations were earned relative to STIM-ON throughout the study (Figure 1A and 1B). In all three FR testing phases, rats in the HL-OFF group responded more than rats in the STIM-ON group (ps<0.001). Responding on the inactive lever did not differ between groups during any phase of the study (Fs≤0.67). The groups earned stimulus presentations with similar frequency during initial FR1 sessions. Although the frequency of stimulus presentations decreased during FR1 testing, the decrease was more pronounced for the STIM-ON group (Group × Session interaction, p=0.04). Further analyses revealed that the HL-OFF group earned significantly more stimulus presentations, relative to the STIM-ON group, during the last 5 sessions of this phase (ps<0.0055). During all remaining phases, the HL-OFF group earned more stimulus presentations than the STIM-ON group (ps<0.0033, FR2; ps<0.0083, FR5).
Figure 1.
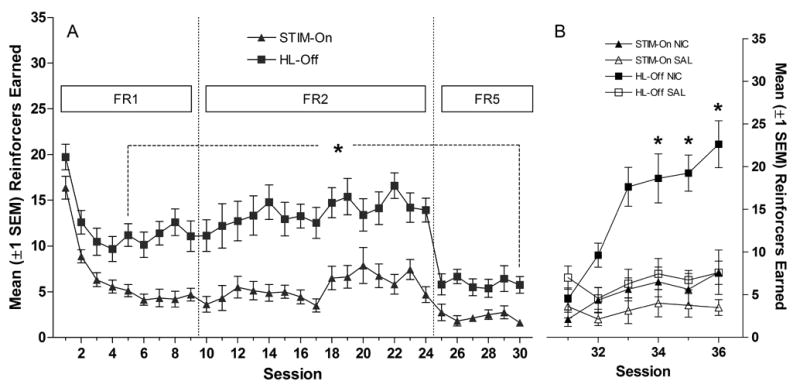
Panel A shows the mean (±1 SEM) number of reinforcers earned during the stimulus comparison phase. Panel B illustrates the mean (±1 SEM) number of reinforcers earned during nicotine/saline testing sessions. * in Panel A indicates reinforcers earned for HL-OFF group differs from STIM-ON group. * in Panel B indicates that reinforcers earned in HL-OFF/NIC group differs from HL-OFF/SAL group.
3.1.2 Effect of Nicotine Treatment
Responding for the more reinforcing stimulus (HL-OFF) was enhanced by nicotine, whereas responding for the less reinforcing stimulus (STIM-ON) was not affected by drug treatment. Neither stimulus was affected by saline treatment (Figures 1C & 1D). All main effects and interactions were significant in the three-way (Drug × Stimulus × Session) ANOVA on stimulus presentations (ps≤0.02). Further analyses revealed that HL-OFF/NIC rats earned more stimulus presentations than HL-OFF/SAL rats on sessions 34–36 (ps<0.0083). In contrast, the number of stimulus presentations did not differ significantly between STIM-ON/NIC and STIM-ON/SAL groups (ps≥0.16, corrected α=0.0083). A nearly identical pattern was evident from analyses of active-lever responding. All main effects and interactions were significant (ps≤0.02), the HL-OFF/NIC group responded more on sessions 33, 35, and 36, relative to HL-OFF/SAL controls (corrected ps<0.0083), and responding did not differ between STIM-ON/NIC and STIM-ON/SAL during any of these test sessions (ps≥0.17, corrected α=0.0083). Responding on the inactive lever (data not shown) did not vary as a function of Group or Drug on these tests (ps≥0.59).
3.2 Experiment 2
3.2.1 HL-OFF Testing
Responding for HL-OFF was similar to Experiment 1 (data not shown). Stability criteria were met on session 6 (FR1) session 11 (FR2) and session 30 (FR5). Average response-rates and reinforcers earned during the last 5 sessions (26–30) are illustrated in the far left portion of Figures 2A and 2B, respectively.
Figure 2.
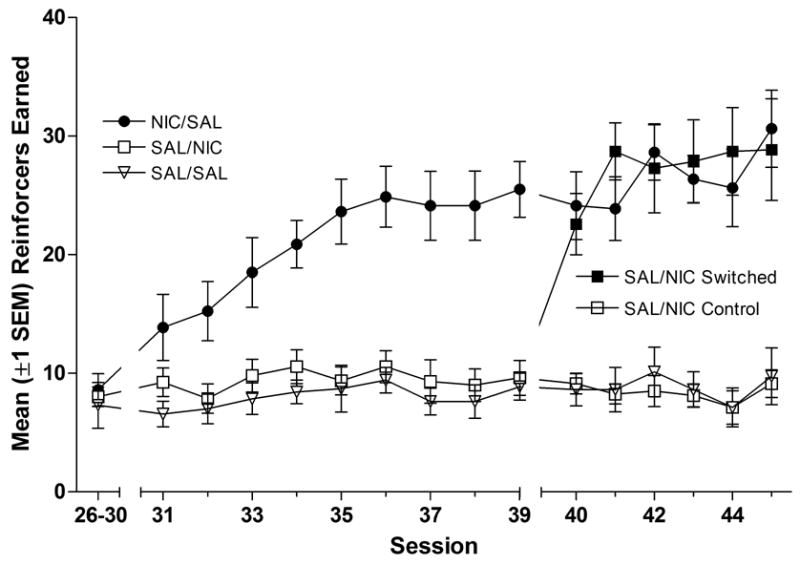
Mean (±1 SEM) number of reinforcers earned during initial testing (average of sessions 26–30), the first 9 nicotine challenge tests (sessions 31–39), and the last 6 nicotine challenge tests (sessions 40–45). Rats in the NIC/SAL group (●) received nicotine injections 5-min before and saline injections 1-h after each session depicted in the figure. Rats in the SAL/SAL group (▽) received saline injections 5-min before and 1-h after each session. The SAL/NIC group (□) received saline injections 5-min before and nicotine injections 1-h after each of the first 9 drug test sessions (31–39). For the next six tests (sessions 40–45), the SAL/NIC group was separated into two subgroups, one receiving nicotine injections 5-min before the session (■) and one that continued to receive nicotine injections 1-h after the session (□).
3.2.2 Pre- vs. Post-Session Nicotine
For rats that experienced the immediate pharmacological effect of nicotine during test sessions (NIC/SAL group) response rates and reinforcers earned increased at a gradual rate (Figure 2A & B) that replicated the findings from Experiment 1 (Figures 1C & 1D). These measures did not change for any other group, indicating that repeated nicotine exposure 22 h prior to each session (SAL/NIC group) had no detectable effect on responding for HL-OFF. These results were confirmed by two-way ANOVAs on response rate and reinforcers earned from sessions 31–39 with significant main effects of Group, Session, and a Group × Session interaction (ps<0.01).
3.2.3 Acquisition of Reinforcement Enhancing Effects
The gradual increase in HL-OFF reinforcement from sessions 31–39 (NIC/SAL group) was dependent on a change in the response to nicotine after repeated exposure, rather than new learning about the value of HL-OFF (right panels of Figures 2A & 2B). Rats that switched from post-session nicotine injections to pre-session nicotine injections (SAL/NIC Switched) immediately increased responding such that response-rate and the number of reinforcers earned were similar to rats in the NIC/SAL condition on the first day of nicotine pretreatment. The stability criterion was met on session 44, but rats were allowed to continue in this phase for 1 additional session reach 15 total nicotine exposures. Three-way ANOVA revealed significant main effects of Group, Phase (sessions 35–39 vs. sessions 40–44), and Group × Phase interactions for both dependent variables (response rate and reinforcers earned, ps<0.01). Further analyses confirmed that SAL/NIC Switched rats were statistically similar to SAL/NIC Control and SAL/SAL groups during post-session nicotine treatments (sessions 31–39, ps≥0.2). However, the SAL/NIC Switched group was statistically similar to the NIC/SAL group on all 5 sessions in which they received pre-session nicotine injections (sessions 40–44, ps≥0.5).
3.2.4 Control for Nicotine Withdrawal
For rats previously receiving pre-session nicotine injections (NIC/SAL and SAL/NIC Switched groups), responding declined to baseline on the first session that saline injections were administered (session 46, Figures 3A & 3B). This was confirmed by the finding that all groups achieved stable performance in 5 test sessions (session 50). Further confirmation was provided by three-way ANOVAs with significant main effects of Phase (nicotine vs. saline), Group, and significant Phase × Group interactions for each dependent measure (response-rate and reinforcers earned, ps≤0.01).
Figure 3.
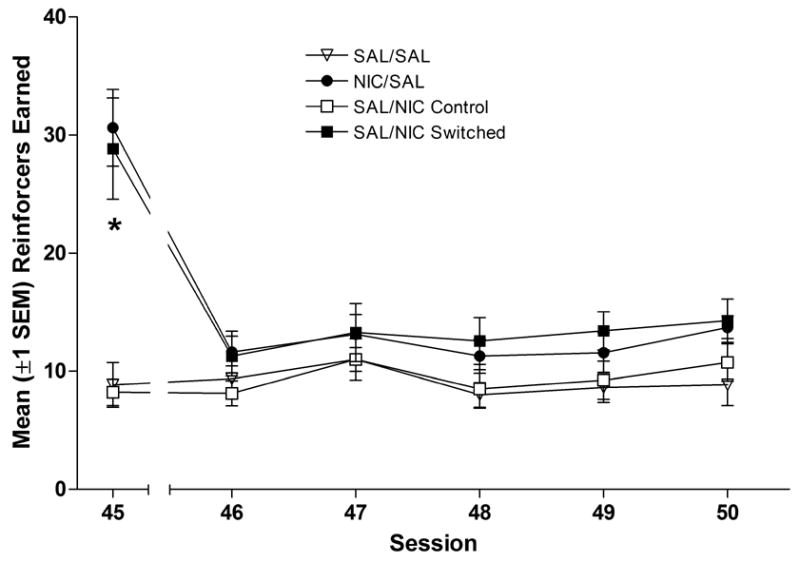
Panel A shows the mean (±1 SEM) number of responses on the active lever during the last nicotine test (session 45), and the five nicotine withdrawal tests (sessions 46–50). Panel B shows the mean (±1 SEM) number of reinforcers earned during the same phases of Experiment 2. Groups that received nicotine injections 5-min before sessions 40–45 are represented with filled symbols. Groups represented by open symbols were never exposed to nicotine (SAL/SAL group) or received nicotine injections 1-h after sessions 40–45. * denotes significant difference between groups receiving nicotine before the session and SAL/SAL control group, p<0.05.
4.0 Discussion
Nicotine can increase responding for reinforcing non-nicotine stimuli under a variety of reinforcement contingencies, schedules, and stimulus conditions (Caggiula et al. 2002; Chaudhri et al. 2006b, c; Donny et al. 2003; Liu et al. submitted; Palmatier et al. 2006a, b). Despite this generality, our working hypothesis imposes certain limitations on this phenomenon; the present studies examined two of them. First, if the enhancing effects of nicotine depend on the reward value of the non-nicotine stimulus, then responding for a stimulus with minimal reward value should be less affected than responding for a stimulus of greater value. Second, if the reinforcement enhancing effect of nicotine is mediated by a contemporaneous pharmacological action of the drug, as suggested by our previous work (Palmatier et al. 2006b), then it should depend primarily on recent drug administration rather than long-term exposure. These studies generated three new findings that extend our understanding of nicotine’s reinforcement enhancing effects. First, the reinforcement enhancing effects of nicotine are more robust for stimuli with moderate reinforcing value compared to weaker reinforcers. Second, the reinforcement enhancing effects of nicotine depend on initial pharmacological action of the drug. Third, the initial pharmacological effect of nicotine is facilitated by repeated drug exposure and/or a sensitized response to nicotine.
The contrast between the enhancing effect of nicotine on HL-OFF and STIM-ON conditions of Experiment 1 confirms our original hypothesis: nicotine inflates reinforcement by increasing the incentive value of the stimulus. Accordingly, drug treatment increases responding for stimuli that support moderate rates of operant behavior (HL-OFF) but has little or no effect on stimuli with less reinforcing value (STIM-ON). There were two reasons why we chose two qualitatively different sensory reinforcers to describe the reinforcement enhancing effects of nicotine. First, previous studies provided estimates of response rates supported by each reinforcer. Experiment 1 directly determined that HL-OFF supported more responding than STIM-ON, from which we have inferred that HL-OFF is more reinforcing. In the present studies, rates of operant behavior for the reinforcers of interest (sensory stimuli) and schedule (FR5/TO1-min) are probably sufficient to detect differences in magnitude of reinforcement. With other reinforcers (i.e., drugs; Richardson & Roberts, 1996) this schedule may not be sufficient to address reinforcement efficacy and other schedules (i.e., progressive ratio) would be needed. A second reason for choosing two qualitatively different stimuli is based on one problem of sensory reinforcement: determining the reinforcing dimensions of the stimuli. One way to circumvent this issue was to acknowledge that a human experimenter has very little control over which sensory dimensions and/or emergent properties will affect ‘value’ as appraised by the rodent subject. Accordingly, we have previously manipulated the value of each individual stimulus by pairing it with food (Chaudhri et al. 2006b; Liu et al. 2005). For both HL-OFF (Chaudhri et al. 2006b) and STIM-ON (Liu et al. 2005) this increased responding for the stimulus (i.e., more conditional value) which enabled (STIM-ON, Liu et al. 2005) or increased (HL-OFF, Chaudhri et al. 2006b) the enhancing effect of non-contingent nicotine infusions. Thus, the reinforcement enhancing effects of nicotine are sensitive to qualitative (Experiment 1) and associative (Chaudhri et al. 2006b; Liu et al. 2005) differences in stimulus ‘value’.
In Experiment 1, the enhancement of responding for HL-OFF appeared to increase with repeated nicotine exposure. Experiment 2 confirmed this observation; for the NIC/SAL group, responding increased gradually, as evidenced by the 9-sessions required to meet the 5-day stability criterion. There are two dissociable explanations for this gradual increase in responding. First, increased responding for the stimulus may depend on an associative process. That is, rats had to learn about the new value of the stimulus when it was contemporaneous with nicotine. In contrast, the increase in responding may reflect changes in sensitivity to the effects of nicotine. That is, pharmacodynamic or neuroplastic changes can increase behavioral responses to nicotine and other psychomotor stimulants (e.g., Bevins & Palmatier, 2003). The present study directly tested these two alternatives by comparing the gradual increase in the NIC/SAL group to a group of rats that had equal nicotine exposure, but no history of stimulus access in the nicotine state (SAL/NIC Switched group). When the SAL/NIC Switched group received nicotine injections before the session, responding for the stimulus immediately reached asymptotic levels. If the increase in responding depended on a learning history between nicotine and the new value of the stimulus, then a more gradual change would have occurred, as in the NIC/SAL group during the first 9 days of nicotine treatment. Thus, the increase observed for this group most likely reflects a change in responsiveness to nicotine. This change in responsiveness could be due to an increase in the appetitive and stimulant effects of nicotine (sensitization) or a decrease in the initial aversive and motor suppressant effects (tolerance).
Experiment 2 also demonstrated that the reinforcement enhancing effects of nicotine do not depend on simple exposure; rather the initial pharmacological effect of the drug must overlap with access to the non-nicotine reinforcer. Although the distinction between initial effect and repeated exposure had not been directly tested before this study, two recent self-administration studies suggested this possibility (Palmatier et al. 2006a, b). Interestingly, another laboratory has demonstrated that nicotine enhances responding for a conditioned reinforcer, and that this effect persists for 15–19 days after nicotine administration is discontinued (Olausson et al. 2003, 2004a). In those studies, rats were exposed to nicotine (0.35 mg/kg base) or saline for 15 consecutive days before a compound audio-visual cue (5-s stimulus light on + tone) was repeatedly paired with water. Both pre-session and post-session nicotine exposure increased the magnitude of anticipatory water seeking (goal tracking) during the 5-s stimulus. The effects of nicotine exposure were also evident after 19 days of imposed abstinence. When the water-paired stimulus was made contingent upon a novel lever press, nicotine-exposed rats responded more for the stimulus than non-exposed controls (Olausson et al. 2004a). In the present study the nicotine dose was slightly higher (0.4 mg/kg base) and rats were not tested for more than 5 days post-withdrawal; however, the only effect of prior nicotine exposure was to facilitate the development of enhancing effects when the post-session nicotine injection was switched to pre-session treatment (SAL/NIC Switched group, Experiment 2). While it is possible that pre-training exposure (Olausson et al. 2004a) may have different effects than post-session exposure (Experiment 2), Olausson and colleagues (2003) found no differences between pre-session, post-session, and discontinued (i.e., pre-training) nicotine treatment. All nicotine-exposure regimes enhanced conditional responding in those studies. The specific parameters contributing to different outcomes in the Olausson studies (Olausson et al. 2003, 2004a) and ours (Experiment 2; Palmatier et al. 2006a, b) will have to be determined experimentally. However, two obvious targets are the potent reward engendered by water in thirsty rats (Olausson et al. 2003, 2004a, b) relative to HL-OFF, and the conditional nature of stimulus value in those studies (Olausson et al. 2003, 2004a, b), versus unconditional reinforcement engendered by a sensory stimulus in the present studies (see also Palmatier et al. 2006a, b).
The reinforcement enhancing effect of nicotine may be related to the decrease in electrical brain stimulation threshold that is observed after nicotine injection (Huston-Lyons & Kornetsky 1992). The change in threshold is also reversed by blocking nicotinic receptors (Harrison et al. 2002), which is analogous to the immediate decrease in the reinforcement enhancing effects of nicotine after mecamylamine pretreatment (Palmatier et al. 2006b). However nicotine’s effect on brain stimulation reward (BSR) is not complemented by changes in rates of operant behavior or progressive ratio break point (e.g. Bespalov et al. 1999); this contrasts with our findings that nicotine increases both response rate and PR breaking point (Experiments 1 & 2; Chaudhri et al. 2006b). Although there may be parametric manipulations that can make BSR and sensory reinforcement more similar, analogies between reinforcement by brain stimulation and sensory stimuli should be drawn cautiously. There are important behavioral differences in brain stimulation, food, sex, and drug reinforcement (see Wise 1987); by extension there is no reason to predict that reinforcement by sensory stimuli is equivalent to reinforcement by brain stimulation or rewards from any other classification.
Nicotine is a psychomotor stimulant and the emergence of these stimulant effects (e.g., Bevins & Palmatier 2003) is comparable to the emergence of nicotine’s reinforcement enhancing effects (Experiment 2), both are dependent on the confluence of time and drug exposure. This change over time and exposure may also be related to the decrement in aversive effects (Iwamoto & Williamson 1984) and/or an increase in rewarding effects (Shoaib et al. 1994) of nicotine that occurs over time. These similarities raise important questions about pharmacological and neurobiological substrates. For example, many theorists (e.g., DiChiara 2000; Everitt & Robbins 2005) have argued that nicotine and other psychomotor stimulants increase activity by exciting dopamine release in nucleus accumbens terminals that project from the ventral tegmental area. They argue that this increased dopamine release influences communication between cortico-limbic structures and other striatal sub-regions, especially those that encode stimulus value. According to these theories, nicotine-induced excitation of regions that mediate psychomotor behavior may in turn amplify communication between structures that encode stimulus value; thereby enhancing reward and primary reinforcement. Although the neurobiology of conditional reinforcement has been well studied (e.g., Cador et al. 1991; Cunningham & Kelley, 1992a, b; Everitt & Robbins, 1991; Kelley & Delfs, 1991a, b; Robbins et al. 1983, 1989), the neurobiology of ‘sensory reinforcement’ has been largely ignored (cf. Ackil et al. 1975). An important future direction of this research will be to determine which brain regions and pharmacological substrates mediate the primary reinforcing effects of sensory reinforcers such as HL-OFF, as well as the reinforcement enhancing effect of nicotine. Such studies promise to add to our understanding of drug reinforcement as well as the neurobiological sources of stimulus value and the dynamic nature of stimulus value. In turn, this understanding can provide insight into the etiology of tobacco dependence and can aid in the development of cessation and relapse-prevention techniques.
Acknowledgments
We thank Leslie Gailey and Prema Chaudhri for their assistance conducting the studies. All experiments followed the “Principles of laboratory animal care” (NIH #85-23, revised 1985) and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Assurance # A3187-01). This research was supported by NIH grants DA-10464, DA-12655, and DA-19278.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackil JE, Levison MJ, Frommer GP. Hypothalamic influences on sensory reinforcement. Physiol Behav. 1975;14:133–142. doi: 10.1016/0031-9384(75)90157-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV TR) Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs in thresholds and breaking points of intracranial self-stimulation in rats. Eur Neuropsychopharm. 1999;9:377–383. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Effects of nicotine preexposure on the conditioned and unconditioned psychomotor effects of nicotine in rats. Behav Brain Res. 2003;143:65–74. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Cador M, Taylor JR, Robbins TW. Potentiation of the effects of reward-related stimuli by dopaminergic-dependent mechanisms in the nucleus accumbens. Psychopharmacology. 1991;104:377–385. doi: 10.1007/BF02246039. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006a;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology. 2006b doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2006c doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelly AE. Evidence for opiate-dopamine cross-sensitization in nucleus accumbens: studies of conditioned rewards. Brain Res Bull. 1992a;29:657–80. doi: 10.1016/0361-9230(92)90137-m. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelly AE. Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Res. 1992b;588:104–14. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits of compulsion. Nature Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Clayton R, Pollin W. Involvement of tobacco in alcoholism and illicit drug use. Brit J Addiction. 1990;85:279–91. doi: 10.1111/j.1360-0443.1990.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Williamson EC. Nicotine-induced taste aversion: characterization and preexposure effects in rats. Pharmacol Biochem Behav. 1984;21:527–532. doi: 10.1016/s0091-3057(84)80034-9. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology. 1991a;103:187–96. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. II. Contrasting effects of amphetamine microinjection into the nucleus accumbens with peptide microinjection into the ventral tegmental area. Psychopharmacology. 1991b;103:197–203. doi: 10.1007/BF02244203. [DOI] [PubMed] [Google Scholar]
- Kono J, Miyata H, Ushijima S, Yanagita T, Miyasato K, Ikawa G, Hukui K. Nicotine, alcohol, methamphetamine, and inhalant dependence: a comparison of clinical features with the use of a new clinical evaluation form. Alcohol. 2001;24:99–106. doi: 10.1016/s0741-8329(01)00143-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Chaudhri N, Sved AF. Program No. 1027.14. Washington, DC: Society for Neuroscience; 2005. Reinforcement enhancing effect of nicotine depends on the reinforcement valence of non-drug stimulus. [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonist. Psychopharmacology. doi: 10.1007/s00213-007-0863-3. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain Res. 2002;924:10–9. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Nil R. A psychopharmacological and psychophysiological evaluation of smoking motives. Rev Environ Health. 1991;9:85–115. doi: 10.1515/reveh.1991.9.2.85. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology. 2004a;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004b;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Caggiula AR, Liu X, Chaudhri N, Donny EC, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement enhancing effects of nicotine in self-administration with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006a;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Caggiula AR, Liu X, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement enhancing effects of nicotine. Neuropsychopharmacology. 2006b doi: 10.1038/sj.npp.1301228. Online: http://www.acnp.org/Docs/RapidPub/NPP091506060226.pdf. [DOI] [PMC free article] [PubMed]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Robbins TW, Watson BA, Gaskin M, Ennis C. Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology. 1983;80:113–119. doi: 10.1007/BF00427952. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Russell MA. Smoking problems: An overview. NIDA Res Monogr. 1977;17:13–33. [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP, Kumar RC. Nicotine-induced place preferences following prior nicotine exposure in rats. Psychopharmacology. 1994;113:445–452. doi: 10.1007/BF02245221. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology. 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- USDHHS. Nicotine addiction: a report of the surgeon general. US Department of Health and Human Services, Office of the Assistant Secretary for Health, Office on Smoking and Health; Rockville, MD: 1988. [Google Scholar]
- Wise RA. Intravenous drug self-administration: A special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. New York: Springer-Verlag; 1987. pp. 117–141. [Google Scholar]


