Abstract
At its most basic level, the function of mammalian sleep can be described as a restorative process of the brain and body; recently, however, progressive research has revealed a host of vital functions to which sleep is essential. Although many excellent reviews on sleep behavior have been published, none have incorporated contemporary studies examining the molecular mechanisms that govern the various stages of sleep. Utilizing a holistic approach, this review is focused on the basic mechanisms involved in the transition from wakefulness, initiation of sleep and the subsequent generation of slow-wave sleep and rapid eye movement (REM) sleep. Additionally, using recent molecular studies and experimental evidence that provides a direct link to sleep as a behavior, we have developed a new model, the Cellular-Molecular-Network model, explaining the mechanisms responsible for regulating REM sleep. By analyzing the fundamental neurobiological mechanisms responsible for the generation and maintenance of sleep-wake behavior in mammals, we intend to provide a broader understanding of our present knowledge in the field of sleep research.
Keywords: Wakefulness, Slow-wave sleep, Rapid-eye movement sleep, Mammals, Wake-promoting structures of the brain, Metabolite homeostasis, Molecular mechanisms of sleep, History of identification of sleep stages, Cellular-Molecular-Network model of REM sleep, Neurotransmitters
1. Introduction
Sleep is a highly evolved global behavioral state in the mammalian species. Over the last fifty years, phenomenological and mechanistic aspects of sleep have been studied more carefully and extensively than the waking states. Many philosophers and scientists have behaviorally defined this state in a variety of terms; yet none of those single-state definitions have succeeded in satisfying all aspects of sleep. This failure to define sleep as a single-state lies in the fact that it is not a homogenous state, rather a continuum of a number of mixed states. The different components of this sleep continuum in the mammalian species could broadly be divided into two major states: non-rapid eye movement (NREM) and rapid eye movement (REM). These two states can be identified objectively using behavioral and physiological signs. For the past fifty years, scientists have debated sleep as either an active or passive process of the brain and body. Recent research, however, reveals initiation of NREM sleep is a passive metabolic process of the body and brain but its maintenance is an active process of the brain. Conversely, both initiation and maintenance of REM sleep are active processes of specific neuronal cell groups that form a network located within the caudal midbrain and pons. Although these two sleep states occur in a relatively predictable manner, this predictability could become highly erratic depending on the existing mental and physical state, time, and surrounding space. Utilizing experimental evidence that provides direct link to sleep as a behavior, the aim of this review is focused on the basic mechanisms involved in the transition from wakefulness, initiation of sleep and the subsequent generation of slow-wave sleep (SWS, the latter part of NREM sleep) and REM sleep. Although, in recent years, a number of excellent reviews have been published, most have focused narrowly on findings that support the reviewer’s theory. By doing so, majority of those review articles ignored some of the most important findings of the last 20 years, especially to describe the mechanisms of REM sleep. Using a holistic approach, this review/commentary article incorporates those important findings in relation to existing literature and views of many different “Pundits”. During the last 20 years, a number of specific functions of sleep have also been discovered, but detailed discussion of those functional advantages and disadvantages of NREM and REM sleep are beyond the scope of this review.
2. Historical background on consciousness and identification of sleep stages
Before the middle of twentieth century, scientists and philosophers have assumed that sleep is a passive unconscious state (reviewed in Gottesmann, 1999). This assumption was based mainly on the subjective experiences during sleep, such as loss of consciousness and inability to recall mental activity. Regrettably, these scientists and philosophers appeared to have ignored consideration of the earliest descriptions regarding the different levels of consciousness across sleep-wake states. An ideal analysis of the states of sleep would include a critical examination of the intricacies of consciousness; however, since most modern research on consciousness is emerging as an independent field, and to describe that research requires considerable space, this article merely provides a description of consciousness as it relates to different stages of sleep.
Philosophical speculation regarding the nature and types of sleep are as old as our recorded history of human civilization (Vedas, undated between 16th and 11th century BC) (see Datta, 2006). In early Hindu civilization, also known as Indus Valley civilization, philosophers divided levels of consciousness into four different states. Of those four states, sleep was divided in two distinctly different types, “Prajna” and “Taijasa”. The first type of sleep, Prajna, meaning dreamless sleep, is considered a state of deep sleep equivalent to modern day NREM sleep. This state of consciousness is characterized by abounding bliss in which a veil of apparent unconsciousness envelops our thought and knowledge, and the subtle impressions of our mind seem to vanish. In short, this is a stage ideally suited for our body and mind to rest and replenish, erasing mental excesses acquired during wakefulness, or “Vaisvanara”. The second type of sleep, “Taijasa”, meaning dreaming sleep, is associated with an internal consciousness equivalent to modern day REM sleep (also known as paradoxical sleep and active sleep). Characterized by a consciousness only of our dreams, we enjoy the mind’s subtle impressions of the deeds we have done in the past. A modern interpretation of this ancient description could be considered a conscious state in which the mind is reactivated to replay or reprocess memories of our past experiences. Vedas also explained that the Prajna (SWS) is the doorway to the Vaisvanara (wakefulness) and Taijasa (REM sleep) states. Correspondingly, with the aid of modern equipment, we now recognize that to enter into REM sleep from wakefulness, we pass through SWS. These two stages of sleep are not exclusive to humans. The progression of sleep research, in conjunction with modern technology, has given us the ability to objectively identify both types of sleep not only in humans, but also in animals. Between Vedic civilization and the actual time of objective identification of sleep stages in the early twentieth century, there were a number of naturalists (namely Lucretius, circa 98-55 BC, Fontana, 1765, and many others), who described individual observations of dreaming sleep in humans and animals. Although worth considering, the views and perceptions of these artists, philosophers, and poets have been examined already in a number of books and, thus, are not repeated in this communication.
The discovery of human electroencephalogram (EEG) was the first important event that signaled the beginning of the modern scientific era of sleep research and provided the framework for our current concepts of objective sleep stage identification. Hans Berger, a German psychiatrist working in Jena, first demonstrated that when his subjects relaxed, closed their eyes or dosed off into drowsiness, the low-voltage brain wave activity associated with alertness gave way to higher voltage, lower frequency patterns (Berger, 1929). Although the first EEG recordings were produced in 1924, because of his secretive nature, Berger withheld publication of his EEG analysis until 1929. Following Berger’s discovery, there ensued a flurry of descriptive and experimental studies aimed at understanding the EEG itself, including the full range of its state-dependent variability and the control of that variability by the brain. Loomis and his coworkers were the first group of scientists to clearly distinguish between waking, sleep, and dreaming EEG in the human (Loomis et al., 1935a, 1935b; Loomis et al., 1937; Loomis et al., 1938). Dividing sleep into five categories (individually identified by letters A–E), Loomis and his colleagues used various stimuli and EEG to correlate subjective experiences of participants with corresponding EEG data (Loomis et al., 1937). Their findings not only defined objective states of waking and sleep, but also a hypothesis that dreaming occurred in the “B” category, denoted by low amplitude waves. In the same year, another German scientist, Klaue, began sleep experiments using animal models. While recording EEG in the cat, Klaue found that sleep progressed in a characteristic sequence: a period of light sleep, during which the cortex produced slow brain waves, followed by a period of deep sleep, in which cortical activity sped up (Klaue, 1937). This deep sleep period of low amplitude EEG, which occurred after a stage with irregular 8 cycles/sec waves, was accompanied by complete muscular relaxation and numerous jerks of single extremities. These initial EEG recording studies indicated that there existed two different patterns of EEG activity during sleep; documenting both characteristic slow cortical waves, but also faster, lower amplitude waves. Not all of the observations of these initial sleep experiments were widely recognized among the research community. The physiological significance of Klaue’s “deep sleep” was not immediately acknowledged by such researchers as Belgian physiologist Frederick Bremer, Swiss neurophysiologist Nobel Laureate W. R. Hess, Italian neurophysiologist Giuseppe Moruzzi, U.S. neurophysiologists Horace W. Mogoun, as well many other scientists of that time (Bremer, 1936; Hess et al., 1953; Hess, 1954; Moruzzi and Magoun, 1949). As a result, for a long-time, faster and low-amplitude EEG activity was generally accepted as the typical EEG sign of arousal--until the first description of REM sleep by Aserinsky and Kleitman (1953).
Eugene Aserinsky, then a Ph.D. student in the laboratory of Professor Nathaniel Kleitman at the University of Chicago, observed while studying the cyclic variations of sleep in infants, the infants’ eyes continued to move under closed lids for some time at the onset of sleep after all major body movement had ceased (Aserinsky, 1996; Aserinsky and Kleitman, 1953a, 1953b). The occurrence of eye movements would fluctuate in duration, and were the first observable body movements to be seen as the infant woke up. Aserinsky realized that, compared to gross body movements, eye movements provided a more reliable means of distinguishing between the active and quiescent phases of sleep. Based on these observations, Aserinsky and Kleitman suggested that eye movements might be used to follow similar cycles in the depth of sleep in adults. To test their hypothesis, using an old electroencephalograph machine, Aserinsky recorded eye movements, as well as EEG, pulse and respiration rates, and gross body movements of adult male subjects, including his mentor, Kleitman. The recordings of the electroencephalograph showed not only the slow movements of the eyes that Aserinsky had observed in infants, but also rapid, jerky eye movements that appeared in clusters. Each individual eye movement took only a fraction of second, but a cluster often lasted, with interruptions, as long as 50 minutes. The first rapid eye movements usually began between 90 min and 120 min after sleep onset, and clusters of eye movements appeared in cyclic fashion through the night. Coinciding with these cyclic rapid eye movements, the EEG from frontal and occipital areas recorded low amplitude (5–30 μV) and showed an irregular light sleep pattern (15–20 cycles/sec). The pulse and respiration rates also increased, and subjects remained motionless (Aserinsky, 1996; Aserinsky and Kleitman, 1953a; Aserinsky and Kleitman, 1955). Aserinsky’s findings were first published in 1953 (Aserinsky and Kleitman, 1953a), hence that year is now considered to mark the discovery of the modern day version of REM sleep. Subsequently, another student of Kleitman, William Dement, contributed tremendously to our early understanding of REM sleep (Dement, 1955; Dement and Kleitman, 1957a, 1957b). Working with Kleitman, Dement correlated the length of REM sleep with the subjective length of dreams, as well as correspondence between visual imagery of dreams and specific eye movement patterns. These observations established an association between spontaneous REM sleep and dreaming. In addition to this connection, Dement and Kleitman clearly showed the strong similarity between the low voltage EEG activity observed during wakefulness and that of REM sleep. The extensive observations of Kleitman and Dement led to the first systematic characterization of the four different stages (stage 1 - stage 4) of non-REM sleep based on EEG activity (Dement and Kleitman, 1957b). Stages 3 and 4 of this non-REM sleep were specifically categorized as SWS.
Following the initial exploration of REM sleep in humans, utilizing EEG activity and careful visual observations, William Dement went on to establish two sleep stages in the cat (Dement, 1958). In that publication, similar to Klaue (1937), he demonstrated two different patterns of cortical EEG activities in the sleeping cats. In the first part of sleep, EEG waves were mostly higher voltage, slow, and some spindle patterns, which he labeled as “sleep.” In the later stage of sleep, EEG waves were low voltage, fast rhythms. Although the observations of Klaue cannot be ignored, this marks the first clear definition of REM sleep in the animal model. Concomitant with the low voltage, fast EEG rhythms, he observed substantial twitching movements of legs, ears and vibrissae, as well as considerable movements of eyeballs (Dement, 1958). Also present was the complete absence of muscle potentials, although he did not mention this in the records. The arousal threshold for the auditory stimuli during periods of REM sleep was also significantly increased. Based on the cortical EEG activity patterns and visual observations, this later stage of sleep was labeled as “activated sleep”, characterizing REM sleep. In fact, this was the first publication to label REM sleep as “activated sleep.” To ensure the cats would sleep easily in the laboratory and not be disturbed by the experimenter’s necessary presence, the cats were sleep deprived for 1–3 days prior to experimental observation. Notable is Dement’s use of the water tank for successful sleep deprivation in the cat. This study was, most likely, the first utilization of this technique.
At the same time in Lyon, France, a neurosurgeon, Michael Jouvet, and a neurologist, Francois Michel, set out to investigate the subcortical activities during sleep in the cat (Jouvet and Michel, 1959; Jouvet et al., 1959a, 1959b). In order to examine the various subcortical areas, the cortex was removed and electrodes were implanted to record EEG from the ventral hippocampus, midbrain reticular formation, and pontine reticularis caudalis nucleus. Additionally, to record EMG, electrodes were implanted in the neck muscle of the cat. During the course of the six-hour recordings, upwards of 4–5 times, electrical activity of the neck muscle disappeared completely for regular periods (about 6 minutes long). Corresponding with these periods of muscle atonia, high voltage spiky waves appeared in the pontine EEG recording electrodes. These spiky waves were correlated with eye movements of the sleeping animals. They also demonstrated that during this incongruous sleep stage, the EEG activity in the ventral hippocampal and midbrain reticular formation are similar to the waking stage (Jouvet and Michel, 1959; Jouvet et al., 1959a, 1959b). In studies of cats with intact brains, EEG and EMG recordings also exhibited a disappearance of muscle tone and a striking correlation between rapid eye movement and fast cortical activity. These findings were curious and certainly a paradoxical phenomenon, because at that time, fast cortical activity was still regarded as the electrophysiological sign of wakefulness and muscle atonia was an invariable sign of sleep. Therefore, Jouvet aptly named this strange state as “paradoxical sleep” (Jouvet, 1965). Aserinsky, Kleitman, Dement, Jouvet, and Michael are all credited equally for the objective identification of REM sleep/activated sleep/paradoxical sleep stage by the present sleep research community. The discovery of REM sleep and its correlation with EEG based cortical and subcortical activations, in conjunction with vivid hallucinatory dreaming, were the scientific evidence supporting the notion that during this part of the sleep, the brain is highly active.
3. Physiological characteristics of wake, NREM, and REM sleep
A combination of electroencephalography (EEG), electromyography (EMG), and electrooculography (EOG) are used in the laboratory setting to objectively identify different stages of sleep (Datta, 1995, 1997). These measurements are collectively known as polysomnography. The waking state is characterized by low-amplitude synchronization of fast oscillations in the cortical EEG (also called activated EEG) in the range of 20 to 60 Hz and presence of muscle tone in the EMG. While we are wake, our voluntary movements are present and thresholds for sensory responses are lowest while thought processes are logical and progressive.
Physiological identification of different stages of NREM sleep requires only cortical EEG recordings; making it relatively easier to identify than REM sleep. In a human, NREM sleep is divided into four stages, each corresponding to an increasing depth of sleep. As the depth of sleep increases, the EEG recordings are progressively dominated by high-voltage, low frequency wave activity. At the deepest stages of NREM sleep (stages III and IV), also termed SWS, only low frequency wave activity is present. Stage II NREM sleep is characterized by slow (<1 Hz) oscillation with distinctive sleep spindles (waxing and waning of 12–14 Hz waves lasting between 0.5–1.0 sec) and K-complex (a negative sharp wave followed immediately by slower positive component) waveforms. Stage I NREM sleep is characterized by relatively low voltage, mixed frequency activity (3–7 Hz) and vertex sharp waves in the EEG. Distinctions between stages of sleep in animal models differ slightly from that of humans. The most common, and preferred, animal models include the mouse, rat, and cat. In these animals, NREM sleep is normally divided into two stages (SWS stages I and II). SWS-I is identified by the presence of sleep spindles in the cortical EEG. SWS-II is considered deep sleep, also termed delta sleep, and is identified by the presence of high amplitude, low frequency waves (0.1–4.0 Hz) in the cortical EEG.
REM sleep is characterized by a constellation of events including the following: 1) Low-amplitude synchronization of fast oscillations in the cortical EEG (also called activated EEG); 2) Very low muscle tone (atonia) in the EMG. The atonia is observed to be particularly strong on antigravity muscles, whereas the diaphragm and extra-ocular muscles retain substantial tone; and 3) Singlets and clusters of rapid eye movements (REMs) in the EOG. Supplemental to these polysomnographic signs, other REM sleep-specific physiological signs are: myoclonic twitches, most apparent in the facial and distal limb musculature; pronounced fluctuations in cardio-respiratory rhythms and core body temperature; penile erection and clitoral tumescence. Two other physiological signs (require surgical implantations of recording electrodes) can also be used to identify REM sleep in the non-human primates, rats, and cats. These two signs are: 1) Theta rhythm in the hippocampal EEG and 2) Spiky field potentials in the pons (P-waves), lateral geniculate nucleus, and occipital cortex (called as ponto-geniculo-occipital (PGO) spikes). In addition to these physiological signs, occurrence of vivid dreaming is an important mental experience of REM sleep.
The cyclic organization of sleep varies within and between species. The period lengths of each REM-NREM sleep epoch increases with brain size across species. Within species, the depth and proportion of the NREM sleep phase in each cycle also increases with brain maturation. In adult humans and non-human primates, circadian distribution of sleep period is mono-phasic, but in the mouse, rat, and cat, circadian distribution of sleep period is poly-phasic. Human NREM and REM sleep alternate throughout each of the four or six sleep cycles that occur every night. Early in the night, NREM sleep is deeper and occupies a disproportionately large amount of time, especially in the first cycle, when the REM epoch might be short or aborted. Later in the night, NREM sleep is shallow, and an increased amount of each cycle is devoted to REM sleep. In the mouse, rat, and cat, NREM-REM sleep cycles are much shorter than the human and non-human primates. These cyclic NREM-REM sleep epochs in rodents and cats continue throughout sleep during the day and night, except when they are engaged in other activities that requires them to stay wake.
4. Regulation of sleep timing
One of the most common observational features of mammalian sleep is that it appears during a predictable time in a 24-hour cycle. In humans, this process is exemplified as daylight decreases and night approaches, increasing feelings of drowsiness eventually lead to the desire to sleep. This overt pattern of sleep indicates that circadian regulatory processes are involved in the regulation of sleep timing. The timing of sleep is species-specific and follows a general rule: organisms remain active during hours when the opportunity to acquire food exceeds the risk of predation and sleep during times when the need for vigilance is minimized. Using rodent and non-human primate models, a number of lesion studies have shown that the suprachiasmatic nucleus (SCN) of the hypothalamus is a critical brain structure that maintains timing of the 24-hour circadian rhythm in sleep drive (Edgar et al., 1993; Ibuka et al., 1977; Ibuka and Kawamura, 1975; Mendelson et al., 2003; Mistlberger et al., 1983; Tobler et al., 1983). Studies of humans in a forced desynchrony protocol, where they experience a 28-hour “day” without any external time cues, have confirmed the persistence of a strong 24-hour circadian rhythm regarding sleep drive (Dijk and Czeisler, 1995).
It is well known fact that the central pacemaker for the mammalian circadian clock is located within the SCN (Drucker-Colin et al., 1984; Moore and Eichler, 1972; Prosser and Gillette, 1989; Ralph et al., 1990; Stephan and Zucker, 1972). Situated directly above the optic chiasm, the SCN is highly integrated with regions of the brain implicated in sleep and arousal. Under normal circumstances, the SCN is reset on a daily basis by light inputs from the retina during the day and by melatonin secretion from the pineal gland during the dark cycle (Cassone et al., 1986; Gillette and McArthur, 1996; Johnson et al., 1988). The light signal is received from a specialized set of retinal ganglion cells that contain the photopigment melanopsin (Berson et al., 2002; Hattar et al., 2003). This light signal is then transmitted from the retina to the SCN via the retinohypothalamic tract (Johnson et al., 1988; Mosko and Moore, 1979; Rusak, 1979). These timing signals keep the clock in synchrony with the external day-night cycle. Recent studies designed to understand the molecular mechanisms of this time-keeping function of SCN have shown that many circadian clock genes encode different types of proteins (BMAL, PERIOD, CRYPTOCHROME, and CLOCK). These proteins act as transcriptional factors to regulate their own transcription. Transcriptional-translational and post-translational regulation of core circadian clock genes form feedback loops that ultimately generate circadian time (Gillette and Sejnowski, 2005; Jin et al., 1999; Lowrey and Takahashi, 2004; Reppert and Weaver, 2002). In summary, sleep timing is tightly governed by the interactions between SCN activity and corresponding output to sleep-wake structures as well as the regulation of circadian clock genes. There are some recent reviews that specifically discuss SCN outputs to the sleep-wake regulating structures (Mistlberger, 2005) and how circadian clock genes may influence timing and total amount of sleep-wake (Albrecht, 2002; Allada et al., 2001; Pace-Schott and Hobson, 2002; Reppert and Weaver, 2002).
5. Wake-promoting systems of the brain
To understand the basic mechanisms for the initiation and maintenance of sleep, it is critical to understand wake-promoting systems of our brain. Activation of these systems results in arousal, thus preventing an organism from falling asleep. The state of wakefulness is a complex, coordinated expression of behaviors that are constantly changing in response to variations in the internal and external milieu. In mammals, there exist multiple systems that promote wakefulness. Individual activation of these wake-promoting systems contributes in specific ways to maintain our general state of wakefulness. Identification of these wake-promoting systems began almost 60 years ago using transection and electrical stimulation experiments in the cat. In these early experiments, Moruzzi and Magoun suggested that the waking state required a critical level of brain activity maintained by a steady flow of ascending impulses arising in the brainstem reticular formation (Moruzzi, 1964, 1972; Moruzzi and Magoun, 1949). At that time, the excitable area of this ascending reticular activating system (ARAS) was broadly localized within the core of pons and midbrain; including the central region of the brainstem extending forward from the bulbar reticular formation, through the pontine and mesencephalic tegmentum, and into the caudal diencephalons (Moruzzi and Magoun, 1949).
Over many years, the advancement of anatomical and physiological techniques has enabled us to identify neurochemically-specific wake-promoting cell groups within the ARAS. These wake-promoting cell groups are: 1) Norepinephrine (NE)-synthesizing (noradrenergic) cells in the locus coeruleus, 2) Serotonin (5-HT)-synthesizing (serotonergic) cells in the raphe nuclei (RN), 3) Acetylcholine (Ach)-synthesizing (cholinergic) cells in the pedunculopontine tegmentum (PPT), 4) Glutamate (Glut)-synthesizing (glutamatergic) cells in the midbrain, and 5) Dopamine (DA)-synthesizing (dopaminergic) cells in the substantia nigra compacta (SNc) and ventral tegmental area (VTA). Projections from these pontine and midbrain wake promoting cells travel dorsally to activate thalamo-cortical system as well as ventrally to activate hypothalamo-cortical and basalo-cortical systems (reviewed in Garcia-Rill, 2002; Morgane and Stern, 1974; Sakai and Crochet, 2003). Spontaneous or experimental activation of these systems results in cortical stimulation necessary to maintain wakefulness.
5.1. Noradrenergic cells of the LC
These neurons project directly to the cerebral cortex, hippocampus, amygdala, and other subcortical areas such as thalamus, hypothalamus, and basal forebrain (Berridge and Waterhouse, 2003; Dahlstrom and Fuxe, 1964; Descarries et al., 1977; Foote et al., 1983; Lewis et al., 1987; Morrison and Foote, 1986). Evidences from single cell recordings and gene expression studies suggest the activation of these noradrenergic cells participates in the process of cortical activation and behavioral arousal. Accordingly, noradrenergic neurons in the LC fire maximally during active wake behavior, steadily decrease firing through quiet wakefulness and SWS, and ultimately cease firing during REM sleep (Aston-Jones and Bloom, 1981b; Chu and Bloom, 1973; Chu and Bloom, 1974; Datta, 1997; Foote et al., 1983; Hobson et al., 1975; Jacobs, 1986; McCarley and Hobson, 1975a). These single cell activity-recording studies have also shown that these noradrenergic cells respond to external arousing stimuli by increasing their firing rate (Aston-Jones and Bloom, 1981b; Foote et al., 1983; Jacobs, 1986). Immediately prior to the physiological and behavioral signs of spontaneous wakefulness, the activity of LC noradrenergic cells increases, suggesting these cells could anticipate spontaneous wakefulness (Aston-Jones and Bloom, 1981a; Datta, 1997). Maximum levels of spontaneously released NE in the forebrain and cerebral cortex occur during wakefulness and stimulation of the LC increases NE release (Berridge and Abercrombie, 1999; Florin-Lechner et al., 1996; Kawahara et al., 1999). In addition, activation and inactivation of LC neurons results in an increase and decrease of wakefulness, respectively (Berridge and Foote, 1991, 1996; Berridge et al., 1993; De Sarro et al., 1987; Sakai and Crochet, 2002). Experimental application of noradrenergic drugs directly into the thalamo-cortical, hypothalamo-cortical, and basalo-cortical activating systems induces cortical activation and promotes wakefulness (Berridge and Waterhouse, 2003; Datta, 1995; Datta et al., 1985; Kayama et al., 1990; Kayama et al., 1982; Kayama and Ogawa, 1987; Kumar et al., 1984). Stressful conditions, induced by forced wakefulness, activate noradrenergic cells in the LC and increase expression of neuronal activity related transcription factors and corresponding genes in the cerebral cortex and other subcortical areas (Cirelli et al., 1996; Cirelli and Tononi, 2000). It has also been reported that, compared to normal mice, mice lacking NE fall asleep more rapidly after a mild stress and low doses of amphetamine (Hunsley and Palmiter, 2003). The results of these molecular and genetic studies could be interpreted to suggest that during wakefulness and periods of stress, normal functioning of the LC noradrenergic cells are critical in the generation of an internally arousing stimulus that ultimately intensifies our general wakefulness.
5.2. Serotonergic cells in the RN
Despite containing both serotonergic and non-serotonergic cells, the RN is the major source of 5-HT in the mammalian brain, particularly the dorsal and median RN (Aghajanian et al., 1978; Belin et al., 1979; Datta, 1997; Descarries et al., 1986; Steinbusch et al., 1980). These serotonergic cells project to almost all of the same brain regions as noradrenergic cell projections (Azmitia and Segal, 1978; Consolazione et al., 1984; Morgane et al., 2005; Morrison and Foote, 1986; Reader et al., 1979; Tork, 1990; Vertes and Martin, 1988); yet, unlike noradrenergic cells, the role of serotonergic neurons in promoting wakefulness and/or sleep is not as clear. A number of single cell recording studies reported that serotonergic cells in the RN fire maximally during wakefulness, decrease firing during SWS and cease firing during REM sleep (Lydic et al., 1983; McGinty and Harper, 1976; Trulson and Jacobs, 1979). Based on these single cell discharge patterns, it has been suggested that the activation of these serotonergic cells may promote wakefulness. A careful reexamination of these activity patterns, however, revealed that these serotonergic cells are unable to anticipate spontaneous changes from sleep to wakefulness (Datta, 1997); an indication the activity of these cells may not be causal for the cortical activation and wakefulness. Independently, this argument is not sufficient to exclude the possible involvement of serotonin in maintaining wakefulness. Supporting evidence illustrates the activation of serotonergic cells in the RN is concomitant with induction of SWS by suppressing wakefulness. For instance, a lesion in the RN and localized inactivation of serotonergic cells in the RN increase wakefulness and suppress SWS (Petitjean et al., 1978; Sakai and Crochet, 2001), demonstrating these cells are involved in the promotion SWS. Further support that the activation of this group of serotonergic cells promotes sleep is illustrated in single cell recording studies identifying a population of RN serotonergic cells that increase firing rate during SWS (Kocsis et al., 2006; Kocsis and Vertes, 1992; Sakai and Crochet, 2003; Urbain et al., 2004). The application of serotonin into the preoptic area results in a decrease in active wakefulness to allow SWS (Datta et al., 1987; Yamaguchi et al., 1963). Similarly, the application of serotonergic agonist has also been shown to inhibit wake-promoting basal forebrain cholinergic cells and suppress cortical activation (Cape and Jones, 1998; Khateb et al., 1993). These pharmacological results suggest that serotonin may suppress the activity of hypothalamo-cortical and basalo-cortical wake-promoting systems. Conversely, a number of other pharmacological studies utilizing various agonists and antagonists of different types of 5-HT receptors and 5-HT1A receptor knock-out mice suggest 5-HT may be involved in maintaining wakefulness (Bjorvatn et al., 1997; Boutrel et al., 2002; Dugovic et al., 1989; Dzoljic et al., 1992; Monti and Jantos, 1992; Ponzoni et al., 1993; Ursin, 1976; Wojcik et al., 1980). Thus, after reviewing the existing evidence, we suggest that serotonergic cells in the RN may participate in both wakefulness and SWS with processes yet to be elucidated. Based on the specific projections of serotonergic cells and their actions on the central nucleus of amygdala and hypothalamus, we suggest that the involvement of 5-HT in maintaining wakefulness, if any, would be an arousal process associated with positive emotion.
5.3. Cholinergic cells in the PPT
The PPT nucleus is one of the major aggregations of cholinergic neurons in the mammalian brainstem capable of synthesizing many different neurotransmitters and peptides (Armstrong et al., 1983; Cuello and Sofroniew, 1984; Datta, 1995; Geula et al., 1993; Kimura et al., 1981; Mesulam et al., 1989; Mesulam et al., 1983; Mizukawa et al., 1986; Rye et al., 1987; Satoh and Fibiger, 1985a, 1985b; Shiromani et al., 1988; Vincent and Reiner, 1987). In addition to different neurotransmitters and peptides, these PPT cholinergic cells also synthesize a gaseous neuromodulator, nitric oxide (NO) (Bredt et al., 1991; Bredt et al., 1990; Bredt and Snyder, 1992; Datta and Siwek, 1997; Hope et al., 1991; Vincent et al., 1983; Vincent et al., 1986). Although NO does not promote wakefulness, locally released NO acts as a paracrine signal that regulates wakefulness by controlling activity levels of PPT cells (Datta, 1997). PPT cholinergic cells project directly to multiple subcortical areas but do not innervate the cerebral cortex (reviewed in Datta, 1995). Activation of PPT cholinergic cells promotes wakefulness by activating thalamo-cortical, hypothalamo-cortical, basalo-cortical, suprachiasmatic, and amygdaloid wake-promoting systems of the forebrain (reviewed in Datta, 1995; Datta et al., 1997). Additionally, electrical stimulation of the PPT region in cats and rats has been shown to promote wakefulness by inducing locomotion (Coles et al., 1989; Garcia-Rill et al., 1986; Garcia-Rill et al., 1983; Skinner and Garcia-Rill, 1984). Following is a brief summary of evidences that are critical in establishing the PPT as one of the most important wake-promoting structures of the brainstem.
Single cell recording studies examining sleep-wake state dependent firing patterns in the PPT of behaving cats identified the presence of several different cell types (Datta et al., 1989; El Mansari et al., 1989, 1990; Saito et al., 1977; Steriade et al., 1990b; Steriade et al., 1990a). Despite similar firing patterns recorded by different groups, the classification of those specific cells varied amongst researchers. To establish a uniform classification, published results of those PPT single cell recording studies of behaving animals were re-examined and re-classified into four major groups: 1) REM-on, 2) Wake-REM-on, 3) Wake-on, and 4) sleep-wake state-unrelated (Datta, 1995). Of those four categories of cells, Wake-REM-on cells are active during both wakefulness and REM sleep (Datta, 1995). In contrast, the Wake-on cells were found to be active only during wakefulness. Based on the presence of Wake-REM-on and REM-on types of cells, the PPT was considered to be involved in the generation of REM sleep. Similarly, the presence of Wake-REM-on and Wake-on types of cells, also suggests the PPT is involved in promoting wakefulness (Datta, 1995). The causal evidence that the PPT is involved in generating both wakefulness and REM sleep came from local chemical stimulation studies (Datta and Siwek, 1997; Datta et al., 2001a). A recent single cell recording study in the freely moving rats has shown that 60% of cells within the cholinergic cell compartment of the PPT are Wake-REM-on type (Datta and Siwek, 2002). This study also demonstrated that the level of activity within the cholinergic cell compartment of the PPT during SWS drops 7.4% from levels of observed wakefulness and during REM sleep cholinergic activity is 65% of wakefulness level. Another interesting observation reveals the Wake-REM-on cell population within the cholinergic cell compartment of the PPT increases neuronal activity as a prelude to wakefulness and remains very active until 5 to 8 sec before the end of wakefulness (Datta and Siwek, 2002). Presently this is the only study to record REM/wake specific single cell activity patterns in the freely moving rats across sleep-wake cycle and careful analysis of this study decisively confirms the PPT cholinergic cells are involved in promoting wakefulness (Datta and Siwek, 2002). Consistent with this analysis, Ach release in the thalamus is highest during waking, slightly less during REM sleep and minimum during SWS (Williams et al., 1994).
5.4. Midbrain reticular formation (MRF)
Over many decades, a vast number of studies have demonstrated that the electrical stimulation of MRF is one of the most reliable techniques to induce cortical activation in anaesthetized cats and rats (Kumar et al., 1989; Matthias et al., 1996; Moruzzi and Magoun, 1949). Single cell recording studies in the cat have shown that majority of the MRF neurons are more active during wakefulness than SWS (Kasamatsu, 1970; Manohar et al., 1972; Steriade et al., 1982). The increase in firing rate of MRF neurons precedes the appearance of EEG activation as animals spontaneously transition from the behavioral state of SWS to wakefulness. It has also been demonstrated that the local microinjection of kainic acid into the MRF causes cortical activation and behavior associated with arousal in the cat (Kitsikis and Steriade, 1981). In parallel to these animal studies, using non-invasive neuroimaging techniques in human subjects, activity in the MRF observed during wakefulness was higher than activity during SWS (Braun et al., 1997; Kajimura et al., 1999; Kinomura et al., 1996; Maquet et al., 1990; Peigneux et al., 2003). It is also known that the cells from MRF send their ascending projections to thalamo-cortical, hypothalamo-cortical, and basalo-cortical pathways (Datta, 1995; Vertes and Martin, 1988; Zemlan et al., 1984). Although the neurotransmitter phenotype of those MRF cells has not specifically been identified, they are most likely to be glutamatergic (Ropert and Steriade, 1981). The wake cellular activity of the MRF and its well-documented significance in cortical stimulation, suggest that glutamatergic and non-glutamatergic neuronal activity in the MRF is critically involved in maintaining behavioral states of wakefulness.
5.5. Dopaminergic cells in the SNc and VTA
The DA-containing neurons in the ARAS are grouped in the SNc and VTA (Dahlstrom and Fuxe, 1964; Hillarp et al., 1966). Dopaminergic neurons heavily innervate the frontal cortex, striatum, limbic areas and basal forebrain (Freeman et al., 2001; Hillarp et al., 1966; Jones, 2005; Trulson and Preussler, 1984; Trulson et al., 1981). In contrast to single cell recording studies of other wake-promoting neurotransmitter systems, dopaminergic neurons of the SNc and VTA do not display robust alterations in firing rate across sleep-wake states (Steinfels et al., 1983; Trulson and Preussler, 1984; Trulson et al., 1981). A few studies, however, have shown DA containing cells exhibit enhanced activity in bursts of spikes associated with aroused, especially rewarding, states and REM sleep (Maloney et al., 2002; Mirenowicz and Schultz, 1996). Interestingly, despite the relatively static firing rate of dopaminergic neurons throughout sleep-wake states, extracellular concentrations of DA are significantly elevated during periods of wakefulness (Feenstra et al., 2000; Trulson, 1985). Microdialysis studies in rats demonstrate during the dark period, when rats are typically active, levels of extracellular DA in the striatum and prefrontal cortex are higher than the light period (Feenstra et al., 2000; Smith et al., 1992). Intracerebroventricular injection of a dopamine receptor agonist increased wakefulness and wake-active behavior while suppressing REM and SWS (Isaac and Berridge, 2003; Monti et al., 1990). Conversely, systemic administration of a dopamine antagonist significantly decreased wakefulness and increased SWS (Monti et al., 1990; Trampus et al., 1991). The stable firing rate of dopaminergic neurons across the sleep-wake cycle combined with the presence of short bursts during positively rewarding states and heightened extracellular levels during wakefulness suggests DA release with positive emotion normally stimulates central arousal.
In addition to brainstem ARAS wake-promoting cell groups, there are at least four other groups of cells in the forebrain that could promote wakefulness independently and/or in coordination with wake-promoting cells of the brainstem. These forebrain cell groups are: 1) histamine (HA)-containing (histaminergic) cells in the posterior hypothalamus (PH), 2) hypocretin (Hcrt, also known as orexins)-containing (hypocretinergic) cells in the lateral hypothalamus (Luque et al.), 3) Ach-containing (cholinergic) cells in the basal forebrain (BF), and 4) cells in the suprachiasmatic nucleus (SCN). Besides these well-known wake-promoting systems in the brainstem and forebrain areas, accumulating evidence indicates that increased activity beyond normal range in the prefrontal cortex (PFC) may also increase the duration of wakefulness by preventing initiation of sleep.
5.6. Histaminergic cells in the PH
Expanding on the research of Baron Constantin von Economo (1930), early lesion studies led to the assumption that the PH functions as a “waking center” in the brain (reviewed in Sakai and Crochet, 2003). During World War I, von Economo came across a group of patients with “Encephalities Lethergica”, a presumed viral infection of the brain. One of the most common clinical symptoms of those patients was a prolonged period of sleepiness with a higher threshold for awaking. These patients could be kept awake only for a brief period before returning to a sleepy state. The symptomatic prolonged sleepiness was reported to be a result of injury between PH and rostral midbrain (von Economo, 1930). Subsequently, this clinical observation of prolonged sleepiness was experimentally reproduced by destruction of PH in monkeys, rats, and cats (McGinty, 1969; Nauta, 1946; Ranson, 1939; Swett and Hobson, 1968).
Modern analysis of the PH, especially the tuberomammillary nuclei (TMN), identified a cluster of histaminergic cells that project diffusely throughout the brain, including the cerebral cortex and wake-promoting structures in the brainstem and forebrain (Brown et al., 2001a; Hass and Panula, 2003; Huang et al., 2001; Inagaki et al., 1988; Lin et al., 1986; Panula et al., 1989; Sakai et al., 1990; Sherin et al., 1998). Histaminergic neurons effect behavioral arousal via efferent projections to the thalamo-cortical and basalo-cortical wake promoting systems. Single cell activity patterns show that the majority of these neurons in the TMN are primarily active only during wakefulness and silent during sleep in freely moving cats and rats ((Ko et al., 2003; Sakai et al., 1990; Steininger et al., 1999; Takahashi et al., 2006; Vanni-Mercier et al., 1984). Subsequent recordings demonstrate these histaminergic cells resume activity during sleep just prior to awakening. Additionally, extracellular levels of histamine (HA) are higher during wakefulness than during SWS (Mochizuki et al., 1992). The increased release of HA during wakefulness and wake anticipatory pattern of activity is suggestive that these cells promote wakefulness. Three alternative experimental paradigms also support a wake promoting function of histaminergic cells in the TMN. First, a microinjection of muscimol, a potent agonist of GABA, into the TMN induces SWS and suppresses wakefulness (Lin et al., 1989; Sakai et al., 1990); indicating inhibition of TMN histaminergic cells suppress wakefulness. Second, drugs that enhance HA signaling increase cortical activation and wakefulness (Monti et al., 1986; Shiromani et al., 1988). Third, knockout mice lacking the histidine decarboxylase gene show a deficit of wakefulness throughout a normal circadian wake period and fail to remain awake when placed in a novel environment (Parmentier et al., 2002). It has also been demonstrated that compared to wild-type, H1 receptor knockout mice are less susceptible to other wake-promoting factors (Huang et al., 2001). Collectively, the studies discussed above indicate the activation of histaminergic cells in the PH produces cortical activation and promotes wakefulness.
5.7. Hypocretinergic cells in the LH
Neurons containing hypocretin (Hcrt) peptides are localized in the LH (Chemelli et al., 1999; Date et al., 1999; de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998). Hypocretinergic neurons project diffusely throughout the central nervous system and heavily innervate the PPT/LDT, LC, RN, SNc, VTA, BF, and TMN (Nambu et al., 1999; Peyron et al., 1998; Taheri et al., 1999). The projection of hypocretinergic neurons to these structures have also been shown to be involved in the promotion of wakefulness and/or cortical activation (Bayer et al., 2001; Brown et al., 2002; Eggermann et al., 2001; Hagan et al., 1999). Interestingly, these Hcrt containing neurons also co-express with two excitatory transmitters, glutamate and pentraxin (Bayer et al., 2002; Reti et al., 2002). Single cell activity recording studies have shown that most Hcrt neurons are more active during wakefulness than SWS (Alam et al., 2002; Koyama et al., 2002; Methippara et al., 2003). Consistent hypocretinergic neuron pattern of activity, Hcrt levels in the cerebrospinal fluid and Fos activation are highest during wakefulness, particularly during periods of motor activity (Espana et al., 2003; Estabrooke et al., 2001; Kiyashchenko et al., 2002; Zeitzer et al., 2003). Local application of Hcrt excites neurons in the LDT (Takahashi et al., 2002), LC (Bourgin et al., 2000; Hagan et al., 1999), RN (Brown et al., 2002), VTA (Li et al., 2002), PH (Bayer et al., 2001), lateral preoptic area (Methippara et al., 2000), basal forebrain area (Eggermann et al., 2001; Espana et al., 2001; Thakkar et al., 2001), and thalamocortical projecting neurons (Bayer et al., 2002). This application indicates Hcrt increases arousal via an excitatory effect on wake promoting neuronal systems in the brain; however, some of these studies also suggest that the local application of Hcrt increases wakefulness by suppressing sleep. Lesioning that selectively eliminates Hcrt-containing neurons in the LH is accompanied by a significant increase in SWS and REM sleep and decrease in wakefulness (Gerashchenko et al., 2003; Gerashchenko and Shiromani, 2004). Transgenic mice with gene-specific ablation of Hcrt containing neurons (Hara et al., 2001) or Hcrt knockout mice (Chemelli et al., 1999) exhibit a phenotype strikingly similar to human narcolepsy. Although the neurotransmitter phenotypes for neurons of the LH have only recently been identified, the discovery of the mammalian feeding center in the LH has long associated this area with wakefulness (Anand and Brobeck, 1951; Anand et al., 1955; Morgane, 1979; Morgane and Panksepp, 1980; Oomura, 1980). Thus, the major wake-promoting function of LH neurons could be integrated with motivated behavior. The evidences discussed above support that the activation of Hcrt neurons in the LH could promote wakefulness by exciting primary wake-promoting neuronal systems of the brain.
5.8. Cholinergic cells in the BF
Cholinergic neurons of the BF have been implicated in a variety of wake-promoting behaviors, including attention, sensory processing, and learning (Bartus et al., 1982; Fibiger, 1991; Hars et al., 1993; Metherate and Ashe, 1993; Muir et al., 1994; Pirch, 1993; Richardson and DeLong, 1990; Sarter and Bruno, 1997; Whalen et al., 1994; Wilson and Rolls, 1990). The BF cholinergic regions are also known to have an important role in hippocampal and neocortical EEG activation (Detari et al., 1984; Detari and Vanderwolf, 1987; Nunez, 1996; Stewart et al., 1984). These cholinergic cells receive input from other brainstem and hypothalamic wake-promoting systems and, in turn, have widespread projections to the cerebral cortex (Detari and Vanderwolf, 1987; Fisher et al., 1988; Gritti et al., 1997; Zaborszky et al., 1986a; Zaborszky et al., 1991; Zaborszky et al., 1986b). A number of studies have shown that Ach levels in the neocortex and hippocampus are higher during wakefulness and wake activities compared to those during SWS (Day et al., 1991; Dudar et al., 1979; Giovannini et al., 1998; Jasper and Tessier, 1971; Kurosawa et al., 1993; Phillis, 1968). Increased levels of Ach in the cerebral cortex are indicative of higher BF cholinergic cellular activity during wakefulness compared to SWS. Indeed, single cell activity recording studies in the cats and rats demonstrate putative cholinergic cells in the BF are more active during wakefulness than during SWS (Alam et al., 1997; Alam et al., 1999; Detari et al., 1984; Szymusiak and McGinty, 1986, 1989). Projections of individual cholinergic neurons and activity patterns across sleep-wake states help to solidify the BF cholinergic cells as part of the forebrain wake-promoting system.
5.9. Cells in the SCN
Various studies have reported that SCN-lesioning causes a slight increase in total sleep time and decrease in wakefulness in mice, rats and monkeys (Easton et al., 2004; Edgar et al., 1993; Mendelson et al., 2003). Single cell activity recording studies in the rats have also shown that during wakefulness, SCN firing rates are much higher than during SWS (Deboer et al., 2003; Glotzbach et al., 1987). The anatomical projections of SCN cells, together with the results of these lesion and single cell recording studies, suggest that the SCN may be involved in promoting wakefulness (Abrahamson et al., 2001; Deurveilher and Semba, 2005; Kriegsfeld et al., 2004; Mistlberger, 2005; Morin et al., 1994; Stephan et al., 1981; Watts, 1991). On the contrary, some studies involving bilateral ablation of the SCN in the rat had little or no effect on total amount sleep or wake (Eastman et al., 1984; Mistlberger et al., 1983; Tobler et al., 1983). These results indicate that the SCN may not be involved in promoting wakefulness. It is possible, however, that those earlier ablation studies may have also lesioned part of the preoptic area/anterior hypothalamus, located dorsally to the SCN. These SCN lesions combined with unintentional damage to the preoptic area might have eliminated additional SWS that is normally observed after SCN lesion. Our suggestion that the SCN is a wake-promoting area is also supported by the recent genetic studies that have shown that the mutation of Bmal1 and Cry1/Cry2 genes increases NREM sleep at the expense of wakefulness (Laposky et al., 2005; Naylor et al., 2000; Wisor et al., 2002). Based on the evidences obtained from recent lesion and molecular studies, it is reasonable to suggest that the SCN is a wake-promoting area of the brain.
5.10. Prefrontal cortex (PFC) in the primate and medial prefrontal cortex (mPFC) in the rodent
In the rat, the mPFC is a heterogeneous and complex structure consisting of four main subdivisions, from dorsal to ventral: the medial agranular (AGm), the anterior cingulate cortex (AC; dorsal and ventral divisions), the prelimbic (PL) cortex and the infralimbic (IL) cortex (reviewed in Vertes, 2006). The various subdivisions of the mPFC appear to serve separate and distinct functions. For example, dorsal regions of the mPFC (AGm and AC) have been linked to various motor behaviors, while ventral regions of the mPFC (PL and IL) have been associated with diverse emotional cognitive and mnemonic processes (Heidbreder and Groenewegen, 2003; Morgane et al., 2005). The mPFC of rat anatomically corresponds to the prefrontal cortex (PFC) in primate (Nauta, 1972; Oomura and Takigawa, 1976). The PFC in the primate is divided into three major regions: orbital, medial and lateral parts (Fuster, 2001; Pandya and Yeterian, 1996; Petrides and Pandya, 2002, 2006; Siwek and Pandya, 1991). The orbital and medial regions (orbitomedial prefrontal cortex; OMPFC) have established roles in emotional behavior and the dorsolateral prefrontal cortex (DLPFC) in ‘executive’ functions of the PFC (Fuster, 2001; Vertes, 2006). Recently it has been suggested that the IL and the PL (and ventral AC) of rats may be functionally homologous to the OMPFC and DLPFC of primates, respectively (Vertes, 2006).
Depending on the species, normal functioning of the mPFC or PFC (mPFC/PFC) is critical for cognitive flexibility (Birnbaum et al., 2004; Bunge et al., 2001; Dalley et al., 2004; Goldman-Rakic, 1987; Lepage et al., 2000; Stuss and Knight, 2002). By utilizing representational knowledge, cognitive flexibility serves to appropriately guide our emotions, thoughts, and behaviors. A classical example of impaired cognitive flexibility is an occasional, although common, situation characterized by a state of mental and physical fatigue accompanied by an inability to fall asleep. Despite conscious intentions to rest and relax, the conflicting mental processes reverberate in a seemingly endless loop. While scientifically attributing a temporary dysfunction of the mPFC to the maladaptive situation above is premature, we suggest that this type of wake experiences might be due to hyperactivity of mPFC. For example, it is known that this type of sleep initiation problem is more frequent in aging population. Normal aging consistently impairs many of the cognitive functions of the mPFC/PFC in humans, monkeys, and rats (Albert, 1997; Chao and Knight, 1997; Herndon et al., 1997; Nielsen-Bohlman and Knight, 1995; West, 1996)). It has also been documented that the protein kinase A (PKA) signaling pathway becomes dis-inhibited in the mPFC/PFC with advancing age, and increased PKA activity in the mPFC/PFC disrupts cognitive flexibility in the rats and monkeys (Ramos et al., 2003). Neuroimaging studies demonstrate a decrease in neuronal activity and metabolic rate of glucose in the human PFC during spontaneous sleep (Maquet et al., 1990; Maquet et al., 1996; Thomas et al., 2000). Also supporting the PFC as a wake-promoting structure are studies examining the unique neuronal circuitry between the PFC and the thalamic reticular nucleus as well as other high-order thalamic nuclei involved in attentional mechanisms (Zikopoulos and Barbas, 2006). Thus, we suggest that the hyperactivity of the mPFC/PFC could increase wakefulness by preventing initiation of sleep. We acknowledge, however, that future experimental work will be necessary to confirm or refute this suggestion that the mPFC is a wake-promoting region of the brain.
6. Initiation of sleep
Utilizing only a macroscopic view of our external behavior, sleep is defined by the absence of wakefulness. Although the timing of sleep is regulated by the suprachiasmatic nucleus, the initiation of sleep is a complex passive process. The notion that the sleep is a passive process was the principle mantra of the reticular deactivation theory (Moruzzi, 1972). The reticular deactivation theory was based on two assumptions. First, “the waking state requires a critical level of brain activity, which is maintained by a steady flow of ascending impulses arising in the brainstem reticular formation”. The second is “a reduction of tonic activity of the ascending reticular system is responsible for physiological sleep”. Over the past three decades, we have gathered more knowledge about widespread changes in physiological functions during the transition from wakefulness to sleep, including electrical activity of the brain, sensory, motor, and metabolic processes. Based on new information, we propose the initiation of sleep is a passive process and this process depends on the homeostatic regulation of the levels of activity-dependent metabolites.
6.1. Activity-dependent metabolites homeostatic theory
During wakefulness, our use-dependent metabolic rate in the brain and body is at a much higher level compared to sleep and/or resting periods. The increased metabolic rate during wakefulness is accompanied by an increased rate of metabolite synthesis that is higher than the rate of clearance; as a result, the levels of specific metabolites tend to accumulate in the brain and body. When these metabolites reach a critical level, our metabolic process responds by slowing down wake-promoting neuronal activities, thus lowering the rate of production until the metabolites return to basal levels. During this period, the rate of metabolite clearance remains unchanged. The diminished activity of wake promoting neuronal systems results in reduced synthesis of metabolites. The reduction of metabolites from critical to basal levels can be considered a process of metabolite homeostasis. This homeostatic demand for a lower metabolic state begins at the cellular level, ultimately affecting behavior at the systemic level and is the primary factor necessary to initiate sleep. The initiation of sleep at the cellular level has some similarity with the “neuronal group theory of sleep function” (Krueger and Obal, 1993, 2003; Rector et al., 2005). Our metabolite homeostatic theory for the initiation of consolidated sleep predicts that the frequency of consolidated sleep periods will have a positive relationship with the accumulation rate of use-dependent metabolites. This theory also predicts that the duration of consolidated sleep period will have an inverse relationship with the rate of metabolite clearance. We would like to emphasize that this theory is applicable only to terrestrial mammals. In the following subsections, analysis is focused on sleep initiating metabolic factors that reduces the activity levels of wake promoting neuronal structures.
6.2. Sleep initiating metabolic factors
Sleep-initiating metabolic factors are endogenous metabolites produced during wakefulness that increase proportionately with an increased duration and intensity of wakefulness. The slow accumulation of these metabolic factors increases sleep inertia and, with sufficient accumulation, facilitates the transition between wakefulness to sleep by suppressing wakefulness. Intra-cerebral application of these factors also initiates sleep. At present, among hundreds of known metabolites, adenosine, neuroinhibitory amino acids (gamma amino butyric acid (GABA) and glycine), prostaglandin D2 (PGD2), and cytokines (interleukin-I beta (IL-1β)) and tumor necrosis factor alpha (TNFα)) have been identified as sleep initiating metabolic factors. Some of the sleep initiating metabolic factors listed above, after the onset of sleep, are also involved in sleep induction. It should be noted that factors only involved in sleep induction are not metabolic factors (not synthesized as a metabolic byproduct). Instead, sleep induction factors are synthesized after SWS has been established. These and many other sleep induction factors have been discussed in great details elsewhere (Obal and Krueger, 2003).
6.2.1. Adenosine
The purine nucleoside adenosine, ubiquitous in daily functioning, is comprised of adenine attached to a ribose moiety. Intracellular adenosine is released when adenosine triphosphate (ATP) is hydrolyzed as a function of cellular metabolic activity (reviewed in Obal and Krueger, 2003). After hydrolysis, excess adenosine is transported out of the cell along its concentration gradient. ATP is also co-released in some neurotransmitter-containing vesicles, such as acetylcholine, glutamate, noradrenaline, and dopamine. The extracellular ATP is then metabolized to adenosine via ectoenzymes. Extracellular levels of adenosine increases with higher neuronal and metabolic activities during wakefulness and decreases during sleep (Basheer et al., 2004; Chagoya de Sanchez et al., 1993; Porkka-Heiskanen et al., 2000; Porkka-Heiskanen et al., 1997; Strecker et al., 2000). Significant levels of adenosine accumulate in the basal forebrain and cortex during a period of forced wakefulness extending beyond the normal onset of sleep (Basheer et al., 2004; Porkka-Heiskanen et al., 2000). Increased adenosine levels resulting from sleep deprivation gradually decline throughout a three-hour post-deprivation recovery period (Porkka-Heiskanen et al., 2000). Administration of adenosine via intracerebral or systemic injection increases sleep duration and enhances EEG slow wave activity (an electrophysiological characteristic of non-REM sleep) in the rat (Radulovacki, 1985; Ticho and Radulovacki, 1991). Conversely, blocking of both adenosine synthesis and/or receptor-mediated action of adenosine in the brain effectively eliminates SWS and increases wakefulness (Kalinchuk et al., 2003; Landolt et al., 1995; Schwierin et al., 1996). More recently it has also been demonstrated, using patch-clamp recording in hypothalamic slices, that application of adenosine inhibits activity of identified Hcrt containing neurons of the LH, a wake-promoting area (Liu and Gao, 2007).
6.2.2. Inhibitory amino acids
GABA and glycine are the major inhibitory amino acids in the central nervous system. GABA acts in all parts of the neuraxis, and glycine acts predominantly in the spinal cord and brainstem. GABA is formed via a metabolic pathway called the GABA shunt. The initial step in this pathway utilizes α–ketoglutarate formed from glucose metabolism via the Krebs cycle. α–Ketoglutarate is then transaminated by α–oxoglutarate transaminase (GABA-T) to form glutamate, the immediate precursor of GABA. Finally, glutamate is decarboxylated to form GABA by enzyme(s) glutamic acid decarboxylase (GAD). For the production of GABA, glutamate is also synthesized from glutamine by glutaminase activity (Paul, 1995; Roberts, 1986). Increased neuronal activity results in an increase in local GABA synthesis and enzymatic activity of the GAD (Erlander and Tobin, 1991). Glycine is synthesized from the degradation of serine by the enzyme serine hydroxymethyltransferase. Serine can be synthesized from the glycolytic intermediate 3-phosphoglycerate via a NAD+-linked dehydrogenase that converts this intermediate to 3-phosphohydroxypyruvate. The latter then undergoes transamination with glutamate to 3-phosphoserine, followed by the irreversible removal of the phosphate by a phosphatase. This cytosolic pathway from 3-phosphoglycerate is distributed widely and is considered the major pathway of serine synthesis in mammals. Once serine is formed from glycolytic intermediates, it can be converted to glycine via serine hydroxymethyltransferase. A limited amount of glycine can be synthesized from catabolism of threonine by the threonine cleavage complex. Glycine can also be produced from metabolism of betaine (or degradation of its precursor, choline) by successive removal of the methyl groups from the amino group of betaine. This leads to formation of dimethylglycine and monomethylglycine (sarcosine) and, ultimately, glycine (Stipanuk, 2000).
There are evidences to indicate that with increased neuronal activities and metabolic demand during spontaneous and/or forced wakefulness, the brain level of GABA and glycine increases as metabolites (Gong et al., 2004; Karadzic et al., 1971; Murck et al., 2002; Stipanuk, 2000). A global increase of GABA via intracerebroventricular infusion promotes electrophysiological signs of non-REM sleep (reviewed in Gottesmann, 2002). Inhibition of GABA resulting from an intraperitoneal injection of a GABAB or GABAC antagonist increases wakefulness and decreases SWS (Arnaud et al., 2001; Gauthier et al., 1997). There are a number of studies that indicate endogenous glycine could suppress motor activities, a prerequisite for the initiation of sleep, by inhibiting motor neurons in the brainstem and spinal cord (Chase et al., 1989; Jonas et al., 1998; Kodama et al., 2003; O’Brien and Berger, 1999; Russier et al., 2002; Soja et al., 1991; Spencer et al., 1989; Yamuy et al., 1999).
6.2.3. Prostaglandin (PG)
The most abundant prostaglandin (PG) in the central nervous system of mammals is PGD2 (Hayaishi, 1991; Matsumura et al., 1994; Obal and Krueger, 2003). Prostaglandins are a family of naturally occurring unsaturated fatty acids containing 20 carbon atoms and a cyclopentane ring. These eicosanoids are produced by the arachidonate cascade system in which arachidonic acid is converted to PGH2 via the cyclooxygenase pathway (COX-I and –II) (reviewed in Obal and Krueger, 2003). The subsequent isomerization of PGH2 to PGD2 is catalyzed by the enzyme PGD synthase (PGDS) (Hayaishi, 1991; Urade et al., 1985). Synthesis of PGD2 is expressed predominantly in the leptomeninges, the epithelial cells of the choroid plexus, and oligodendrocytes (Urade et al., 1993). As a result, a significant amount of PGDS activity is observed in the cerebral spinal fluid (CSF) between the arachnoid membrane and pia mater (Hayaishi, 1991). An increase in PGD2 and PGDS is associated with neuron-glia interactions and in several glial functions such as metabolism and myelin maintenance (Urade et al., with increased intensity of wakefulness. As duration of imposed sleep deprivation increases, rats exhibit a higher concentration of PGD2 in the CSF (Ram et al., 1997). The higher concentrations of PGD2 are significant at 2.5h and persist at significant levels after 10h of sleep deprivation (Ram et al., 1997). A significant increase in non-REM sleep is also observed after injection of PGD2 into the preoptic area or continuous infusion into lateral or third ventricles (Inoue et al., 1984; Onoe et al., 1988). It has also been demonstrated that the infusion of PGD2 into the outer surface of the rostral basal forebrain increases SWS in the rat (Hayaishi, 1991; Matsumura et al., 1994). Non-REM sleep is also significantly reduced in rats following inhibition of PGDS via inhibition of the COX pathways or an injection of inorganic selenium compounds (Matsumura et al., 1991; Naito et al., 1988).
6.2.4. Cytokines
Produced from various cells, including neurons, cytokines stimulate subtle changes in cellular metabolism (Botchkina et al., 1997; Cheng et al., 1994; Dinarello, 1994; Vitkovic et al., 2000). It is presumed that cytokines are produced in response to neural activity and mainly effect input-output relationships within the neural circuits where they originate (Krueger et al., 2001). Two cytokines that have been extensively studied in regards to sleep regulation are IL-1β and TNFα (Alam et al., 2004; Krueger et al., 2001; Krueger et al., 1984; Nistico et al., 1992; Opp et al., 1991; Shoham et al., 1987).
The structure of IL-1 exhibits a beta-trefoil topology characterized by six beta-strands forming a beta-barrel, which is closed at one end by another six beta-strands (Vigers et al., 1994). IL-1β, one of the three major IL ligands, is produced by glia, endothelial cells, and neurons (Breder et al., 1988; Obal and Krueger, 2003). After pre-IL-1β is cleaved via IL-1β converting enzyme (ICE), the biologically active IL-1β is transported out of the cell (Dinarello, 1994; Obal and Krueger, 2003). Varying concentrations of IL-1β are detected throughout the sleep-wake cycle in both the brain and blood. Hypothalamic levels of IL-1β in the rat are highest at the beginning of daylight hours, a time when homeostatic demand for the non-REM sleep is maximal (Nguyen et al., 1998). In human blood and CSF analysis, levels of IL-1β have been shown to peak at the onset and initial hours of sleep and decline during the night and morning hours to minimum levels (Hohagen et al., 1993; Moldofsky et al., 1986). Following sleep deprivation, a significant increase in IL-1β mRNA occurs in the hypothalamus and cortex of rats (Taishi et al., 1997). Levels of IL-1β in the bloodstream also increase following sleep deprivation in humans (Hohagen et al., 1993). The amount of time spent in non-REM sleep is enhanced following an injection of IL-1β directly into brain areas, intravenously, or intraperitoneally (Fang et al., 1998; Krueger et al., 2001; Opp and Krueger, 1994a; Tobler et al., 1984). In addition to inducing sleep, intravenous injection of IL-1β enhances EEG activity during non-REM sleep in rabbits (Krueger et al., 1984); although this effect is species and route of administration dependent. If IL-1β is applied locally to the cortex EEG delta power is enhanced locally during non-REM sleep but not during REM sleep or wakefulness suggesting state-specific paracrine actions of IL-1β in the brain (Yasuda et al., 2005). These actions involve cortical-reticular thalamic communication as well as activation of VLPO and other hypothalamic areas. Substances that inhibit IL-1β production, such as IL receptor antagonist (ILRA), CRH and anti-IL-1 antibodies, or inhibition of cleavage of biologically active IL-1, decrease spontaneous sleep in the rabbit (Imeri et al., 2006; Opp and Krueger, 1994b; Takahashi et al., 1996a).
Similar to IL-1, TNFα is produced by glia, astrocytes, and neurons in the CNS (Breder et al., 1993; Obal and Krueger, 2003). Mature TNFα is a 157 amino acid cytokine composed of a beta-sandwich containing two sheets, with five beta strands each, and a disulfide bridge (Eck and Sprang, 1989; Spriggs et al., 1992). The highest level of TNFα in rats occurs at daybreak, just before the onset of sleep, and is 10-fold greater than minimal night-time values (Floyd and Krueger, 1997). Hypothalamic TNFα mRNA and circulating levels of TNF increase with sleep deprivation in rats (Tahishi et al., 1999; Yamasu et al., 1992). Human blood plasma samples also exhibit a significant increase in the TNFα soluble receptor after total sleep deprivation (Shearer et al., 2001). A microinjection of TNFα into the preoptic area significantly enhances non-REM sleep in rats (Kubota et al., 2002). The duration of non-REM sleep also increases following intracerebroventricular, intravenous, or intraperitoneal injection of TNFα (reviewed in Obal and Krueger, 2003). Direct injection of TNF into the POA enhances NREMS while injection of an inhibitor of TNF inhibits non-REM sleep (Kubota et al., 2002). Furthermore, microinfusion of TNFα into the subarachnoid space beneath the basal forebrain in rats, an area implicated in PGD2 production, enhances non-REM sleep (Terao et al., 1998). In addition to an increased amount of time spent in non-REM sleep, intraperitoneal injection of TNFα has been shown to strengthen EEG slow wave activity in rabbits (Shoham et al., 1987); this effect, like that of IL-1, is species and route of administration dependent. Also like IL-1, microinjection of TNF onto the surface of the cerebral cortex enhances EEG delta power locally. Further, microinjection of the TNF soluble receptor reduces sleep-loss enhanced EEG delta power locally (Yoshida et al., 2004). Inhibition of TNFα by means of anti-TNF antibodies or TNF soluble receptor fragments decreases spontaneous sleep in rats and rabbits (Takahashi et al., 1995; Takahashi et al., 1996b).
In summary, during wakefulness, neuronal activity-dependent metabolic activities produce adenosine, GABA, glycine, PGD2, IL-1β and TNF and many other substances in the brain as metabolic end product. These excess levels of metabolites then passively act on the wake-promoting neuronal systems of our brain to dampen their activities. The initiation of sleep is a consequence of the dampened activities in our wake-promoting brain systems. After the initiation of sleep, some of these sleep initiating factors also are involved in the induction of sleep. Here we would like to emphasize that in addition to these well-known metabolic factors (discussed above), activation of many other enzymes, that are involved in the synthesis of metabolic factors, may also be involved in the initiation of sleep. Due to the lack of experimental evidence, the role of specific enzymes in the sleep initiation process is not formally discussed in this communication.
7. Mechanisms for the generation and maintenance of SWS
Occurring after the initiation of sleep, SWS is considered the most quiescent state of the brain. As explained above, the reduction of neuronal activities in wake-promoting brain regions is one of the most important events immediately preceding SWS. Coinciding with the reduced neuronal activity, the transmission of incoming sensory signals to the cortex via thalamic sensory neurons is suspended. Sensory gating at the level of the thalamus is achieved when thalamic relay neurons are hyperpolarized by bursting activities of GABAergic neurons in the thalamic reticularis (Llinas and Steriade, 2006). Unlike the complex mechanisms responsible for the generation REM sleep, SWS is generated simply via the activation of GABA-containing neurons in the preoptic area (POA) of the hypothalamus. Synthesis of a specific hormone, growth hormone-releasing hormone (GHRH), that intensifies GABA-mediated activities in the brain also increase the depth and duration of SWS (reviewed in Krueger and Obal, 2003; Obal and Krueger, 2004).
A hypnogenic role for the POA was first suggested by von Economo (1929) more than seventy-five years ago. In post mortem brain tissue analysis of patients exhibiting insomnia in association with viral encephalitis, von Economo documented inflammatory lesions within the region recognized as the POA. Nauta (1946) supported this hypothesis by experimentally replicating behavioral insomnia in rats using bilateral knife cut lesions in the POA. Furthermore, polygraphic recordings of sleep-wake stages in cats with localized electrolytic lesions of the anterior hypothalamus objectively demonstrated the role of the POA in SWS generation (McGinty and Sterman, 1968). The advancement of experimental techniques have confirmed, as well as expanded, the findings of these early lesion studies. For example, specific lesioning of cell bodies in the preoptic area of the anterior hypothalamus has been shown to effectively suppress SWS in mammals (John and Kumar, 1998; John et al., 1994; Kumar et al., 1996; Lu et al., 2000; Srividya et al., 2006). Utilizing single cell recording techniques, various researchers have identified a large population of cells within the preoptic area that are more active during the electrophysiological and behavioral signs of SWS (Alam et al., 1995; Findlay and Hayward, 1969; Glotzbach and Heller, 1984; Kaitin, 1984; Koyama and Hayaishi, 1994; Kumar et al., 1989; Lincoln, 1969; McGinty and Szymusiak, 1990, 2000; Suntsova et al., 2002; Szymusiak et al., 1998). A number of local microinjection studies have also suggested a critical role for the preoptic area in the generation of SWS (Datta et al., 1988; Datta et al., 1985; Kumar et al., 1986; Mendelson and Martin, 1992; Ticho and Radulovacki, 1991).
Some of the recent studies have claimed that the sole regions responsible for the generation of SWS are located within the ventro-lateral (VLPOA) and/or median (MnPOA) preoptic areas (Gaus et al., 2002; Gvilia et al., 2006; Lu et al., 2002; Saper et al., 2001; Sherin et al., 1998; Sherin et al., 1996). This assumption was primarily based on a significant increase of c-Fos immunoreactivity occurring during SWS in the VLPOA and MnPOA (Gong et al., 2004; Gong et al., 2000). In our laboratory, using an identical experimental paradigm, we have also observed an increase in c-Fos activity within these two specific regions; however, a more extensive analysis reveals that surrounding areas also display heightened c-Fos immunoreactivity during SWS (Unpublished observation of Datta Laboratory). In fact, our observations indicate that the numbers of c-Fos labeled cells in the medial preoptic area (mPOA), as well as some parts of the basal forebrain, were relatively higher compared to the VLPOA and MnPOA. Also notable is a study using functional magnetic resonance imaging (fMRI) in the behaving rats that elegantly demonstrated the mPOA is more active than other parts of the hypothalamus and basal forebrain during SWS (Khubchandani et al., 2005). The results of a few studies suggest that small lesions in the VLPOA and MnPOA effectively suppressed SWS (Gerashchenko et al., 2003; Lu et al., 2000); however, similar lesion experiments in our laboratory demonstrated only a slight reduction in SWS that lasted approximately two days. Additionally, a larger lesion that includes the entire POA reduced SWS by 50% for approximately fourteen days. Indeed, a number of studies have shown that, compared to VLPOA, a lesion in the mPOA is more effective to reduce SWS (John and Kumar, 1998; John et al., 1994; Srividya et al., 2006). Together these results suggest a more inclusive role with regard to various regions of the POA in the generation of SWS.
Immunohistochemical analysis of sleep-active neurons in the preoptic area has revealed that a majority contain the inhibitory neurotransmitters GABA and galanin (Gaus et al., 2002; Gong et al., 2004; Gvilia et al., 2006; Sherin et al., 1998; Sherin et al., 1996). These sleep-active cells innervate many wake-promoting areas of the brain, including the TMN, LH, LC, DRN, and PPT/LDT (Gritti et al., 1994; Sherin et al., 1998; Steininger et al., 2001; Zardetto-Smith and Johnson, 1995). Thus, it is possible that the increased activity of SWS-active GABAergic cells in the preoptic area could release GABA to targets within the wake-promoting areas of the brain. Released GABA could suppress activity in these areas in two different ways: 1) GABA receptor-activation mediated inhibition of wake-promoting cells or 2) Inhibition of presynaptic neurotransmitter release that is necessary for the activation of wake-promoting cells (Gottesmann, 2002; Ulloor et al., 2004). Neuropharmacological studies have shown that SWS is also induced by a number of sedating and hypnotic drugs that involve potentiation of preoptic area GABAergic neurotransmission (Mendelson, 2001; Sallanon et al., 1989; Tung et al., 2001; Tung and Mendelson, 2004). Heightened activity of GABAergic neurons throughout the entire preoptic area during SWS substantiates the primary factor responsible for the induction of SWS in terrestrial mammals is the activation of GABA-containing POA neurons.
Given this conclusion that the activation of POA GABAergic cells causes induction of SWS, a numbers of pharmacological studies have demonstrated that the application of noradrenergic and serotonergic drugs in the POA induces wakefulness (Datta et al., 1987; Datta et al., 1985; Kumar et al., 1986; Kumar et al., 1984; Yamaguchi et al., 1963). Iontophoretic application of both norepinephrine and serotonin also activates cells in the POA (Beckman and Eisenman, 1970; Cunningham et al., 1967; Jell, 1973, 1974; Knox et al., 1973; Murakami, 1973). Although these results are paradoxical to the interpretation discussion above, suggesting that the activation of GABAergic POA cells could induce wakefulness. This interpretation implies another precondition(s) may be involved in the POA GABAergic cells activation-mediated induction of SWS. Indeed, recent research has indicated that the presence of a sleep inducing factor, growth hormone-releasing hormone (GHRH), may be critical for the generation of GABAergic POA cells activation-mediated SWS.
The GHRH is a peptide composed of 40–44 amino acid residues and is a member of the secretin-glucagon peptide family (Mayo et al., 1995). There are two distinct clusters of GHRHergic (GHRH-synthesizing) neurons in the hypothalamus: one cluster, containing majority of GHRHergic cells is located in the arcuate nucleus and another cluster, with less number of cells, is located around the ventromedial nucleus and the paraventricular nucleus (Daikoku et al., 1986; Merchenthaler et al., 1984; Sawchenko et al., 1985). In the rat, hypothalamic GHRH mRNA levels peak around light onset, decrease towards the end of the light period and remain at very low levels throughout the night (Bredow et al., 1996; Toppila et al., 1997). The light onset is followed by a short rise in GHRH contents suggesting that the transcribed mRNA is translated into protein very rapidly (Gardi et al., 1999). The results of these GHRH mRNA levels and GHRH content could be interpreted as the time of the day where maximum GHRH synthesis and release occur corresponds to the period of deepest SWS. It has also been shown that sleep deprivation increases hypothalamic GHRH mRNA levels and depletes GHRH peptide (Gardi et al., 1999; Toppila et al., 1997; Zhang et al., 1999). This anatomical and temporal expression of GHRH mRNA and GHRH synthesis data suggest the possibility that GHRH is involved in the induction of SWS.
The GHRHergic neurons in the arcuate nucleus are the major source of GHRH released at the median eminence; thus, the control of pituitary GH secretion is the major function of this group of neurons (reviewed in Obal and Krueger, 2004). The anterior pituitary somatotroph cells produce growth hormone (GH); its secretion occurs in pulses throughout the day but, after sleep onset, deep SWS is associated with large bursts of GH secretion. The GH secretion during SWS can amount to two thirds of the total GH secreted in young males. The majority of the extra-arcuate GHRHergic neurons as well as part of the arcuate GHRHergic neurons project predominantly to the POA. This GHRHergic neuronal projection to the POA is significant because the activation of POA GABAergic cells is shown to be involved in the generation and maintenance of SWS. Indeed, systemic injection of GHRH increased SWS in the humans (Kerkhofs et al., 1993; Marshall et al., 1999; Schussler et al., 2006; Steiger et al., 1992) and rats (Obal et al., 1996). Intracerebroventricular administration of GHRH results in an increase SWS in the rat (Ehlers et al., 1986; Nistico et al., 1987; Obal et al., 1988). Inhibition of endogenous GHRH by using either a peptide antagonist (Obal et al., 1991) or anti-GHRH antibodies (Obal et al., 1992) suppresses SWS. Inhibition of endogenous GHRH by feedback inhibition after application of GH also suppresses SWS (Stern et al., 1975). Studies have also shown that the SWS is reduced in the transgenic animal models with mutation in the GHRH gene as dw/dw rat (Obal et al., 2001) and lit/lit mice (Obal et al., 2003) compared to their wild type of rats and mice. Based on the above evidences, there is no doubt that GHRH is an important hypothalamic peptide for the induction of SWS. Another study has demonstrated that the application of GHRH directly into the mPOA increases SWS (Zhang et al., 1999). The same study also demonstrated that spontaneous and rebound SWS after 3 hr total sleep deprivation is suppressed when GHRH antagonist is microinjected into the mPOA. This study indicated that induced SWS generating mechanisms by application of GHRH most likely involves the mPOA. More recently, it has also been demonstrated that the application of GHRH in the hypothalamic cell culture increases intracellular calcium level in the GABAergic cells (De et al., 2002). Collectively, these studies discussed above could be interpreted that, for the induction of SWS, GHRH is released in the POA and binds to the GHRH receptors to activate POA GABAergic cells.
8. Mechanisms for the generation and maintenance of REM sleep
This section includes a historical perspective of early studies and theories that were critical for the initiation of modern research on the mechanisms of REM sleep, the “Cellular-Molecular-Network” (CMN) model of REM sleep regulation, and a discussion of studies that are the experimental backbone of the CMN model.
8.1. Historical perspective
Although REM sleep only occupies approximately 20% of our total sleep time, its regulation is a relatively more complex process than that of SWS. To fully appreciate the history that has guided modern research on the neurobiological mechanisms of REM sleep regulation, it is important to mention early transection studies in the cat by Jouvet and his colleagues (Jouvet, 1962; Jouvet and Michel, 1959, 1960; Jouvet and Mounier, 1960) and the original “reciprocal interaction” model by McCarley and Hobson (1975). Transection studies by Jouvet and collaborators demonstrated when the neuraxis was cut rostral to the pons, resulting in destruction of the caudal portion of the midbrain, the activated EEG signs of REM sleep were absent in the forebrain. This procedure, called “pontine preparation”, eliminated REM sleep EEG signs but various observable signs of REM sleep persisted. Muscle atonia capable of abolishing decerebrate rigidity, a classical sign of pontine preparation, and periodic rapid eye movements were still present in post-transection sleep. Also notable was the appearance of spiky waves in the pons, the pontine component of PGO waves. These signs of REM sleep suggested that brainstem structures located caudal to the transection of the midbrain were sufficient for the generation of REM sleep signs and periodicity. More importantly, these results inspired the next generation of studies enabling the precise localization of cellular and network components for REM sleep phenomena.
Publication of reciprocal-interaction model (Hobson et al., 1975; McCarley and Hobson, 1975a) was one of the most powerful catalysts for the initiation of modern research on the neurobiological mechanisms involved in REM sleep. In this model, Lotka-Volterra equations were used to mathematically describe the possible interactions between populations of REM-on and REM-off neurons in the brainstem. This structural and mathematical model, for the first time, proposed that aminergic and cholinergic neurons of the mesopontine junction interact in a reciprocal manner that results in the ultradian alteration of mammalian REM and non-REM sleep. According to this model, REM-on cells of the medial pontine reticular formation (mPRF) are cholinergic and are postsynaptically excited by the activation of cholinergic receptors. Conversely, REM-off cells are aminergic (noradrenergic cells in the LC and serotonergic cells in the DR) and are postsynaptically inhibited by the activation of noradrenergic and serotonergic receptors. During wakefulness, the aminergic REM-off system is tonically activated, thus inhibiting the cholinergic REM-on system. Throughout non-REM sleep, the aminergic inhibition wanes and cholinergic excitation waxes as a result of the gradual withdrawal of aminergic inhibitory influences from cholinergic REM-on system. At REM sleep onset, aminergic inhibition is turned off and cholinergic excitability peaks, while other outputs are inhibited. The behavioral predictions of this reciprocal-interaction model might have been influenced by the contemporary anatomical, single cell recordings, and local microinjection studies of that time (Amatruda et al., 1975; Chu and Bloom, 1973; George et al., 1964; Hobson et al., 1974; Hobson et al., 1975; McCarley and Hobson, 1971; Shute and Lewis, 1967).
Although this original reciprocal-interaction model initiated a new era of research on the neurobiological mechanisms of REM sleep regulation, subsequent studies utilizing specific monoclonal antibodies of choline acetyltransferase (Bernard et al. 1999) on multiple brain regions, identified brainstem cholinergic cell groups in the PPT and LDT but not the mPRF (Armstrong et al., 1983; Cuello and Sofroniew, 1984; Mesulam et al., 1989; Mesulam et al., 1983, 1984). Accordingly, single cell recording studies have shown that a subset of cholinergic cells in the PPT and LDT are the principal REM-on neurons in the brainstem (reviewed in Datta, 1995; Datta and Siwek, 2002). To accommodate these new findings, a modified reciprocal-interaction model shifted the location for the major locus of cholinergic REM-on neurons to the LDT/PPT from its original postulated location in the mPRF (Pace-Schott and Hobson, 2002; Steriade and McCarley, 1990).
Over the last 25 years of research, especially during the last decade, numerous studies are directed towards understanding the basic mechanisms of REM sleep. Based on the results of earlier studies and findings of the last decade, researchers have suggested that the regulation of REM sleep involves a distributed network rather than a single center (Datta and Hobson, 1995; Vertes, 1984). The network for REM sleep generation and primary cell groups are located within the pons and midbrain. Depending on the homeostatic, as well as some patho-physiological demands, this brainstem network could also be modulated by many other cell groups in the forebrain. The current status on our knowledge of the mechanisms of REM sleep is summarized in the following description of “Cellular-Molecular-Network model of REM sleep regulation”.
8.2. Cellular-Molecular-Network model of REM sleep regulation
Utilizing previous studies focused on REM sleep, a “structural and functional model for REM sleep regulation” (Datta, 1995) was developed to formally acknowledge the idea of a distributed network theory (Vertes, 1984). Since this earlier publication, the research and knowledge of REM sleep regulation has grown tremendously. To update the “structural and functional model for REM sleep regulation” and maintain a degree of flexibility for future developments in cellular and molecular aspects of REM sleep regulation, the “structural and functional” model is now renamed as “CMN model of REM sleep regulation”.
According to the CMN model, the individual events of REM sleep are generated by distinct cell groups located in the brainstem. They are discrete components of a widely distributed network rather than a single REM sleep “center.” For example, muscle atonia is executed by the activation of neurons in the locus coeruleus alpha (LCα), rapid eye movements result from the activation of neurons in the peri-abducens reticular formation (PAb), PGO waves emerge by the activation of neurons in the caudo-lateral peribrachial area (C-PBL) of predator mammals and in the dorsal part of the nucleus subcoeruleus (Sub C) of prey mammals, hippocampal theta rhythm is produced via the activation of neurons in the pontis oralis (PO), muscle twitches appear with the activation of neurons in the nucleus gigantocellularis (especially the caudal part), and increased brain temperature and cardio-respiratory fluctuations occur via the activation of neurons in the parabrachial nucleus (PBN). The cortical EEG activation sign of REM sleep, however, is executed jointly by the activation of neurons in the mesencephalic reticular formation (MRF) and rostrally-projecting bulbar reticular formation (also called medullary magnocellular nucleus (MN)). We would like to emphasize here that these particular cell groups are simply the executive neurons for an individual sign. For the final expression of an individual sign, the relevant executive neurons employ a specific neuronal circuit unique to that REM sleep sign. In essence, each of these REM sleep signs has a separate, specialized network. Thus, each of these REM sleep signs could be modulated with multiple neurotransmitters at multiple sites of their circuit.
Turn-on or turn-off conditions of REM sleep generating executive neurons are regulated by the ratios of available aminergic and cholinergic neurotransmitters within those cell groups. The source of aminergic neurotransmitters is the LC and RN, while cholinergic neurotransmitters originate from the LDT and PPT. The activity of both aminergic and cholinergic cells is approximately equal during wakefulness and the onset of SWS results in an equal reduction in activity. Therefore, during wakefulness and SWS the ratio of aminergic to cholinergic neurotransmitters in REM sleep generators is proportionate. During REM sleep, however, aminergic cell activities are markedly reduced or absent and cholinergic cell activities are comparatively high. The level of cholinergic cell activity during REM sleep is roughly thirty-five percent less than that of wakefulness. Thus, when a hypothetical ratio of aminergic and cholinergic neurotransmitters is 1:1, the REM sleep sign-generator remained in turned-off condition; however, when this ratio is 0:0.65, the generator is turned-on to express REM sleep signs.
8.3. REM sleep sign-generators
In order for brainstem site to be considered a specific REM sleep sign-generator, we suggest following three basic criteria. First, lesioning of a particular cell group in the brainstem eliminates a particular REM sleep sign during natural REM sleep episodes. Second, stimulation of the same cell group would elicit that particular REM sleep sign in a state-independent manner (i.e. exhibition of that REM sleep sign during wakefulness and SWS). Finally, the single cell activity patterns of those cells should exhibit a discharge profile consistent with causality (i.e. for the tonic signs, there should be an appropriate increase in tonic discharge rate and for the phasic signs, there should be an increase in tonic and/or bursting type of discharges prior to that particular sign of REM sleep). Following are the descriptions of individual generators for the six most important signs of REM sleep.
8.3.1. Cortical EEG activation generator
High frequency, low amplitude cortical EEG is present in both wakefulness and REM sleep, making the identification of this generator challenging. Jouvet (1962) proposed that the nucleus pontis caudalis was the region most directly involved in the activation of cortical EEG during REM sleep. As such, initial studies examining the neural substrates controlling active cortical EEG during REM sleep were concentrated in the pons rather than in the midbrain or medulla. A series of studies placed electrolytic lesions throughout the pons to examine changes in REM sleep architecture (Carli et al., 1963, 1965; Carli and Zanchetti, 1965; Zanchetti, 1967). The collective results demonstrated that activation of cortical EEG during REM sleep was unaffected by lesions in the anterior, dorsal, and median raphe nuclei as well as the locus coeruleus and subcoeruleus nuclei, nucleus reticularis tegmenti pontis, dorsal and ventral tegmental nuclei of Gudden, and pontis caudalis. Since these lesion studies decreased the likelihood that the pons was involved in REM sleep cortical activation, researchers focused attention on the midbrain as a possible generator for REM sleep EEG. Two early lesion/transection studies provided evidence indicating the midbrain may be more important than pons in the generation of REM sleep cortical EEG activity (Candia et al., 1967; Hobson, 1965). The role for midbrain cells in cortical EEG activation was further strengthened by the demonstration that midbrain reticular formation cooling, which temporarily blocks neuronal activities, leads to a reversible suppression of cortical EEG activation (Jones and Bickford, 1977; Skinner, 1970). More recent studies, utilizing kainic acid lesions, confirm the activated cortical EEG sign of REM sleep is not generated by the pons (Sastre et al., 1981). Another chemical stimulation study demonstrated that a microinjection of kainic acid into the midbrain immediately (latencies between 20 and 30 sec) activates the cortical EEG in the behaving cats (Kitsikis and Steriade, 1981). This induced state-independent cortical EEG activity lasted for approximately 12–24 hrs. Therefore, it is possible the activation of this midbrain injection site is associated with the cortical EEG activation of REM sleep.
The importance of the midbrain in the generation of cortical EEG, specifically during REM sleep, was firmly established by analyzing state-dependent activity of individual neurons. A series of studies recording single cell activity patterns demonstrated that, when compared to the slower EEG state of SWS, the tonic firing rates of midbrain reticular formation neurons are significantly higher during the active EEG states of wakefulness and REM sleep (Kasamatsu, 1970; Manohar et al., 1972; Steriade et al., 1982). It is also been reported that these cells begin to increase their firing rate preceding changes in cortical EEG activity during transitions from SWS-REM sleep (Steriade et al., 1982). Another single cell recording study identified a population of rostrally-projecting neurons in the medullary reticular formation (magnocellular nucleus) that discharge tonically during REM sleep (Steriade et al., 1984). These cells begin to increase their firing rate about a minute before the transition between SWS and REM sleep and fire maximally during REM sleep. Based on these activity patterns, Steriade et al. (1984) proposed that these medullary reticular formation cells act synergistically with MRF neurons to control the EEG activation during REM sleep.
We suggest for the generation of REM sleep associated cortical EEG activity, glutamatergic cells in the MRF and magnocellular nucleus are activated, resulting in stimulation of the thalamo-cortical pathway. Ultimately, activation of thalamo-cortical pathway excites the cerebral cortex resulting in the generation of active EEG signs.
8.3.2. Muscle atonia generator
Absence of postural muscle tone, or muscle atonia, is a well-documented characteristic of REM sleep. During periods of REM sleep, all terrestrial mammals remain paralyzed by the active inhibition of motoneurons controlling the tone of antigravity muscles. The previously mentioned study by Jouvet (1962) demonstrated that the muscle atonia during REM sleep remained intact following a transection between rostral pons and midbrain. Additionally, this study illustrated that a transection between caudal pons and rostral medulla abolished REM sleep atonia. These two observations established the primary brain structure(s) responsible for the generation of muscle atonia during REM sleep to be located within the pons. This important hypothesis was confirmed by other investigators in subsequent studies (Matsuzaki, 1969; Villablanca, 1966). One particular lesion study by Jouvet and Delorme (1965) intended to further localize the specific part of the pons that may be responsible for the generation of atonia during REM sleep in the cat; however, a remarkably interesting phenomenon occurred. Commonly described as “REM sleep without atonia”, bilateral lesions centered in the caudal part of the LC eliminated muscle atonia while all other signs of REM sleep remained intact. Based on this observation, these investigators suggested that the LC is involved in the generation of atonia during REM sleep (Jouvet and Delorme, 1965). Subsequent lesion studies in the cat revealed that smaller lesions ventromedial to the LC were more effective in disrupting the atonia of REM sleep than those directly on the LC (Hendricks et al., 1982; Henley and Morrison, 1974; Morrison, 1979; Sastre and Jouvet, 1979; Sastre et al., 1978). This REM sleep atonia generating site in the ventromedial to LC was later termed the peri-LC-alpha in the cat (Sakai, 1980). Recent lesion studies in cats and rats have also confirmed that the REM sleep atonia generating site is located in the peri-LC-alpha (Lu et al., 2006; Sanford et al., 1994). In the rat, this area has been designated as sublaterodorsal nucleus (SLD) (Boissard et al., 2002; Lu et al., 2006). Besides evidences from lesion studies, three other lines of evidences have also demonstrated that REM sleep atonia generating neurons are located within the peri-LC-alpha. The first involves experiments recording single cell activity patterns in cats that report the cells of the peri-LC-alpha are predominantly REM-on cells and half of those REM-on cells discharge selectively and at tonic rates throughout the atonia of REM sleep (Sakai, 1980; Sakai et al., 1981). Secondly, electrical stimulation of pontine areas that overlap the peri-LC-alpha results in hyperpolarization of lumber motor neurons in the cat during REM sleep (Fung et al., 1982). Finally, inhibition of peri-LC-alpha cells by local microinjection of clonidine eliminates muscle atonia during REM sleep (Datta et al., 1993a). More recently, it has been shown that neurons in the peri-LC-alpha/SLD are of glutamatergic type (Lu et al., 2006).
We suggest that the activation of muscle atonia generating neurons in the peri-LC-alpha activates medullary inhibitory area of Magoun and Rhines (1946) via the tegmento-reticular tract (Datta et al., 1993a; Datta et al., 1993b; Hendricks et al., 1982; Sakai, 1980; Sakai et al., 1981); which, in turn, activates GABAergic and/or glycinergic neurons in the spinal cord (Chase et al., 1989; Taepavarapruk et al., 2002). Ultimately, the activation of those GABAergic and/or glycinergic cells inhibits spinal motor neurons (Chase et al., 1981; Chase et al., 1989; Curtis et al., 1968; Fung et al., 1982; Maxwell and Riddell, 1999; Taal and Holstege, 1994; Taepavarapruk et al., 2002; Walmsley et al., 1987; Watson and Bazzaz, 2001). This inhibition results in muscle atonia that characterizes REM sleep.
8.3.3. Rapid eye movements generator
Although the expression of rapid eye movements (REMs) are a distinguishing feature of REM sleep, very few studies have been devoted to localizing the specific brainstem site containing cells that generate REMs. To effectively describe the REMs generator, we will first focus on some studies devoted to identifying brainstem cells that control saccadic eye movements during wakefulness. The involvement of the brainstem in eye movement control was initially proposed after stimulation of the ponto-medullary reticular formation resulted in conjugate eye movements and small unilateral lesions eliminated ipsilateral eye movements in the conscious monkeys (Cohen and Komatsuzaki, 1972; Goebel et al., 1971). Correspondingly, a number of single cell recording studies have identified many different cell types in the brainstem whose firing patterns were temporally synchronized with different aspects of saccadic eye movements (Curthoys et al., 1981; Henn and Cohen, 1976; Hikosaka and Kawakami, 1977; Igusa et al., 1980; Kaneko et al., 1981; Keller, 1974; Luschei and Fuchs, 1972; Yoshida et al., 1982). Of those different types of cells, a group of excitatory neurons discharge a burst of action potentials immediately preceding ipsilateral horizontal saccades that continues for the approximate duration of the saccade. Moreover, these neurons are virtually silent in the absence of eye movements, during slow pursuit eye movements, during vertical saccades, and during contralateral horizontal saccades. These excitatory burst neurons are localized within a relatively restricted region of the dorsomedial pons just rostral to the abducens nucleus, also known as peri-abducens area (Curthoys et al., 1981; Gottesmann, 1997; Igusa et al., 1980; Kaneko et al., 1981). Stimulation of the burst neuron region evokes monosynaptic excitatory postsynaptic potentials (EPSPs) in the abducens motor neurons (Highstein et al., 1976) and these burst neurons can be antidromically activated from the ipsilateral abducens nucleus (Igusa et al., 1980; Kaneko et al., 1981). Additionally, a field potential in the abducens nucleus at monosynaptic delays can be induced by repetitive firing of these burst neurons (Igusa et al., 1980). Applying both anterograde and retrograde anatomical techniques, cells from the burst neuron region have been shown to directly project to the abducens nucleus (Buttner-Ennever and Henn, 1976; Gacek, 1979; Graybiel, 1977; Maciewicz et al., 1977). Since the discharge is tightly phase locked to individual saccades and excitation of these cells monosynaptically excite the abducens nucleus, these burst neurons have been suggested to be the immediate pre-motor elements that generate horizontal saccades (Kaneko et al., 1981). Based on the anatomical and physiological characteristics of these excitatory burst neurons, it is reasonable to speculate that these cells could also be involved in the generation of REMs associated with REM sleep. In fact, a population of cells in the chronically prepared decerebrate cat exhibit brief, phasic bursts of activity correlated with discrete bursts of REMs during spontaneous and eserine-induced REM sleep-like states (Hoshino et al., 1976). Similar to the excitatory burst neurons, considered the immediate pre-motor elements generating horizontal saccades during wakefulness, these REM specific burst neurons are also located within the peri-abducens area (Hoshino et al., 1976). In another single cell recording study, it was reported that 32 of 306 pontomedullary cells discharge in association with the rapid (saccadic) eye movements of both waking and REM sleep (Siegel and Tomaszewski, 1983). Interestingly, all of these 32 neurons were found clustered within the peri-abducens area. Based on the collective findings of above discussed studies, it is reasonable to suggest that the peri-abducens area is the REMs generator. As such, we suggest that the activation of REMs generating cells in the peri-abducens area stimulate occulomotor neurons in the abducens nucleus, which activate occulomotor muscles to cause the expression of REMs associated with REM sleep. In addition to this primary generator (peri-abducens area), REM sleep associated clusters of REMs are also positively modulated by the activation of vestibular nuclei and phasic P-wave generating cells through a feed-forward monosynaptic network (Datta and Hobson, 1994; Datta et al., 1998; Mergner and Pompeiano, 1978; Morrison and Pompeiano, 1966; Pompeiano and Morrison, 1965).
8.3.4. Hippocampal theta-wave generator
The hippocampal theta-wave rhythm is a sinusoidal pattern of electrical activity in the frequency range of 5–10 Hz (Vertes and Kocsis, 1997). Green and Arduini (1954) were the first to draw widespread attention to existence of the prominent theta rhythm in the rabbit hippocampus. These investigators and many others have demonstrated that theta rhythm could be elicited by both natural sensory stimulation and direct activation of the brainstem reticular formation (Green and Arduini, 1954; Kahana et al., 2001; Vanderwolf, 1969; Xu et al., 2004). Hippocampal theta-wave rhythm is present continuously throughout REM sleep and during specific waking conditions (reviewed in Vertes, 1982; Vertes and Kocsis, 1997). Although theta-wave rhythm is present during certain waking conditions, these waking conditions vary between species (Vanderwolf, 1969; Winson, 1972). The short episodes of theta-wave rhythm during specific waking are also not as synchronized as REM sleep (Vertes and Kocsis, 1997). Between 1954 and 1982, a number of studies were devoted to localizing the specific brainstem and/or forebrain nuclei involved in generation of hippocampal theta rhythm (reviewed in Vertes, 1982, 2005; Vertes et al., 2004). Different studies have contributed to different aspects of the theta-wave generator and its ascending pathways (Macadar et al., 1974; Robinson and Vanderwolf, 1978; Robinson et al., 1977); however, the careful mapping studies of Vertes (Vertes, 1980, 1981) and Macadar et al. (1974) have conclusively demonstrated that the hippocampal theta-wave generator is located in the nucleus pontis oralis. Using single cell recording techniques, Vertes (1977 Vertes (1979) also identified a specific type of neuron with theta-associated discharge characteristics within the nucleus pontis oralis of the rat. These cells, designated as tonic MOV-REM neurons, maintained a selectively high tonic rate of discharge during waking-motor behavior and REM sleep. More interestingly, these cells exhibited changes in firing rate that corresponded directly to fluctuations in the regularity of the theta rhythm during REM sleep. The MOV-REM type of cells is also present in the pontine reticular formation of the freely moving cat (Sakai, 1980). Furthermore, another study by Vertes and his colleagues (Vertes et al., 1993) induced hippocampal theta-wave rhythms in the urethane-anesthetized rats via cholinergic activation of nucleus pontis oralis.
Thus, based on the collective results from the above studies, REM sleep associated hippocampal theta wave rhythms are generated primarily by the activation of specific cells in the nucleus pontis oralis. This activating signal from the nucleus pontis oralis to the hippocampus involves a polysynaptic interplay between GABAergic and cholinergic cells of the septo-hippocamapal pathway (reviewed in Vertes and Kocsis, 1997; Xu et al., 2004). Recently, it has been demonstrated that the activation of P-wave generating cells could also reset the frequency and amplitude of hippocampal theta-wave rhythms through monosynaptic connections between the P-wave generator and dorsal hippocampus (Datta, 2006; Datta et al., 1998).
8.3.5. Fluctuations in autonomic systems
Body temperature, blood pressure, heart and respiratory rates exhibit fluctuations beyond normal limits during REM sleep (Baust et al., 1972; Parmeggiani, 1980; Parmeggiani and Rabini, 1967, 1970; Snyder et al., 1964). Since all of these variables are coordinated by the autonomic nervous system, it is assumed that mammalian autonomic system suspends regulatory functions throughout REM sleep. For example, hypothalamic temperature regulating systems reduce and/or suspend their role in the homeostatic control of body temperature. As a result, body temperature could raise or fall depending on the environmental temperature (Glotzbach and Heller, 1976; Parmeggiani, 1980; Parmeggiani and Rabini, 1967). In essence, homeothermic mammals become temporarily poikilothermic. A number of studies have suggested that REM sleep associated changes in the rates and patterns of neuronal activity in the brainstem parabrachial area (PBN) is responsible for the disruption of thermostatic functions of hypothalamic thermoregulatory systems (reviewed in Datta, 1995; Parmeggiani, 1980). Breathing also becomes shallow, more frequent and irregular during REM sleep (Gottesmann, 1969; Orem et al., 1977; Phillipson, 1978; Radulovacki and Carley, 2003; Remmers et al., 1976; Sullivan, 1980). The central control of respiration is a complex process involving several brainstem areas, each controlling a different aspect of breathing. Using lesion, stimulation, and single cell activity recording techniques, a number of studies have provided strong evidences supporting the altered activity of the PBN neurons associated with REM sleep is the causal factor for respiratory fluctuations during REM sleep (Bertrand and Hugelin, 1971; Cohen, 1971; Harper and Sieck, 1980; Knox and King, 1976; Lydic and Orem, 1979). The most striking cardiovascular changes in REM sleep involve phasically occurring increases in heart rate and blood pressure that coincide with the other phasic events of REM sleep (Baust and Bohnert, 1969; Gassel et al., 1964; Gottesmann, 1969; Mancia and Zanchetti, 1980; Spreng et al., 1968). Similar to the respiratory system, several cell groups of the brainstem, cerebellum, and forebrain have been implicated in cardiovascular control (Alexander, 1946; Armour, 2004; Calaresu et al., 1976; Chai and Wang, 1962; Ciriello and Calaresu, 1977; Evans, 1980; Gordon and Sved, 2002; Levy and Martin, 1979; Loewy et al., 1979; Lowie and Spyer, 1990; Miura and Reis, 1969; Ward and Gunn, 1976). Using stimulation and single cell recording techniques, it has been suggested that REM sleep associated changes in the rate and patterns of the PBN neuronal activity is responsible for the heart rate and blood pressure changes during REM sleep (Mraovitch et al., 1982; Sieck and Harper, 1980). In summary, executive neurons producing REM sleep associated fluctuations in the autonomic functions are located within the PBN.
8.3.6. PGO/P-wave generator
PGO/P-waves are considered one of the most definitive physiologic signs of REM sleep. Initial experiments in the cat identified a field potential that originates in the pons (P) and propagates to the lateral geniculate body (G) and occipital cortex (O); thus termed the PGO wave (Brooks and Bizzi, 1963; Jouvet, 1965). This field potential was subsequently recorded from many other parts of the brain which receive excitatory inputs from the PGO-wave generation site (Datta, 1997; Datta et al., 1998). In addition to cats, the PGO wave has been documented and studied in other mammals including non-human primates, humans, and rodents (reviewed in Datta, 1997). Since the field potential is absent in the LGB of the rat (Stern et al., 1974), due to the lack of afferent inputs from P-wave generating cells, this field potential is called a P-wave (Datta et al., 1999). The P-wave in the rat is equivalent to the pontine component of the PGO wave in the cat (Datta et al., 1999; Datta et al., 1998). Lasting for 75–150 msec, the P-wave appears during REM sleep as clusters containing a variable number of P-waves (3–5 waves/burst) or a singlet with amplitudes ranging from 100–150μV and a frequency range of 30–60 spikes/min (Datta, 2000; Datta et al., 1998; Datta et al., 2001a).
Transection, lesion, electrical stimulation, and thermal cooling experiments have directly and indirectly demonstrated that the PGO/P-wave generator is located within the pons (reviewed in Datta, 1997). For example, a number of studies recording single cell activity patterns in and around the PPT/LDT observed a small population of neurons (about 3–5%) that discharged in bursts of 3–5 spikes preceding individual LGB PGO waves (McCarley et al., 1978; Nelson et al., 1983; Saito et al., 1977; Sakai and Jouvet, 1980; Steriade et al., 1990b; Steriade et al., 1990a). Based on this observation, these cells were considered to be PGO-wave generating neurons (McCarley et al., 1978; Steriade et al., 1990b). Recent studies, however, clearly indicate that the burst cells in the PPT/LDT are not PGO-wave generating neurons (reviewed in Datta, 1995). Instead, these cells, called transferring neurons, are responsible for conveying information from the pontine PGO to the forebrain in the cat (Datta, 1997). In the rat, P-wave generating cells transmit P-wave information directly to the forebrain (Datta et al., 1998). Therefore, this type of transferring neurons is absent in the rat (Datta and Siwek, 2002). Using chemical microstimulation, cell-specific lesions, and single cell recording techniques, the PGO-wave generator in the cat was localized in the caudo-lateral part of the peribrachial area; whereas, the P-wave generator in the rat is located within the dorsal part of the subcoeruleus nucleus (Datta, 1995; Datta and Hobson, 1994, 1995; Datta et al., 1998). Analysis utilizing specific monoclonal antibodies has identified P-wave generating cells to be glutamatergic (Datta, 2006). Single cell recording studies have shown that these P-wave generating neurons discharge high-frequency (>500 Hz) spike bursts (3–5 spikes/burst) in the background of tonically increased firing rates (30–40 Hz) during the transition from SWS to REM sleep (tS-R) and REM sleep (Datta, 1997; Datta and Hobson, 1994). These glutamatergic P-wave generating cells normally remain silent during W and SWS (Datta and Hobson, 1994). A neuroanatomical pathway tracing study has demonstrated that functionally identified P-wave generator cells project to the hippocampus, amygdala, entorhinal cortex, visual cortex and many other regions of the brain known to be involved in cognitive processing (Datta et al., 1998). Using microdialysis technique, we have also observed that the cholinergic activation of the P-wave generator increases glutamate release in the dorsal hippocampus (Datta, 2006). Likewise, it has been demonstrated that the P-wave activity has a positive influence on the hippocampal theta-wave activities in the dorsal hippocampus (Datta, 2006; Karashima et al., 2002; Karashima et al., 2004).
8.4. Regulation of state-dependent cholinergic tone in the REM sleep sign-generators
While progressing towards REM sleep, receptor activation-mediated aminergic tone slowly reduces and cholinergic tone begins to increase in the REM sleep sign-generators. The net result of aminergic tone withdrawal and increased cholinergic tone is the activation of each individual REM sleep sign-generator to express specific REM sleep signs (Datta, 1995; Datta et al., 1997). There are three different evidences to support the cholinergic involvement in REM sleep sign-generator activation. First, anatomical studies have shown that each of these individual REM sleep sign generating nuclei receives anatomical inputs from the PPT (Hallanger and Wainer, 1988; Mitani et al., 1988; Moon-Edley and Graybiel, 1983; Rye et al., 1988; Semba, 1993; Woolf and Butcher, 1986, 1989; Woolf et al., 1990). Second, in a number of studies, it has been shown that the cholinergic activation of individual REM sleep sign-generating nuclei results in state independent expression of that particular REM sleep sign (Datta et al., 1992; Datta et al., 2004; Datta et al., 1993b; Datta et al., 1998; Kodama et al., 1998; Vertes et al., 1993). Finally, REM sleep is increased and suppressed by activation and inhibition of PPT cholinergic cells, respectively (Datta, 2002; Datta and Siwek, 1997; Datta et al., 2002; Datta et al., 2001a; Ulloor et al., 2004). Thus, the regulation of state-dependent cholinergic tone in the REM sleep sign-generators depends directly on the regulation of state-dependent activity patterns of the PPT cholinergic cells. Following are brief descriptions of state-dependent activity patterns of PPT cholinergic cells and receptor activation-mediated intracellular events responsible for the regulation of REM sleep.
8.4.1. Neuronal activity patterns of the PPT cholinergic cells
Although examination of the state-dependent neuronal activity patterns of brainstem cells began in late 1960’s, studies specifically designed to record state-dependent activity patterns of cells within the anatomically defined cholinergic nuclei of the brainstem (PPT and LDT) did not begin until 1987. During this period, two researchers (Datta and El-Mansari) from two different laboratories (Steriade and Sakai) began, for the first time, to record single cell activity patterns of PPT and LDT cholinergic cells in the cat (Datta et al., 1989; El Mansari et al., 1989). The PPT and LDT were only established as the major cholinergic nuclei of the brainstem by choline acetyltransferase immunohistochemistry studies during the 1980’s; thus accounting for the relative delay in recording activity of these cells (Armstrong et al., 1983; Cuello and Sofroniew, 1984; Kimura et al., 1981; Mesulam et al., 1983, 1984; Mizukawa et al., 1986; Satoh and Fibiger, 1985a, 1985b; Shiromani et al., 1988; Vincent and Reiner, 1987; Woolf and Butcher, 1986). Perhaps another factor delaying the recording of these cells involves the surgical removal of tentorium, a bony structure overlying the PPT and LDT. Without this procedure it was almost impossible to approach cells of the entire PPT and LDT with a microelectrode in the cat. During this period, however, surgical removal of tentorium was perfected by Datta et al. (Datta et al., 1989) for the single cell recordings in chronically prepared behaving cats (Steriade et al., 1990b; Steriade et al., 1990a).
A detailed description of state-dependent activity patterns of PPT/LDT cholinergic cells and specific criteria for the classification of these cells has been previously published in a review article (Datta, 1995). Briefly, based on the activity patterns, single cell activity recording studies have identified several different types of cholinergic cells in the PPT/LDT area of behaving cats (Datta et al., 1989; El Mansari et al., 1989, 1990; Steriade et al., 1990b; Steriade et al., 1990a). Of those cells, the firing rates of two types of neurons correlate well with the initiation and maintenance of REM sleep. The firing rate of the first type of neuron, called REM-on cells, increases as the animal transitions from wakefulness to SWS and then to REM sleep. The firing rate of the second group of neurons, called Wake-REM-on cells, increases during both wakefulness and REM sleep. Two other single cell recording studies sampled a much smaller population of cells and recorded similar types of activity patterns in the LDT of rat (Kayama et al., 1992) and LDT/PPT of cat (Thakkar et al., 1998). More recently, single cell activity patterns in the freely moving rat across multiple sleep-wake cycles were recorded from the neurons in the cholinergic cell compartment of the of the PPT (Datta and Siwek, 2002). For several reasons, the results of PPT neuronal activity analysis of this study were more informative and interesting than earlier studies. This is the first study that recorded state-dependent single cell activity patterns of PPT cholinergic cells in the freely moving rats. Of those 70 cells recorded from the cholinergic cell compartment of the PPT, 12.86% were of REM-on type and 60.0% were of Wake-REM-on type. Interestingly, mathematical analysis of this study revealed, for the first time, that the level of population activity within the cholinergic cell compartment of the PPT is one of the most critical factors for the induction of REM sleep (Datta and Siwek, 2002). This study also revealed that unlike in the cat (El Mansari et al., 1990; Steriade et al., 1990b) and brainstem slices of rats and guinea pigs (Leonard and Llinas, 1994; Luebke et al., 1992; Williams and Reiner, 1993), cholinergic cells in the PPT of adult freely moving rats do not fire in a bursting mode (Datta and Siwek, 2002). Although only a small amount of LDT neurons were examined, none recorded in the head-restrained adult rats showed a bursting type of neuronal activity (Kayama et al., 1992). Thus, the bursting type of neuronal activity in the rodent slice preparation could be the slice-specific experimental preparation effect rather than a natural phenomenon.
8.4.2. Receptor activation-mediated intracellular events within the cholinergic cell compartment of the PPT
Based on some indirect evidences, the involvement of PPT/LDT cholinergic cells the generation REM sleep has been suggested for the long time (Steriade and McCarley, 1990). During the last decade, however, direct evidences have shown that when the activity level in the cholinergic cell compartment of the PPT reaches to about 65% level by tonic release of glutamate, REM sleep is induced (Datta et al., 1997; Datta and Siwek, 2002; Datta et al., 2001a). Throughout SWS, activation of GABA-B receptors inhibits cells in the cholinergic cell compartment of the PPT maintaining a population activity level of approximately 7.4% (Datta and Siwek, 2002; Ulloor et al., 2004). Activation of low-threshold, kainate-type glutamate receptors on the PPT cholinergic cells results in a population activity level increase in the cholinergic cell compartment of the PPT to about 65%, resulting in REM sleep generation (Datta, 2002; Datta et al., 2002). When high-threshold NMDA-type receptors on those PPT cholinergic cells are also activated, the population activity in the cholinergic cell compartment reaches approximately 100%, resulting in wakefulness (Datta et al., 2001b; Datta and Siwek, 2002; Datta et al., 2002). In summary, PPT cholinergic cell activity and REM sleep are regulated by the interactions of neurotransmitters glutamate and GABA as well as the activation of kainate, NMDA, and GABA-B receptors.
It is a well-established fact that the neurotransmitter-mediated excitation and inhibition of PPT cells are important processes for the regulation of REM sleep. This receptor-mediated action involves intracellular molecular signaling mechanisms for the transcription, gene activation, and protein synthesis ultimately expressing REM sleep behavior. To this end, the results of some recent experiments are very promising for understanding the molecular mechanisms of REM sleep regulation (Bandyopadhya, 2006; Datta, 2006; Datta and Prutzman, 2005). One study has demonstrated that the activation of GABA-B receptors within the cholinergic cell compartment of the PPT suppresses REM sleep in the freely moving rat (Ulloor et al., 2004). It is known that the GABA-B receptors couple to Gi/Go G proteins (Couve et al., 2000; Kerr and Ong, 1995; Mody et al., 1994; Robbins et al., 2001; Sivilotti and Nistri, 1991; Takahashi et al., 1998; Thompson, 1994), and Gi/Go G proteins inhibit adenylyl cyclase (AC); which, in turn, prevents activation of the cAMP-PKA signal transduction pathway (Gilman, 1987; Marinissen and Gutkind, 2001). Therefore, the suppression of REM sleep, initiated by the activation of GABA-B receptors in the PPT, may be due to inhibition of the cAMP-PKA signal transduction pathway. Recent pharmacological and physiological studies have also shown that the activation of PPT kainate-type glutamate receptors induces REM sleep (Datta, 2002; Datta et al., 2002). It is also well known that the activation of kainate receptors increases the cytoplasmic free calcium concentration (Bernard et al., 1999; Bleakman and Lodge, 1998; Haak et al., 1997; Kovacs et al., 2000; Michaelis, 1998). In neurons, calcium ions can stimulate the production of cAMP and the activation of PKA via the activation AC (Cali et al., 1994; Ginty et al., 1991; Waltereit et al., 2001; Xia et al., 1996). Thus, it is possible that the activation of kainate receptors in the PPT could also activate the cAMP-PKA signal transduction pathway to induce REM sleep. Indeed, one recent study has demonstrated that the inhibition of AC in the cholinergic cell compartment of the PPT suppressed REM sleep in the freely moving rat (Datta and Prutzman, 2005). Yet another recent study demonstrated that REM sleep increases with increased catalytic subunit of PKA and PKA enzymatic activity in the cholinergic cell compartment of the PPT (Bandyopadhya et al., 2006). This study also demonstrated that the pharmacological inhibition of PPT intracellular cAMP-PKA activity suppressed REM sleep. Accordingly, it appears that the cAMP-PKA intracellular signaling pathway is critically involved in the cholinergic cell compartment of the PPT for the regulation of cholinergic tone in the REM sleep sign-generators. Rapid desensitization (in seconds) is one of the important characteristics of the kainate receptors (GluR6) but, increased cytosolic PKA activity can phosphorylate GluR6 subunits and modulate channel function for about 3–25 minutes (Wang et al., 1993). In turn, this cytosolic PKA activity mediated phosphorylation increases the number of active receptors and effectively enhances the gating properties of the channels (Wang et al., 1993). So, the increased PKA activity may also be responsible for the sustained activity of the PPT cells and maintenance of normal REM sleep episodes.
8.4.3. Relationship between the PPT cholinergic cells and glutamatergic cells in the mPRF
A number of sleep researchers believe that the mPRF is the “effector zone” for the REM sleep generation (reviewed in McCarley, 2004). According to our model (CMN model of REM sleep regulation), there exist multiple effector zones (REM sleep sign-generators) rather than a single effector zone. Following is the description and our interpretation of some important studies to understand the relationship between PPT and mPRF for the regulation of REM sleep. This would also clarify the philosophical as well as scientific difference between single and multiple effector zone(s).
Neuroanatomical studies using retrograde tracer have shown that the axons of PPT and LDT cholinergic neurons terminate in the mPRF of cat (Leichnetz et al., 1989; Mitani et al., 1988; Shiromani et al., 1988) as well as in the rat (Semba, 1993). More importantly, it has also been demonstrated that functionally identified REM sleep induction zone in the mPRF receives anatomical projections from the PPT and LDT (Quattrochi et al., 1989). Similarly, neuroanatomical pathway tracing studies have also shown that the neurons from the mPRF REM sleep induction zone project to the PPT and LDT (Leichnetz et al., 1989; Semba and Fibiger, 1992; Steininger et al., 1992). It is also known that the neurons in the mPRF REM sleep induction zone are glutamatergic type (Lai et al., 1993; Liu et al., 1995). Collective results of these anatomical studies provide evidence for the existence of bidirectional anatomical connections between glutamatergic cells in the mPRF REM sleep induction zone and cholinergic cells in the PPT/LDT.
The involvement of a cholinergic neurotransmitter system in the regulation of REM sleep was first suggested during the early part of 1960’s (Cordeau et al., 1963; George et al., 1964; Hernandez-Peon et al., 1962; Mitler and Dement, 1964). These studies have shown that the application of acetylcholine or cholinergic agonist, carbachol, into the brainstem induces REM sleep-like state in the cat. A number of studies from various laboratories confirmed, as well as expanded, those original cholinergic stimulation studies by inducing a REM sleep-like state in the cat via local microinjection of pharmacological agents that increase acetylcholine by blocking its natural degradation or via activation cholinergic receptors in the mPRF (Amatruda et al., 1975; Baghdoyan et al., 1984a; Baghdoyan et al., 1984b, 1987; Baxter, 1969; Hobson et al., 1983; Kostowski, 1971; Quattrochi et al., 1989; Shiromani and McGinty, 1986; Vanni-Mercier et al., 1989; Velazquez-Moctezuma et al., 1989; Velazquez-Moctezuma et al., 1991; Yamamoto et al., 1990a; Yamuy et al., 1993). Microinjection of similar agents in rats also yields a comparative induction of a REM sleep-like state (Bourgin et al., 1995; Gnadt and Pegram, 1986; Kubin, 2001; Marks and Birabil, 1998; Shiromani and Fishbein, 1986). Some other studies have shown that application of carbachol results in the excitation of mPRF neurons (Greene and Carpenter, 1985; Greene et al., 1989; Shiromani and McGinty, 1986; Yamamoto et al., 1990b). It has been demonstrated that the electrical stimulation of the PPT increases acetylcholine release in the mPRF of cat (Lydic and Baghdoyan, 1993). Acetylcholine release in the mPRF of cats is also increased during natural REM sleep (Kodama et al., 1990). Single cell recording studies in the behaving cats have also shown that the majority of the mPRF neurons increase firing rates during natural REM sleep (Hobson et al., 1974; Hobson et al., 1975; McCarley and Hobson, 1975a, 1975b). Similarly, intracellular recordings of mPRF neurons in the behaving cats demonstrated that during natural REM sleep, membrane potential decreased (sign of depolarization) to a level less than −50 mV (Ito et al., 2002). Concurrent with this depolarized state were a storm of depolarizing postsynaptic potentials accompanied by high frequency single spiked action potentials. Our recent studies have demonstrated that both exogenous and endogenous glutamate in the PPT cholinergic cell compartment increases REM sleep by activating kainate type of glutamate receptors (Datta, 2000, 2002; Datta and Siwek, 1997; Datta et al., 2001a). It has also been demonstrated that the carbachol microinjection into the mPRF, activates PPT and LDT cholinergic cells (Shiromani et al., 1992) and increases acetylcholine release in the mPRF (Lydic and Baghdoyan, 1992; Lydic et al., 1991). Combining microdialysis with microinjection techniques, another study established that glutamate release is increased during natural REM sleep as well as post-microinjection of acetylcholine into the mPRF (Kodama et al., 1998).
Based on the experimental evidences discussed above, it is reasonable to suggest that PPT cholinergic cells are activated by the kainate type of glutamate receptors to initiate REM sleep. Activation of PPT cholinergic cells releases acetylcholine in the mPRF and each of the individual REM sleep sign-generators. This endogenously released acetylcholine from the PPT neurons then activates glutamatergic cells in the REM sleep sign-generators to generate REM sleep signs. Similarly, endogenously released acetylcholine from the PPT neurons also activates glutamatergic cells in the mPRF. Cholinergically activated mPRF glutamatergic cells then release glutamate in the cholinergic cell compartment of the PPT. This glutamate from the mPRF activates PPT cholinergic cells to continue release of acetylcholine into the mPRF and individual REM sleep sign-generators. Once REM sleep is initiated by the kainate receptor activation-mediated excitation of PPT cholinergic cells, the mutually excitatory positive feedback relationship between PPT cholinergic cells and mPRF glutamatergic cells helps to maintain REM sleep episodes. According to the single “effector zone” theory, the mPRF is the effector zone; but, according to our CMN model, the mPRF is the “excitatory positive feedback zone”. In our CMN model, there are multiple effector zones -- each of the individual REM sleep sign-generators is considered an effector zone.
8.5. Modulation of monoaminergic tone in the REM sleep sign-generators
Since the cholinergic activation of REM sleep sign generating neurons occurs only if aminergic tone is overpowered by the cholinergic tone (Datta, 1997; Datta et al., 1992; Datta et al., 1998), the withdrawal of monoaminergic tone from the REM sleep generators would be an ideal condition for the expression of REM sleep signs (Datta, 1995). The main sources of these monoaminergic inputs to these REM sleep generators are serotonergic cells in the RN and noradrenergic cells in the LC.
8.5.1. Serotonergic cells in the RN
Dahlstrom and Fuxe (1964) identified nine brainstem serotonergic cell groups, which they termed B1-B9. Except for B9, all the serotonergic nuclei were localized to the midline of the two halves of the brainstem. Based on the seam-like appearance of this structure with serotonin-containing cells, this part of the brain was designated as raphe nuclei (RN). The alpha-numeric designations were subsequently replaced with names that reflect anatomical location or individual characteristics (e.g. dorsal raphe, median raphe, nucleus linearis centralis, centralis superior, raphe pallidus, supraleminiscal nucleus, raphe magnus, etc). Since its identification, there have been a number of excellent reviews and original articles describing basic anatomy of each nucleus and number of serotonergic cells in those individual nucleuses of the raphe nuclei (Azmitia and Gannon, 1986; Baker et al., 1991; Dahlstrom and Fuxe, 1964; Felten and Sladek, 1983; Ishimura et al., 1988; Jacobs et al., 1984; Steinbusch and Nieuwenhuys, 1983; Vertes and Crane, 1997). Although raphe nuclei are mainly identified as serotonergic nuclei, over the years a significant proportion of cells in the raphe nuclei were found to contain GABA and other neurotransmitters (Aznar et al., 2004; Belin et al., 1979; Descarries et al., 1986; Li et al., 2001; Nanopoulos et al., 1982; Steinbusch et al., 1980).
Serotonergic cells of the raphe nuclei send efferent projections to many different parts of the brain, including each of the REM sleep sign-generators in the brainstem (Bobillier et al., 1976; Morrison and Foote, 1986; Parent et al., 1981; Pierce et al., 1976; Semba and Fibiger, 1992; Steinbusch, 1981; Steininger et al., 1992; Vertes, 1991; Vertes et al., 1999; Vertes and Kocsis, 1994; Vertes and Martin, 1988). The projection of serotonergic cells to the REM sleep sign-generators provides important anatomical evidence to our suggestion that the activity of those serotonergic cells might modulate activity of neurons in the REM sleep sign-generators. Allan Hobson (Hobson, 1999), along with Robert McCarley and Peter Wyzinski, recorded brainstem cells in the cat that seemed to stop firing as soon as the animal entered REM sleep (Hobson et al., 1975; McCarley and Hobson, 1975b). Further histological examination revealed that those cells were located in the raphe nuclei. Almost at the same time, McGinty and Harper (1976) published a paper describing single cell activity patterns of dorsal raphe nucleus neurons in the cat across sleep-wake cycle. This publication described a group of neurons that had a very slow but regular discharge rates during waking (2–6 spikes/sec), displayed progressively lower firing rates as the animal moved from waking state to SWS, and ultimately stopped firing during REM sleep. Since these cells stopped firing during REM sleep, they are often called as REM-off cells. Presence of REM-off type of cells in the dorsal raphe nucleus was confirmed by many other researchers (Datta, 1997; Lydic et al., 1983, 1985; Trulson and Jacobs, 1979). Neurons with the same REM-off discharge patterns have also been recorded in the nucleus linearis centralis (Hobson et al., 1983), centralis superior (Rasmussen et al., 1984), raphe magnus (Cespuglio et al., 1981; Fornal et al., 1985), and raphe pallidus (Sakai et al., 1983). Since putative serotonergic cells in the RN stop firing during REM sleep, and these cells project to the individual REM sleep sign-generators, it could be suggested that serotonergic REM-off neurons play a permissive, disinhibitory role in regard to the REM sleep sign-generators. It is also possible that this REM-off cell discharge profile is simply an epiphenomenon. We will discuss three other types of evidences to suggest that REM-off type discharge properties of serotonergic neurons in the RN is not simply an epiphenomenon; rather it is an important physiological step for the activation of cells in the REM sleep sign-generators to express specific REM sleep signs. At the outset, we acknowledge that these evidences are based primarily on the PGO/P-wave generator; but, also provided are some indications of similar effects on other REM sleep sign-generators.
The first line of evidences show that pharmacological manipulations aimed at reducing the availability of 5-HT in the brain promotes appearances of the PGO waves, while administration of pharmacological agents which increase brain 5-HT suppresses PGO waves (Brooks and Gershon, 1971, 1977; Brooks et al., 1972a, 1972b; Delorme et al., 1965; Quattrochi et al., 1993). Thus, the activity of the PGO wave generator, one of the REM sleep sign-generators, is negatively correlated with increased serotonergic neurotransmission. Second, a midline lesion, destroying the raphe nuclei and parasaggital transections and interrupted the connections between the raphe nuclei and the P-wave generator, produced a state-independent increase in PGO wave discharge (Simon et al., 1973). Analogous to the results of knife cut lesions (mentioned above), some success in increasing PGO wave frequency with localized cooling of the RN has also been reported (Cespuglio et al., 1979). These findings support that the serotonergic cells in the RN provide inhibitory control or gating of the PGO wave generator. Indeed, a recent study has demonstrated that the activation of 5-HT receptors in the P-wave generator blocks P-wave activities during REM sleep without altering the total amount of REM sleep (Datta et al., 2003). Another pharmacological study has demonstrated that the suppression of serotonergic cell activity within the RN induces state-independent theta rhythm (another REM sleep sign) in the rat (Vertes and Kocsis, 1994). Some other experiments have shown that the local application of a selective 5-HT1A receptor agonist, 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) into the dorsal raphe nucleus increases REM sleep in the cat (Portas et al., 1996) and rat (Bjorvatn et al., 1997). Since the application of 8-OH-DPAT suppresses serotonergic cell activity (Sprouse and Aghajanian, 1987), 8-OH-DPAT microinjection-induced increases in different signs of REM sleep in the cat and rat (Bjorvatn et al., 1997; Portas et al., 1996) indirectly suggest that the withdrawal of serotonergic tone facilitates the activity of cells in the REM sleep sign-generators.
8.5.2. Noradrenergic cells in the LC
In the mammalian brain, most of the noradrenergic cells are located in the LC. The LC noradrenergic cells send efferent projections to numerous regions of the brain including the REM sleep sign-generators in the brainstem (Aston-Jones et al., 1986; Berridge and Waterhouse, 2003; Dahlstrom and Fuxe, 1964; Descarries et al., 1977; Foote et al., 1983; Jones and Yang, 1985; Lewis et al., 1987; McBride and Sutin, 1976; Morrison and Foote, 1986). As such, it is possible that the activation of these noradrenergic cells could release norepinephrine in the REM sleep sign-generators to modulate the activity of REM sleep sign generating neurons and, ultimately, the expression of REM sleep signs. A number of single cell recording studies in the cat and rat have shown that most of the neurons of the LC decrease their firing rate at sleep onset, nearly stop firing during the transition between SWS and REM sleep and remain completely silent until 10-5 sec before the beginning of wakefulness (Aston-Jones and Bloom, 1981a, 1981b; Chu and Bloom, 1973; Chu and Bloom, 1974; Datta, 1997; Foote et al., 1983; Jacobs, 1986). These single cell activity patterns suggest that the reduction of norepinephrine release due to withdrawal of noradrenergic cell activity could be an important step for the induction of REM sleep. The significance of this cessation of noradrenergic cells activity, immediately before and during REM sleep, was expanded by the demonstration that unilateral and bilateral cooling of the LC in the behaving cats induced a transitional stage between SWS and REM sleep within three minutes and within four minutes total REM sleep was achieved (Cespuglio et al., 1982). This study was significant because the localized cooling suppresses neuronal activity in the LC and reduction of noradrenergic cell activity induced REM sleep. Thus, the results of this study suggested a causal relationship between withdrawal of noradrenergic cells activity and induction of REM sleep. Consistent to this interpretation, another study has demonstrated that unilateral electrolytic lesions of LC increased REM sleep in the cat (Caballero and De Andres, 1986). These results and many other lesion studies, however, should be interpreted with care because large and non-specific lesions in the brainstem often may not produce the desired effects (Jones and Bickford, 1977). Since many of our vital functions are controlled by the brainstem, complete elimination of any parts of the brainstem will obviously affect those vital functions (Datta, 1995). Disruption of any vital function would also disrupt our sleep and, as a result, it is difficult to study the function of any brainstem sites by completely knocking down any structures of the brainstem. Indeed, during the last 15 years of research in the Datta laboratory (reviewed in Datta, 1997, 2006) have shown that the best way to study the functions of the brainstem is to approach a structure unilaterally, rather than bilaterally, and to approach the midline structures partially, rather than completely. Contrary to the brainstem, bilateral approaches are more desirable than the unilateral approaches for the forebrain structures.
In summary, evidences discussed above suggest equivocally that to generate REM sleep, serotonergic cells of the RN and noradrenergic cells of the LC stops their neuronal activities causing decreased release of monoamines that ultimately leads to withdrawal of inhibitory tone from the REM sleep sign generating neurons in the brainstem. The next obvious question is: how these noradrenergic and serotonergic REM-off cells are inhibited?
8.6. Mechanisms for regulation of noradrenergic and serotonergic REM-off cell activity
Based on the evidences above, it is clear that the regulation of electrophysiological activities of REM-off cells is critical for the modulation of REM sleep. Unlike REM-on cells, the mechanisms for the regulation of REM-off cells activity are not as clearly defined. Given this, we would like to discuss three possible mechanisms for the regulation of REM-off type of activities: 1) GABAergic mechanism, 2) Pacemaker mechanism, and 3) withdrawal of histaminergic and hypocretinergic excitatory tone.
8.6.1. GABAergic mechanism
To understand the mechanisms for the regulation of REM-off cell activity during REM sleep, most of the research over the last ten years has been focused on the GABAergic activation-mediated inhibition of REM-off cells. According to the GABAergic hypothesis, the activity of REM-off cells during REM sleep is inhibited by increased GABA release in the RN and LC. To support this hypothesis, Gervasoni et al. (2000) provided anatomical evidence showing that the RN receives afferent projections from GABAergic cells located in the lateral preoptic area (LPOA) and ventro-lateral periaqueductal gray (vlPAG). In addition to these GABAergic afferent projections, there are evidences to suggest that the RN itself contains GABAergic cells (Allers and Sharp, 2003; Aznar et al., 2004; Li et al., 2001; Morgane et al., 2005; Nagai et al., 1983; Nanopoulos et al., 1982; Steinbusch et al., 1980; Varga et al., 2001). Anatomical studies have shown that another REM-off site, the LC, also receives GABAergic afferent projections from the GABAergic cells located in the dorsomedial rostral medulla (Ennis and Aston-Jones, 1989; Mugnaini and Oertel, 1985). It has also been reported that some of the LC cells are GABAergic (Ford et al., 1995; Iijima and Ohtomo, 1988). In addition to the presence GABAergic cells and GABAergic afferent inputs, RN and LC also contain GABA receptors (Gao et al., 1993; Luque et al., 1994). Thus, collectively the results of these anatomical studies provided evidence to suggest that in the RN and LC REM-off cells have access to endogenously released GABA that can act on REM-off cells.
Microdialysis studies have demonstrated that the level spontaneously released GABA in the LC and RN is maximum during REM sleep and minimum during wakefulness (Nitz and Siegel, 1997b; Nitz and Siegel, 1997a). These studies have also indicated that the GABA release during SWS was slightly higher than during wakefulness. It has also been reported that the microiontophoretic application of bicuculline, a GABA-A receptor specific antagonist, on REM-off neurons in the RN and LC could restore tonic firing of those REM-off neurons during SWS and REM sleep in the head-restrained rat (Gervasoni et al., 1998; Gervasoni et al., 2000). The results of these microdialysis and microinontophoretic studies suggest a possibility that during SWS and REM sleep, REM-off cells are actively inhibited by the GABAergic influence. This possibility could also be supported by the results presented in some recent single cell recording studies that have shown the local GABAergic cells in the dorsal raphe nucleus increase firing rate during REM sleep and hippocampal EEG sign of REM sleep (Kocsis and Vertes, 1992; Li et al., 2005; Urbain et al., 2006). Collectively, old and recent single cell recording studies show that during REM sleep, serotonergic and GABAergic cells in the dorsal raphe nucleus discharge reciprocally. This reciprocal neuronal activity between serotonergic and GABAergic cells indicate that the serotonergic REM-off cell activity may also be regulated through a circuit with local GABAergic cells. Indeed, the structural and functional existence of this local circuit could also be supported by the evidences that in the dorsal raphe GABA-A and GABA-B receptors containing serotonergic cells receive synaptic inputs from the local GABAergic cells (Boothman and Sharp, 2005; Gao et al., 1993; Liu et al., 2000; Rodriguez-Pallares et al., 2001; Wang et al., 1992; Wirtshafter and Sheppard, 2001).
8.6.2. Pacemaker mechanism
Noradrenergic cells in the LC are equipped with pacemaker activity which is regulated by the cAMP-dependent PKA activity (Alreja and Aghajanian, 1991, 1993, 1995). It is also suggested that the activity of dorsal raphe serotonergic cells are directly regulated by the pacemaker activity of LC noradrenergic cells. Since, during REM sleep, GABA release increases in the LC (Nitz and Siegel, 1997b) and the activation of GABA-B receptors may suppress cAMP-dependent PKA activity by inhibiting adenylyl cyclase (Datta and Prutzman, 2005; Gilman, 1987; Marinissen and Gutkind, 2001), it is possible that the activity of dorsal raphe nucleus REM-off cells are turned-off by the withdrawal of pacemaker type inputs from the LC during REM sleep. The regulation of activity of dorsal raphe nucleus REM-off cells by the activity of LC noradrenergic cells was also demonstrated in the cat by Sakai and Crochet (2000). Using combination of single cell recordings and microdialysis technique, Sakai and Crochet (2000) demonstrated that the application of phenylepinephrine, an alpha-1 noradrenergic receptor specific agonist, completely blocked the cessation of REM sleep discharge in a subpopulation of DRN REM-off neurons. This study also demonstrated that the application of prazosin, an alpha-1 noradrenergic receptor specific antagonist, suppressed spontaneous discharge of DRN REM-off neurons. Based on this observation, Sakai and Crochet (2000) concluded that the suppression of REM-off neuronal discharge was, in part, by the disfacilitation of excitatory noradrenergic projections to the DRN.
8.6.3. Withdrawal of histaminergic and hypocretinergic excitatory tone
The third possible mechanism for the regulation of REM-off type of activity patterns of LC noradrenergic and RN serotonergic cells might be due to the withdrawal of histaminergic and hypocretinergic excitatory tone. In support of this possibility, anatomical studies have shown that LC and RN receive afferent projections from the histamine synthesizing cells in the PH (Inagaki et al., 1988; Panula et al., 1989) and hypocretin synthesizing cells in the LH (Chemelli et al., 1999; Date et al., 1999; de Lecea et al., 1998; Nambu et al., 1999; Peyron et al., 1998; Sakurai et al., 1998; Taheri et al., 1999). Single cell recording studies have shown that cells in the PH (Ko et al., 2003; Sakai et al., 1990; Steininger et al., 1999; Vanni-Mercier et al., 1984) and LH (Alam et al., 2002; Koyama et al., 2002; Lee, 2005; Lee et al., 2005; Methippara et al., 2003) exhibit relatively less neuronal activity during REM sleep compared to wakefulness. Thus, it is likely that the availability of histamine and hypocretin in the LC and RN decreases progressively while transitioning from SWS to REM sleep. It has also been demonstrated that hypocretin receptors are present in the RN and LC and application of hypocretin results in excitation of cells in the LC and DRN (Bourgin et al., 2000; Brown et al., 2001b; Brown et al., 2002; Hagan et al., 1999; Horvath et al., 1999). More interestingly, it has been demonstrated that the application of histamine blocks the cessation of discharge of suspected serotonergic DRN REM-off cells during REM sleep (Sakai and Crochet, 2000). On the contrary, application of mepyramine, a specific H1 histamine receptor antagonist, suppresses spontaneous discharges during quiet wake and SWS. These results support hypothesis that the cessation of neuronal activity of REM-off cells during REM sleep may be due to the withdrawal of histaminergic and orexinergic excitatory tone.
8.6.4. Presumed REM-off cells in the ventrolateral periaqueductal gray (vlPAG) and lateral pontine tegmentum (LPT)
Based on the suggestion that periaqueductal gray (PAG) as a possible source of the GABAergic input to REM-off cells in the dorsal raphe nucleus (Gervasoni et al., 2000) and Cliff Saper’s hypothalamic flip-flop switch model for the sleep cycle control (Saper et al., 2001; Saper et al., 2005), Lu et al. (2006) suggested that REM-off cells are located in the ventrolateral part of the PAG (vlPAG) and lateral pontine tegmentum (LPT). Involvement of vlPAG in the regulation of REM sleep was also suggested in two other studies that have shown both vlPAG lesions (Petitjean et al., 1975) and microinjection of muscimol into the vlPAG (Sastre et al., 1996) increases REM sleep in the cat. The direct methods that could identify REM-off cells in the brain, however, do not support the suggestion that a population of REM-off cells is located in the vlPAG and/or LPT. For example, single cell activity patterns of vlPAG cells in the freely moving cats do not show any REM-off type of activity (Thakkar et al., 2002). While recording brainstem REM-on and REM-off cells in the behaving cats and freely moving rats (Datta, 1995; Datta and Siwek, 2002; Steriade et al., 1990b; Steriade et al., 1990a; Unpublished observations of Datta Laboratory), we rarely encountered REM-on or REM-off types of cells in the LPT area. Likewise, in the vlPAG, a very small amount of REM-off type cells were encountered in the cat and none were identified in the rat. Those REM-off cells were then suspected to be serotonergic cells, displaced from the dorsal raphe nucleus. In general, the activity of cells in the vlPAG and LPT are state-independent. One exception is in the junction between vlPAG and LPT, where some cells discharge randomly as single spike (3–6 spikes) while the animals were scratching and/or grooming during wakefulness. Otherwise, these cells remain silent during wakefulness, SWS, or REM sleep. Thus, it is unlikely that a significant number of REM-of cells are located in the vlPAG and/or LPT. Since, the PAG is already known for its involvement in the regulation of pain (Fardin et al., 1984; Gray and Dostrovsky, 1983; Mason, 2005; Reynolds, 1969) and PAG neurons interact with the dorsal raphe nucleus for the regulation of pain (Auerbach et al., 1985; Dostrovsky et al., 1983; Mason, 2001), it is not surprising that a vlPAG lesion and microinjection of muscimol into the vlPAG could change in the total amount of time spent in REM sleep (Petitjean et al., 1975; Sastre et al., 1996). Based on these evidences, there is no valid reason to entertain the notion that the vlPAG and LPT contain REM-off type cells and/or that these cells are involved in the modulation of REM sleep sign-generators.
9. Summary
Critical and speculative analyses of sleep behavior date back to the beginning of recorded history. Multiple philosophers, authors and poets from an array of cultures have examined sleep in an attempt to discover its underlying purpose. Scientific research over the past fifty years, however, has yielded important results that have significantly contributed to our larger understanding of sleep regulation and function. The pioneering efforts of early researchers such as Kleitman, Aserinsky, Dement, Jouvet, Hobson, McCarley and many others have laid the foundation for current sleep studies. Building upon their novel studies, combined with the advent of constantly improving research techniques, sleep research has expanded into a prominent field that has important benefits and applications in the general and scientific community.
Wakefulness and wake-associated behaviors are accompanied by heightened global neuronal activity and increased activity of the wake-promoting regions in the brain. Throughout this period of arousal, specific metabolites slowly accumulate in various regions of the brain. The accumulation of these metabolites to a critical level is a cornerstone of the metabolic homeostatic theory and the immediate predecessor to the passive initiation of sleep. At the initation of sleep, a decrease global neuronal activity concomitant with a decrease in metabolite production is associated with the generation of SWS. The induction of SWS is simply generated by the activity of GABAergic cells in the POA, which are facilitated by the increased synthesis of GHRH. During this sleep stage, bursting activity of GABAergic neurons in the thalamus abolishes transmission of incoming signals to the cortex. Active maintenance of SWS is dependent on increased GABA release that continuously suppresses activity in the various wake-promoting regions of the brain.
The generation and maintenance of REM sleep involves a complex system of neuronal connections that form the basis for the CMN model of REM sleep regulation. According to the CMN model, distinct cell groups within the brainstem are responsible for the expression of individual events that characterize REM sleep (e.g. P-wave, cortical activation, muscle atonia). The turn-on, turn-off conditions for the executive neurons unique to each cell group is regulated by the ratios of available aminergic and cholinergic neurotransmitters. Accordingly, the expression of REM sleep signs is a result of a significant reduction in aminergic tone and a comparatively high level of cholinergic tone within each individual REM sleep sign-generator. The induction of REM sleep is facilitated by two types of cholinergic cells located in the PPT and LDT. REM-on cells increase firing rate during the transition periods from wakefulness to SWS and then to REM sleep. The second group of neurons, Wake-REM-on cells, increases firing activity during both wakefulness and REM sleep. Activation of low-threshold, kainate-type glutamate receptors on the PPT generates REM sleep episodes by increasing the activity level in the cholinergic cell compartment of the PPT. Recent research has indicated that the generation of REM sleep also involves the cAMP-PKA signal transduction pathway. Activation of kainate receptors increases cytoplasmic free calcium concentration which activates AC; thus stimulating the production of cAMP and activation of PKA. As a result, it is possible that the activation of kainate receptors in the PPT induce REM sleep via the activation of the cAMP-PKA signaling pathway. Anatomical projections from the PPT then supply the cholinergic tone to each of the REM sleep sign-generators for the expression of each REM sleep sign. Endogenously released acetylcholine from the PPT also activates glutamatergic cells in the mPRF. These activated mPRF cells subsequently release glutamate in the cholingeric compartment of the PPT, causing the continued release of acetylcholine into the mPRF and REM sleep sign generators. Therefore, once REM sleep is initiated by the kainate receptor activation-mediated excitation of PPT cholinergic cells, REM sleep episodes are maintained via a mutually excitatory positive feedback loop between the PPT cholinergic and the mPRF glutamatergic cells.
The continuance of research regarding the mechanisms of sleep regulation is imperative to elucidating the importance of functional sleep. Studies that seek to expand our knowledge of sleep on the molecular and biochemical level have the potential to give new insight into diagnostic criteria and possible treatment strategies for many disorders of which disturbed sleep is symptomatic. Progressive research in intracellular signaling pathways holds perhaps the greatest potential to fully understanding the mechanisms by which mammals generate and maintain normal sleep.
Figure 1.
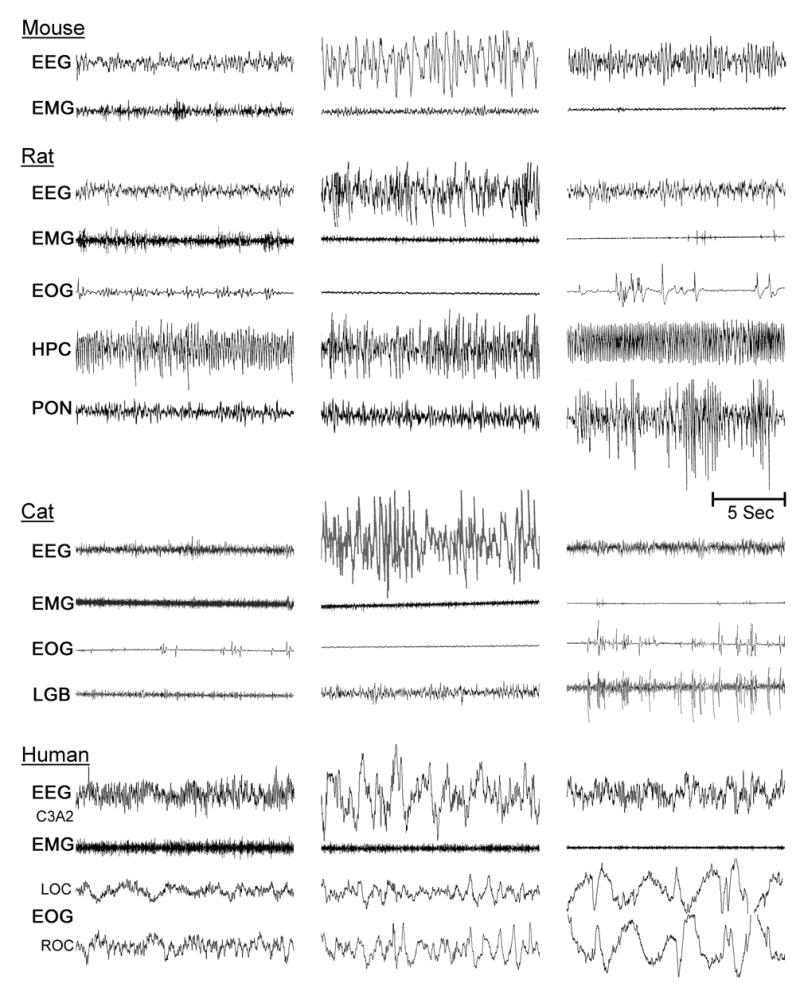
Typical polygraphic appearance of wakefulness (left column), slow-wave sleep (middle column), and REM sleep (right column) in the adult mouse, rat, cat, and human, showing similarities in behavioral state-specific physiological signs across species. During wakefulness, cortical electroencephalogram (EEG) presents low amplitude, high frequency waves (sign of activated cortex) and electromyogram (EMG) shows presence of muscles tone in the antigravity muscles. During slow-wave sleep (SWS, part of NREM sleep), low amplitude, high frequency EEG waves are replaced with high amplitude, low frequency waves and muscle tone is still present (EMG), but slightly reduced compared to wakefulness. During REM sleep, cortical EEG is as activated (low amplitude, high frequency waves) as during wakefulness, but the EMG shows marked reduction or absence of muscle tone (atonia). During REM sleep, electrooculogram (EOG) records the presence of rapid eye movements. Due to its smaller size, the EOG recodings are normally not utilized to identify the sleep-wake states in the mouse. Two additional physiological signs of REM sleep are the rhythmic theta-wave activity (Theta) in the hippocampal EEG (HPC, shown only in the recordings of rat) and the phasic pontine-wave (P-wave)/Ponto-geniculo-occipital wave (PGO-wave). Occasionally, hippocampal theta wave is also present during wakefulness, but theta wave epochs are short lasting and are not as rhythmic as during REM sleep. Theta wave is completely absent during the period of SWS. The P-wave is represented by spiky waves shown in the rat pontine EEG (PON) and are the physiological equivalent to PGO-waves (LGB) shown only in the cat. Normally, P/PGO-waves are absent during wakefulness and SWS. Time bar (for all species and all states) = 5 sec. Polygraphic recordings of mouse, rat and cat are from Datta Laboratory. Human records are kindly provided by Dr. E. F. Pace-Schott.
Figure 2.
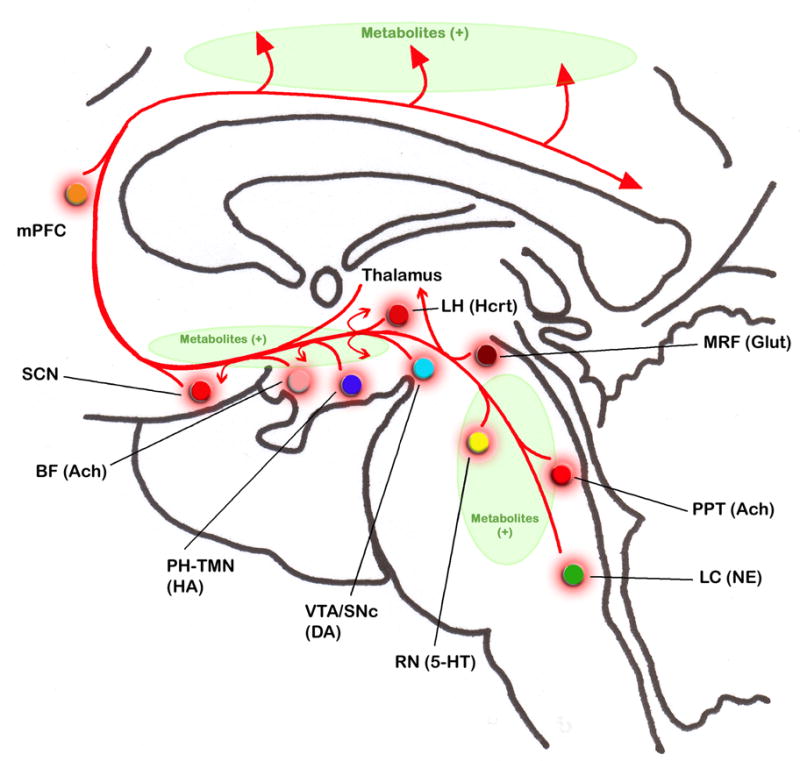
Schematics of the sagittal view of human brain depicting the location of brain regions and their neurotransmitters, as well as pathways that are involved in the generation and maintenance of wakefulness. Ascending projections originating from the brainstem cholinergic cells (synthesize neurotransmitter acetylcholine, Ach) in the pedunculopontine tegmentum (PPT) travel dorsally to the thalamus to activate thalamo-cortical network. Projections from PPT cholinergic cells also travel ventrally to the basal forebrain (BF, to both cholinergic and GABAergic cells) to relay activating signals to the cortex. Ascending brainstem aminergic projections originating from the noradrenergic cells (synthesize neurotransmitter norepinephrine, NE) in the locus coeruleus (LC) and from the serotonergic cells (synthesize neurotransmitter serotonin, 5-HT) in the raphe nucleus (RN) travel both dorsally to the thalamus to activate thalamo-cortical network and ventrally to the hypothalamus and basal forebrain to activate hypothalamo-cortical and basalo-cortical networks. Projections from these aminergic cell groups also travel directly to the cortex. Projections from the brainstem glutamatergic cells (synthesize neurotransmitter glutamate, Glut) in the mesencephalic reticular formation (MRF) travel dorsally to the thalamus to activate thalamo-cortical and ventrally to the hypothalamus to activate hypothalamo-cortical network. Projections from the dopaminergic cells (synthesize neurotransmitter dopamine, DA) in the ventral tegmental area (VTA) and substantia nigra compacta (SNc) also travel to the thalamus, hypothalamus, and basal forebrain to activate thalamo-cortical, hypothalamo-cortical, and basalo-cortical network. The projections from these dopaminergic cells also travel directly to the cortex. Histaminergic cells (synthesize neurotransmitter histamine, HA) in the posterior hypothalamic tuberomammillary nucleus (PH-TMN) and cholinergic cells (Ach) in the basal forebrain (BF) project directly to the cortex. Activation of cells in the suprachiasmatic nucleus (SCN; both glutamatergic and neuropeptide-Y cells), lateral hypothalamus (LH-Hcrt; hypocretinergic/orexinergic cells synthesize hypocretin/orexin), and medial prefrontal cortex (mPFC; glutamatergic cells) could also directly activate the entire cortex. Activation of these brain regions both directly and/or indirectly through the thalamo-cortical, hypothalamo-cortical, and/or basalo-cortical ascending pathways causes activation of cortex as well as global activation of the brain to promote and maintain behavioral states of wakefulness. Although, at this time, we understand very little about different states of wakefulness, it is likely that those various states of wakefulness are the result of differential activation of specific wake-promoting regions in the brain. The synthesis and accumulation of brain metabolites (shown as Metabolites (+), green) are directly proportional to the intensity and duration of activation of those wake-promoting areas and global activation of the brain.
Figure 3.
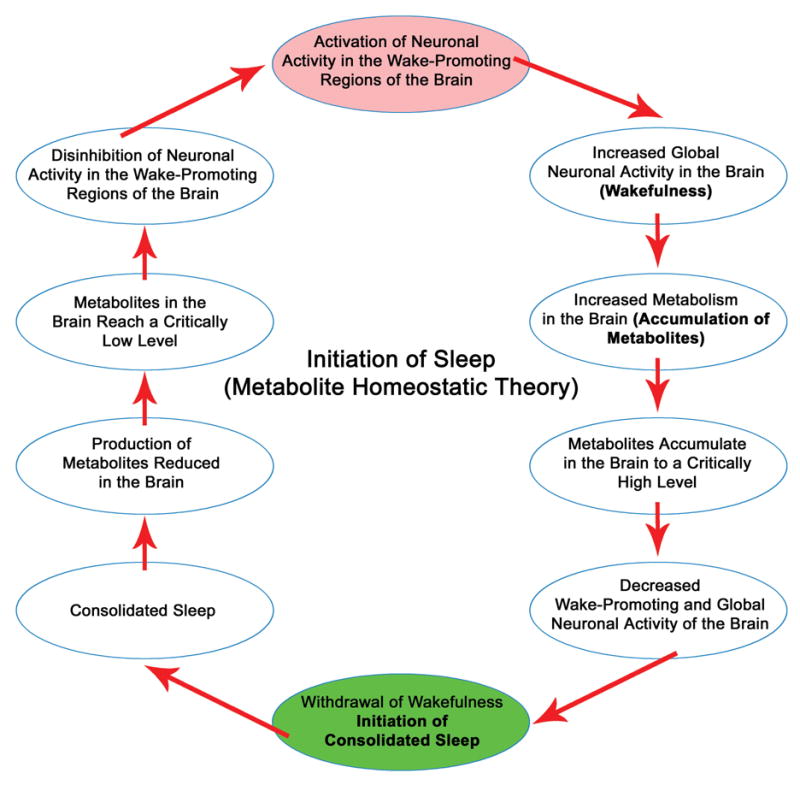
Metabolite Homeostatic model of physiological mechanisms for the initiation of sleep. Throughout wakefulness metabolites accumulate in the brain as a result of increased neuronal activity in wake-promoting structures and increased global neuronal activity. When these metabolites accumulate to a critically high level the brain responds by decreasing neuronal activities in the wake-promoting regions and ultimately global neuronal activities. This decreased neuronal activity causes withdrawal of wakefulness that ultimately initiates consolidated sleep (green). Thus, the initiation of sleep is a passive process caused by the withdrawal of wakefulness. Conversely, during consolidated sleep, metabolites reach a critically low level which results in a disinhibition of neuronal activity in the wake-promoting regions of the brain (red). This disinhibition is the direct predecessor to the increased neuronal activity in the wake-promoting regions of the brain and behavioral states of wakefulness.
Figure 4.
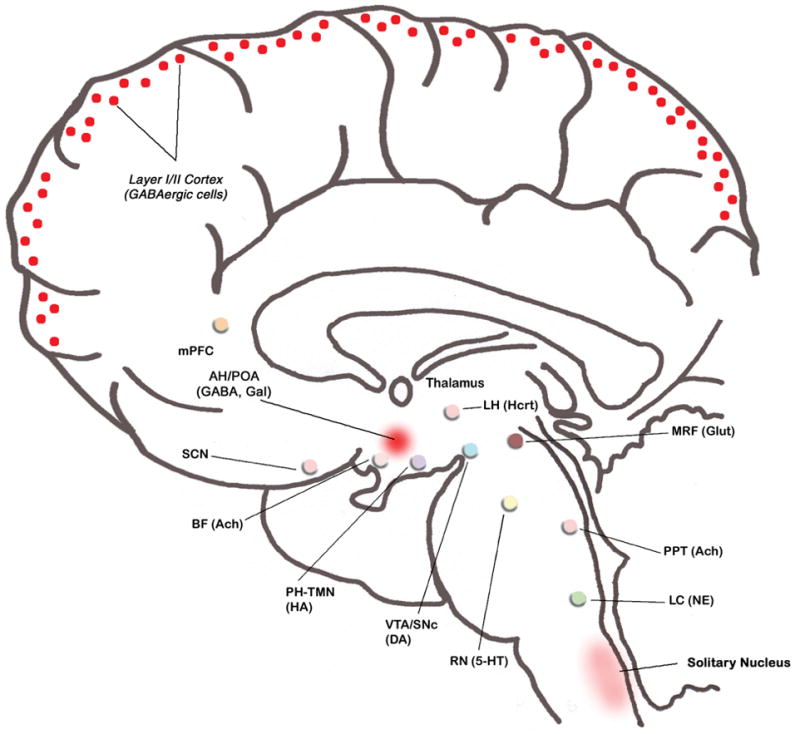
The schematic of human brain depicting regions that are involved in the generation and maintenance slow-wave sleep (SWS). Following initiation of sleep, suppressed neuronal activity in the wake-promoting regions of the brain allow for the activation of γ-aminobutyric acid (GABA) and Galanin (Gal) synthesizing cells in the anterior hypothalamus/preoptic area (AH/POA). Increased activity of GABA and Galanin synthesizing cells in the AH/POA (red) ultimately generates slow wave sleep (SWS). Those GABA and Galanin synthesizing cells innervate the major wake-promoting cell groups of the brain including: histaminergic cells (HA) in the tuberomammilary nucleus (PH-TMN), hypocretin/orexinergic cells (Hcrt) in the lateral hypothalamus (LH), noradrenergic cells (NE) in the locus coeruleus (LC), serotonergic cells (5-HT) in the raphe nucleus (RN) and cholinergic cells (Ach) in the pedunculopontine tegmentum (PPT). The continued inhibition of these wake-promoting structures, via GABA and Galanin release, maintains episodes of SWS. Due to increased GABA and Galanin release, neuronal activity remains suppressed in other wake-promoting regions including: the medial prefrontal cortex (mPFC), suprachiasmatic nucleus (SCN), basal forebrain (BF), ventral tegmental area (VTA)/substantis nigra compacta (SNc) and medial reticular formation (MRF). In addition to AH/POA GABA and Galanin synthesizing cells, increased activity of GABAergic cells in layers I and II of the cortex and cells in the solitary nucleus also contribute to the maintenance of SWS.
Figure 5.
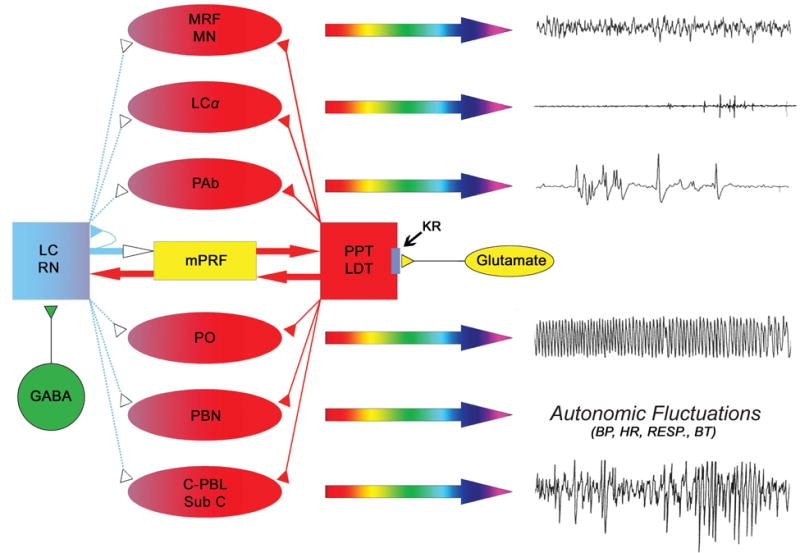
Cellular-Molecular-Network model of physiological mechanisms for the generation of REM sleep. Each of the individual signs of REM sleep (right column, polygraphic signs) is executed by the increased activation of distinct cell groups in the brainstem (red-colored oval shapes). For example, cortical EEG activation sign of REM sleep is executed jointly by the activation of neurons in the mesencephalic reticular formation (MRF) and rostrally-projecting bulbar reticular formation (medullary magnocellular nucleus, MN), muscle atonia is executed by the neurons in the locus coeruleus alpha (LCα), rapid eye movements is executed by the neurons in the peri-abducens reticular formation (PAb), PGO/P-waves are executed by the neurons in the caudo-lateral peribrachial area (C-PBL) of predator mammals and in the dorsal part of the nucleus subcoeruleus (Sub C) of prey mammals, hippocampal theta rhythm is executed by the neurons in the pontis oralis (PO) and increased brain temperature and cardio-respiratory fluctuations are executed by the neurons in the parabrachial nucleus (PBN). These REM sleep sign generating executive neurons are excited by the increased release of cholinergic neurotransmitter while the releases of aminergic neurotransmitter are reduced and/or absent. The sources of the cholinergic neurotransmitter (red) are the cholinergic neurons in the pedunculopontine tegmentum (PPT) and lateral dorsal tegmentum (LDT). The sources of the aminergic neurotransmitters (blue) are the noradrenergic neurons in the locus coeruleus (LC) and serotonergic neurons in the raphe nucleus (RN). For the initiation of REM sleep, kainate receptors on the cholinergic cells are activated by the increased release of glutamate (yellow) that ultimately activates cholinergic cells and increase release of acetylcholine in each of the REM sleep sign-generators and in the cholinoceptive REM sleep inducing site in the medial pontine reticular formation (mPRF). While, PPT/LDT cholinergic cells are activated, local GABAergic cells (green) in the LC and RN are also activated. Activation of these local GABAergic cells actively inhibits aminergic cells in the LC and RN. Active inhibition of those aminergic cells reduces and/or stops releasing aminergic neurotransmitters to those REM sleep sign-generators. For the maintenance of REM sleep episodes, increased acetylcholine release in the mPRF activates glutamatergic cells that continue to release glutamate in the PPT/LDT to maintain activity of cholinergic cells. Thus, PPT/LDT cholinergic cells and mPRF glutamatergic cells create a positive feedback loop to maintain REM sleep. Activation of mPRF glutamatergic cells also releases glutamate in the LC and RN. This glutamate could also activate both aminergic cells and local GABAergic cells in the LC and RN. Activation of GABAergic cells intensifies their inhibitory response to those aminergic cells. But, the possibility of mPRF glutamate activating these aminergic cells is eliminated by the increased release of GABA in the LC and RN and also auto-inhibition.
Figure 6.
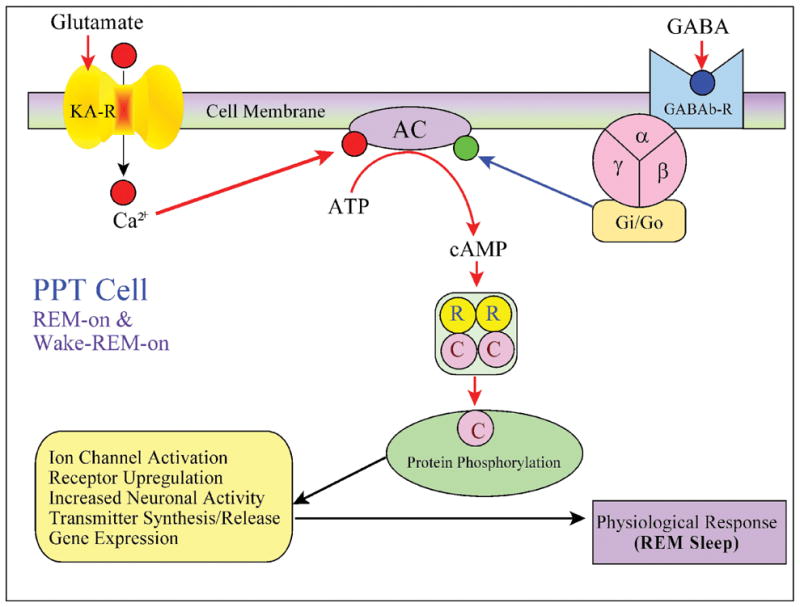
Molecular mechanisms of the Cellular-Molecular-Network model based on recent findings. REM-on and Wake-REM-on cells in the cholinergic cell compartment of the PPT contain both glutamate and γ-aminobutyric acid (GABA) receptors. When endogenously released or exongenously applied glutamate binds to a kainate receptor, intracellular Ca2+ increases through activation of ion channels. Kainate receptor activation-mediated increase in intracellular Ca2+ activated adenylate cyclase (AC). Activation of AC increases synthesis of cAMP, increasing the amount of substrate available to activate protein kainase A (PKA). The phosphorylation of intracellular proteins by PKA elicits the physiological response (REM sleep) via numerous possible intermediate processes (some of which are shown boxed). Similarly, endogenously released or exongenously applied GABA binds to the GABA-B receptor. Activation of GABA-B receptor inhibits AC via G proteins (Gi/GO). The GABA-B receptor mediated inhibition of AC blocks the production of cAMP, limiting the amount of substrate available to activate PKA. By reducing the production of cAMP/PKA, the GABA-B receptor in the PPT cell inhibits REM sleep.
Figure 7.
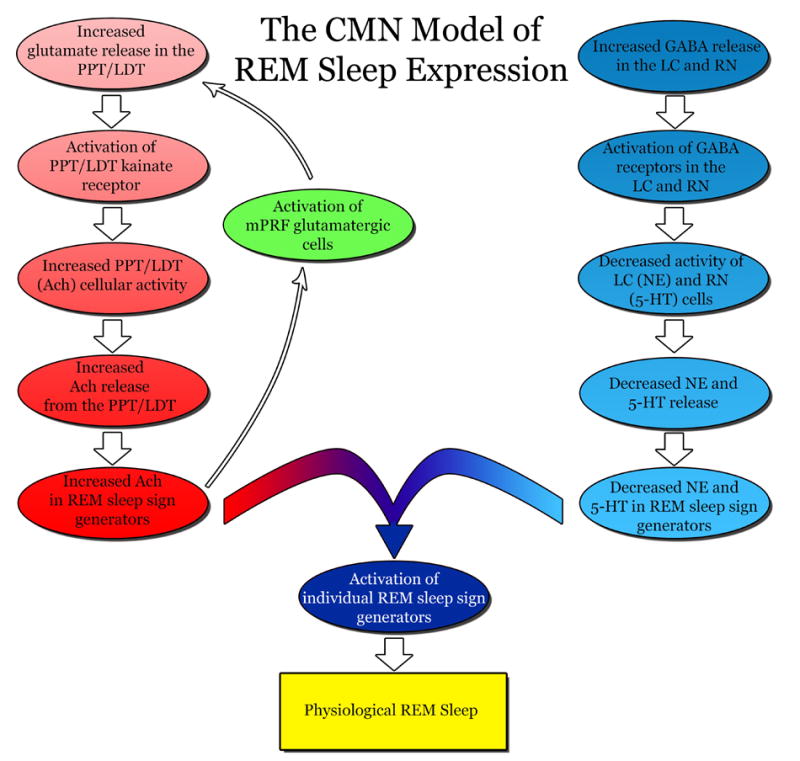
Cellular-Molecular-Network model of cholinergic and aminergic activity for the expression of physiological REM sleep. For the activation of an individual REM sleep sign generator, an increase in acetylcholine (Ach) is accompanied by a decrease in norepinephrine (NE) and serotonin (5-HT) within each generator. An increase in Ach (red) is the result of increased glutamate release in the pedunculopontine tegmentum (PPT) and the lateral dorsal tegmentum (LDT). This increased glutamate activates kainate receptors on the PPT/LDT and signals the release of Ach. The increased Ach in the sleep sign generators also activate medial pontine reticular formation (mPRF) cells that release glutamate (green) to the PPT/LDT and facilitate the continued release of Ach during REM sleep (maintenance of REM sleep episode). A decrease in NE and 5-HT (blue) is the result of increased γ-aminobutyric acid (GABA) release in the locus coeruleus (LC) and raphe nucleus (RN), respectively. The increased GABA reduces the presence of NE and 5-HT by inhibiting the cellular activity of the LC and RN. The combination of decreased NE and 5-HT and increased Ach in each individual REM sleep sign generator ultimately generates the expression of physiological REM sleep.
Acknowledgments
This work was supported by National Institutes of Health Research Grants MH59839 and NS34004. I would like to acknowledge the contributions from all of my students and co-workers who worked with me at Harvard Medical School and Boston University School of Medicine. The authors thank Dr. Robert P. Vertes for his valuable discussion on this manuscript. Professor Subimal Datta would like to gratefully acknowledge Dr. Peter J. Morgane for providing his wisdom and unconditional support to the research program of the Datta laboratory at Boston University School of Medicine. This publication is dedicated to Dr. Morgane for his critical and novel contributions to our understanding of the basic mechanisms of sleep regulation and its functions over the past five decades.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang RY, Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–175. doi: 10.1016/0006-8993(78)91140-x. [DOI] [PubMed] [Google Scholar]
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, Szymusiak R. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in rapid eye movement sleep. Am J Physiol. 1995;269:R1250–1257. doi: 10.1152/ajpregu.1995.269.5.R1250. [DOI] [PubMed] [Google Scholar]
- Alam MN, McGinty D, Szymusiak R. Thermosensitive neurons of the diagonal band in rats: relation to wakefulness and non-rapid eye movement sleep. Brain Res. 1997;752:81–89. doi: 10.1016/s0006-8993(96)01452-7. [DOI] [PubMed] [Google Scholar]
- Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521(Pt 3):679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS. The ageing brain: normal and abnormal memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U. Invited review: regulation of mammalian circadian clock genes. J Appl Physiol. 2002;92:1348–1355. doi: 10.1152/japplphysiol.00759.2001. [DOI] [PubMed] [Google Scholar]
- Alexander RS. Tonic and reflex function of medullary sympathetic cardiovascular centers. Journal of Neurophysiology. 1946;9:205–217. doi: 10.1152/jn.1946.9.3.205. [DOI] [PubMed] [Google Scholar]
- Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Activation of locus coeruleus (LC) neurones by cholera toxin: mediation by cAMP-dependent protein kinase. Neurosci Lett. 1991;134:113–117. doi: 10.1016/0304-3940(91)90520-4. [DOI] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Opiates suppress a resting sodium-dependent inward current and activate an outward potassium current in locus coeruleus neurons. J Neurosci. 1993;13:3525–3532. doi: 10.1523/JNEUROSCI.13-08-03525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alreja M, Aghajanian GK. Use of the whole-cell patch-clamp method in studies on the role of cAMP in regulating the spontaneous firing of locus coeruleus neurons. J Neurosci Methods. 1995;59:67–75. doi: 10.1016/0165-0270(94)00195-m. [DOI] [PubMed] [Google Scholar]
- Amatruda TT, III, Black DA, McKenna TM, McCarley RW, Hobson JA. Sleep cycle control and cholinergic mechanisms: differential effects of carbachol injections at pontine brain stem sites. Brain Res. 1975;98:501–515. doi: 10.1016/0006-8993(75)90369-8. [DOI] [PubMed] [Google Scholar]
- Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77:323–324. doi: 10.3181/00379727-77-18766. [DOI] [PubMed] [Google Scholar]
- Anand BK, Dua S, Shoenberg K. Hypothalamic control of food intake in cats and monkeys. J Physiol. 1955;127:143–152. doi: 10.1113/jphysiol.1955.sp005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Arnaud C, Gauthier P, Gottesmann C. Study of a GABAC receptor antagonist on sleep-waking behavior in rats. Psychopharmacology (Berl) 2001;154:415–419. doi: 10.1007/s002130000653. [DOI] [PubMed] [Google Scholar]
- Aserinsky E. The discovery of REM sleep. J Hist Neurosci. 1996;5:213–227. doi: 10.1080/09647049609525671. [DOI] [PubMed] [Google Scholar]
- Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953a;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Aserinsky E, Kleitman N. Eye movements during sleep. Fed Proc. 1953b;12:6–7. [Google Scholar]
- Aserinsky E, Kleitman N. Two types of ocular motility occurring in sleep. J Appl Physiol. 1955;8:1–10. doi: 10.1152/jappl.1955.8.1.1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Nonrepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. doi: 10.1126/science.3775363. [DOI] [PubMed] [Google Scholar]
- Auerbach S, Fornal C, Jacobs BL. Response of serotonin-containing neurons in nucleus raphe magnus to morphine, noxious stimuli, and periaqueductal gray stimulation in freely moving cats. Exp Neurol. 1985;88:609–628. doi: 10.1016/0014-4886(85)90075-5. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv Neurol. 1986;43:407–468. [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian ZX, Knudsen GM. Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience. 2004;124:573–581. doi: 10.1016/j.neuroscience.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Monaco AP, Rodrigo-Angulo ML, Assens F, McCarley RW, Hobson JA. Microinjection of neostigmine into the pontine reticular formation of cats enhances desynchronized sleep signs. J Pharmacol Exp Ther. 1984a;231:173–180. [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984b;306:39–52. doi: 10.1016/0006-8993(84)90354-8. [DOI] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. doi: 10.1016/0006-8993(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RG, Tork I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991;42:757–775. doi: 10.1016/0306-4522(91)90043-n. [DOI] [PubMed] [Google Scholar]
- Bandyopadhya RS, Datta S, Saha S. Activation of pedunculopontine tegmental protein kinase A: a mechanism for rapid eye movement sleep generation in the freely moving rat. J Neurosci. 2006;26:8931–8942. doi: 10.1523/JNEUROSCI.2173-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Baust W, Bohnert B. The regulation of heart rate during sleep. Exp Brain Res. 1969;7:169–180. doi: 10.1007/BF00235442. [DOI] [PubMed] [Google Scholar]
- Baust W, Noppeney H, Schlosser A. [The influence of REM sleep deprivation on heart rate and respiration] Arztl Forsch. 1972;26:302–308. [PubMed] [Google Scholar]
- Baxter BL. Induction of both emotional behavior and a novel form of REM sleep by chemical stimulation applied to cat mesencephalon. Exp Neurol. 1969;23:220–229. doi: 10.1016/0014-4886(69)90059-4. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M, Serafin M. Selective action of orexin (hypocretin) on nonspecific thalamocortical projection neurons. J Neurosci. 2002;22:7835–7839. doi: 10.1523/JNEUROSCI.22-18-07835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, Muhlethaler M. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- Beckman AL, Eisenman JS. Microelectrophoresis of biogenic amines on hypothalamic thermosensitive cells. Science. 1970;170:334–336. doi: 10.1126/science.170.3955.334. [DOI] [PubMed] [Google Scholar]
- Belin MF, Aguera M, Tappaz M, McRae-Degueurce A, Bobillier P, Pujol JF. GABA-accumulating neurons in the nucleus raphe dorsalis and periaqueductal gray in the rat: a biochemical and radioautographic study. Brain Res. 1979;170:279–297. doi: 10.1016/0006-8993(79)90107-0. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkepahalogramm des Menschen. Arch Psychiat Nervenkr. 1929;87:527–570. [Google Scholar]
- Bernard A, Ferhat L, Dessi F, Charton G, Represa A, Ben-Ari Y, Khrestchatisky M. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–616. doi: 10.1046/j.1460-9568.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience. 1999;93:1263–1270. doi: 10.1016/s0306-4522(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–393. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Bertrand F, Hugelin A. Respiratory synchronizing function of nucleus parabrachialis medialis: pneumotaxic mechanisms. J Neurophysiol. 1971;34:189–207. doi: 10.1152/jn.1971.34.2.189. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Fagerland S, Eid T, Ursin R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997;770:81–88. doi: 10.1016/s0006-8993(97)00758-0. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. 1998;37:1187–1204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- Bobillier P, Seguin S, Petitjean F, Salvert D, Touret M, Jouvet M. The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976;113:449–486. doi: 10.1016/0006-8993(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–1973. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- Boothman LJ, Sharp T. A role for midbrain raphe gamma aminobutyric acid neurons in 5-hydroxytryptamine feedback control. Neuroreport. 2005;16:891–896. doi: 10.1097/00001756-200506210-00004. [DOI] [PubMed] [Google Scholar]
- Botchkina GI, Meistrell ME, 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3:765–781. [PMC free article] [PubMed] [Google Scholar]
- Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation in the rat. Neuroreport. 1995;6:532–536. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Monaca C, Hen R, Hamon M, Adrien J. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci. 2002;22:4686–4692. doi: 10.1523/JNEUROSCI.22-11-04686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120(Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Breder CD, Tsujimoto M, Terano Y, Scott DW, Saper CB. Distribution and characterization of tumor necrosis factor-alpha-like immunoreactivity in the murine central nervous system. J Comp Neurol. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- Bredow S, Taishi P, Obel F, Jr, Guha-Thakurta N, Krueger JM. Hypothalamic growth hormone-releasing hormone mRNA varies across the day in rats. Neuroreport. 1996;7:2501–2505. doi: 10.1097/00001756-199611040-00020. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Bremer F. Nouvelles recherches sur le mécanisme du sommeil. C R Soc Biol. 1936;122:460–464. [Google Scholar]
- Brooks DC, Bizzi E. Brain Stem Electrical Activity During Deep Sleep. Arch Ital Biol. 1963;101:648–665. [PubMed] [Google Scholar]
- Brooks DC, Gershon MD. Eye movement potentials in the oculomotor and visual systems of the cat: a comparison of reserpine induced waves with those present during wakefulness and rapid eye movement sleep. Brain Res. 1971;27:223–239. doi: 10.1016/0006-8993(71)90250-2. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Gershon MD. Amine repletion in the reserpinized cat: effect upon PGO waves and REM sleep. Electroencephalogr Clin Neurophysiol. 1977;42:35–47. doi: 10.1016/0013-4694(77)90149-3. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Gershon MD, Simon RP. Brain stem serotonin depletion and ponto-geniculo-occipital wave activity in the cat treated with reserpine. Neuropharmacology. 1972a;11:511–520. doi: 10.1016/0028-3908(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Gershon MD, Simon RP. An analysis of the effects of reserpine upon pontogeniculooccipital wave activity in the cat. Neuropharmacology. 1972b;11:499–510. doi: 10.1016/0028-3908(72)90005-6. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001b;40:457–459. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001a;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Buttner-Ennever JA, Henn V. An autoradiographic study of the pathways from the pontine reticular formation involved in horizontal eye movements. Brain Res. 1976;108:155–164. doi: 10.1016/0006-8993(76)90171-2. [DOI] [PubMed] [Google Scholar]
- Caballero A, De Andres I. Unilateral lesions in locus coeruleus area enhance paradoxical sleep. Electroencephalogr Clin Neurophysiol. 1986;64:339–346. doi: 10.1016/0013-4694(86)90158-6. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Ciriello J, Mogenson GJ. Identification of pathways mediating cardiovascular responses elicited by stimulation of the septum in the rat. J Physiol. 1976;260:515–530. doi: 10.1113/jphysiol.1976.sp011529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994;269:12190–12195. [PubMed] [Google Scholar]
- Candia O, Rossi GF, Sekino T. Brain stem structures responsible for the electroencephalographic patterns of desynchronized sheep. Science. 1967;155:720–722. doi: 10.1126/science.155.3763.720. [DOI] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–2666. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli G, Armengol V, Zanchetti A. Electroencephalographic desynchronization during deep sleep after destruction of midbrain-limbic pathways in the cat. Science. 1963;140:677–679. doi: 10.1126/science.140.3567.677. [DOI] [PubMed] [Google Scholar]
- Carli G, Armengol V, Zanchetti A. Brain stem-limbic connections, and the electrographic aspects of deep sleep in the cat. Arch Ital Biol. 1965;103:725–750. [PubMed] [Google Scholar]
- Carli G, Zanchetti A. A study of pontine lesions suppressing deep sleep in the cat. Arch Ital Biol. 1965;103:751–788. [PubMed] [Google Scholar]
- Cassone VM, Chesworth MJ, Armstrong SM. Entrainment of rat circadian rhythms by daily injection of melatonin depends upon the hypothalamic suprachiasmatic nuclei. Physiol Behav. 1986;36:1111–1121. doi: 10.1016/0031-9384(86)90488-9. [DOI] [PubMed] [Google Scholar]
- Cespuglio R, Faradji H, Gomez ME, Jouvet M. Single unit recordings in the nuclei raphe dorsalis and magnus during the sleep-waking cycle of semi-chronic prepared cats. Neurosci Lett. 1981;24:133–138. doi: 10.1016/0304-3940(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Cespuglio R, Gomez ME, Faradji H, Jouvet M. Alterations in the sleep-waking cycle induced by cooling of the locus coeruleus area. Electroencephalogr Clin Neurophysiol. 1982;54:570–578. doi: 10.1016/0013-4694(82)90042-6. [DOI] [PubMed] [Google Scholar]
- Cespuglio R, Gomez ME, Walker E, Jouvet M. [Effect of cooling and electrical stimulation of nuclei of raphe system on states of alertness in cat] Electroencephalogr Clin Neurophysiol. 1979;47:289–308. doi: 10.1016/0013-4694(79)90281-5. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sanchez V, Hernandez Munoz R, Suarez J, Vidrio S, Yanez L, Diaz Munoz M. Day-night variations of adenosine and its metabolizing enzymes in the brain cortex of the rat--possible physiological significance for the energetic homeostasis and the sleep-wake cycle. Brain Res. 1993;612:115–121. doi: 10.1016/0006-8993(93)91651-8. [DOI] [PubMed] [Google Scholar]
- Chai CY, Wang SC. Localization of central cardiovascular control mechanism in lower brain stem of the cat. Am J Physiol. 1962;202:25–30. doi: 10.1152/ajplegacy.1962.202.1.25. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cereb Cortex. 1997;7:63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Chase MH, Enomoto S, Murakami T, Nakamura Y, Taira M. Intracellular potential of medullary reticular neurons during sleep and wakefulness. Exp Neurol. 1981;71:226–233. doi: 10.1016/0014-4886(81)90084-4. [DOI] [PubMed] [Google Scholar]
- Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Cheng B, Christakos S, Mattson MP. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Chu N, Bloom FE. Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science. 1973;179:908–910. doi: 10.1126/science.179.4076.908. [DOI] [PubMed] [Google Scholar]
- Chu NS, Bloom FE. Activity patterns of catecholamine-containing pontine neurons in the dorso-lateral tegmentum of unrestrained cats. J Neurobiol. 1974;5:527–544. doi: 10.1002/neu.480050605. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Descending hypothalamic pathways with cardiovascular function in the cat: a silver impregnation study. Exp Neurol. 1977;57:561–580. doi: 10.1016/0014-4886(77)90089-9. [DOI] [PubMed] [Google Scholar]
- Cohen B, Komatsuzaki A. Eye movements induced by stimulation of the pontine reticular formation: evidence for integration in oculomotor pathways. Exp Neurol. 1972;36:101–117. doi: 10.1016/0014-4886(72)90139-2. [DOI] [PubMed] [Google Scholar]
- Cohen MI. Switching of the respiratory phases and evoked phrenic responses produced by rostral pontine electrical stimulation. J Physiol. 1971;217:133–158. doi: 10.1113/jphysiol.1971.sp009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles SK, Iles JF, Nicolopoulos-Stournaras S. The mesencephalic centre controlling locomotion in the rat. Neuroscience. 1989;28:149–157. doi: 10.1016/0306-4522(89)90239-x. [DOI] [PubMed] [Google Scholar]
- Consolazione A, Priestley JV, Cuello AC. Serotonin-containing projections to the thalamus in the rat revealed by a horseradish peroxidase and peroxidase antiperoxidase double-staining technique. Brain Res. 1984;322:233–243. doi: 10.1016/0006-8993(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Cordeau JP, Moreau A, Beaulnes A, Laurin C. Eeg and Behavioral Changes Following Microinjections of Acetylcholine and Adrenaline in the Brain Stem of Cats. Arch Ital Biol. 1963;101:30–47. [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Cuello VM, Sofroniew MV. The anatomy of the CNS cholinergic neurons. Trends. 1984;7:74–78. [Google Scholar]
- Cunningham DJ, Stolwijk JA, Murakami N, Hardy JD. Responses of neurons in the preoptic area to temperature, serotonin, and epinephrine. Am J Physiol. 1967;213:1570–1581. doi: 10.1152/ajplegacy.1967.213.6.1570. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Nakao S, Markham CH. Cat medial pontine reticular neurons related to vestibular nystagmus: firing pattern, location and projection. Brain Res. 1981;222:75–94. doi: 10.1016/0006-8993(81)90941-0. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Hosli L, Johnston GA. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6:1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Daikoku S, Kawano H, Noguchi M, Nakanishi J, Tokuzen M, Chihara K, Nagatsu I. GRF neurons in the rat hypothalamus. Brain Res. 1986;399:250–261. doi: 10.1016/0006-8993(86)91515-5. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Neuronal activity in the peribrachial area: relationship to behavioral state control. Neurosci Biobehav Rev. 1995;19:67–84. doi: 10.1016/0149-7634(94)00043-z. [DOI] [PubMed] [Google Scholar]
- Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–365. doi: 10.1023/A:1026398402985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- Datta S. Activation of phasic pontine-wave generator: A mechanism for sleep-dependent memory processing. Sleep and Biological Rhythms. 2006;4:16–26. [Google Scholar]
- Datta S, Calvo JM, Quattrochi J, Hobson JA. Cholinergic microstimulation of the peribrachial nucleus in the cat. I. Immediate and prolonged increases in ponto-geniculo-occipital waves. Arch Ital Biol. 1992;130:263–284. [PubMed] [Google Scholar]
- Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- Datta S, Hobson JA. Suppression of ponto-geniculo-occipital waves by neurotoxic lesions of pontine caudo-lateral peribrachial cells. Neuroscience. 1995;67:703–712. doi: 10.1016/0306-4522(95)00081-s. [DOI] [PubMed] [Google Scholar]
- Datta S, Kumar VM, Chhina GS, Singh B. Effect of application of serotonin in medial preoptic area on body temperature and sleep-wakefulness. Indian J Exp Biol. 1987;25:681–685. [PubMed] [Google Scholar]
- Datta S, Kumar VM, Chhina GS, Singh B. Interrelationship of thermal and sleep-wakefulness changes elicited from the medial preoptic area in rats. Exp Neurol. 1988;100:40–50. doi: 10.1016/0014-4886(88)90199-9. [DOI] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Patterson EH, Ulloor J. Regulation of rapid eye movement sleep in the freely moving rat: local microinjection of serotonin, norepinephrine, and adenosine into the brainstem. Sleep. 2003;26:513–520. doi: 10.1093/sleep/26.5.513. [DOI] [PubMed] [Google Scholar]
- Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. J Neurosci. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Mohan Kumar V, Chhina GS, Singh B. Tonic activity of medial preoptic norepinephrine mechanism for body temperature maintenance in sleeping and awake rats. Brain Res Bull. 1985;15:447–451. doi: 10.1016/0361-9230(85)90034-6. [DOI] [PubMed] [Google Scholar]
- Datta S, Pare D, Oakson G, Steriade M. Thalamic-projecting neurons in the brainstem cholinergic nuclei increases their firing rates one minute in advance of EEG desyncronization associated with REM sleep. Soc Neurosci Abstr. 1989;15:452. [Google Scholar]
- Datta S, Patterson EH, Siwek DF. Endogenous and exogenous nitric oxide in the pedunculopontine tegmentum induces sleep. Synapse. 1997;27:69–78. doi: 10.1002/(SICI)1098-2396(199709)27:1<69::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Datta S, Patterson EH, Siwek DF. Brainstem afferents of the cholinoceptive pontine wave generation sites in the rat. Sleep Res Online. 1999;2:79–82. [PubMed] [Google Scholar]
- Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001b;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- Datta S, Phillipson O, Hobson JA. Effect of clonidine microinjection in the locus subcoeruleus of cat: REM sleep without atonia. Sleep. 1993a;22:5. [Google Scholar]
- Datta S, Prutzman SL. Novel role of brain stem pedunculopontine tegmental adenylyl cyclase in the regulation of spontaneous REM sleep in the freely moving rat. J Neurophysiol. 2005;94:1928–1937. doi: 10.1152/jn.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Quattrochi JJ, Hobson JA. Effect of specific muscarinic M2 receptor antagonist on carbachol induced long-term REM sleep. Sleep. 1993b;16:8–14. [PubMed] [Google Scholar]
- Datta S, Siwek DF. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J Neurophysiol. 1997;77:2975–2988. doi: 10.1152/jn.1997.77.6.2975. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- Datta S, Siwek DF, Patterson EH, Cipolloni PB. Localization of pontine PGO wave generation sites and their anatomical projections in the rat. Synapse. 1998;30:409–423. doi: 10.1002/(SICI)1098-2396(199812)30:4<409::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel role of pedunculopontine tegmental kainate receptors: a mechanism of rapid eye movement sleep generation in the rat. Neuroscience. 2002;114:157–164. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001a;280:R752–759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- De A, Churchill L, Obal F, Jr, Simasko SM, Krueger JM. GHRH and IL1beta increase cytoplasmic Ca(2+) levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–685. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Vansteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- Delorme F, Jeannerod M, Jouvet M. Effets remarquables de la résperine sur l’activité EEG phasique ponto-geniculo-occipitale. C R Soc Biol (Paris) 1965;159:900–903. [Google Scholar]
- Dement W. Dream recall and eye movements during sleep in schizophrenics and normals. J Nerv Ment Dis. 1955;122:263–269. doi: 10.1097/00005053-195509000-00007. [DOI] [PubMed] [Google Scholar]
- Dement W. The occurrence of low voltage, fast, electroencephalogram patterns during behavioral sleep in the cat. Electroencephalogr Clin Neurophysiol Suppl. 1958;10:291–296. doi: 10.1016/0013-4694(58)90037-3. [DOI] [PubMed] [Google Scholar]
- Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol. 1957a;53:339–346. doi: 10.1037/h0048189. [DOI] [PubMed] [Google Scholar]
- Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol Suppl. 1957b;9:673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- Descarries L, Berthelet F, Garcia S, Beaudet A. Dopaminergic projection from nucleus raphe dorsalis to neostriatum in the rat. J Comp Neurol. 1986;249:511–520. 484–515. doi: 10.1002/cne.902490407. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Lapierre Y. Noradrenergic axon terminals in the cerebral cortex of rat. III. Topometric ultrastructural analysis. Brain Res. 1977;133:197–222. doi: 10.1016/0006-8993(77)90759-4. [DOI] [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T. Firing properties of cat basal forebrain neurones during sleep-wakefulness cycle. Electroencephalogr Clin Neurophysiol. 1984;58:362–368. doi: 10.1016/0013-4694(84)90062-2. [DOI] [PubMed] [Google Scholar]
- Detari L, Vanderwolf CH. Activity of identified cortically projecting and other basal forebrain neurones during large slow waves and cortical activation in anaesthetized rats. Brain Res. 1987;437:1–8. doi: 10.1016/0006-8993(87)91521-6. [DOI] [PubMed] [Google Scholar]
- Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. The interleukin-1 family: 10 years of discovery. Faseb J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- Dostrovsky JO, Shah Y, Gray BG. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:948–960. doi: 10.1152/jn.1983.49.4.948. [DOI] [PubMed] [Google Scholar]
- Drucker-Colin R, Aguilar-Roblero R, Garcia-Hernandez F, Fernandez-Cancino F, Bermudez Rattoni F. Fetal suprachiasmatic nucleus transplants: diurnal rhythm recovery of lesioned rats. Brain Res. 1984;311:353–357. doi: 10.1016/0006-8993(84)90099-4. [DOI] [PubMed] [Google Scholar]
- Dudar JD, Whishaw IQ, Szerb JC. Release of acetylcholine from the hippocampus of freely moving rats during sensory stimulation and running. Neuropharmacology. 1979;18:673–678. doi: 10.1016/0028-3908(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Wauquier A, Leysen JE, Marrannes R, Janssen PA. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology (Berl) 1989;97:436–442. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- Dzoljic MR, Ukponmwan OE, Saxena PR. 5-HT1-like receptor agonists enhance wakefulness. Neuropharmacology. 1992;31:623–633. doi: 10.1016/0028-3908(92)90140-k. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Mistlberger RE, Rechtschaffen A. Suprachiasmatic nuclei lesions eliminate circadian temperature and sleep rhythms in the rat. Physiol Behav. 1984;32:357–368. doi: 10.1016/0031-9384(84)90248-8. [DOI] [PubMed] [Google Scholar]
- Easton A, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep. 2004;27:1307–1318. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- Eck MJ, Sprang SR. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989;264:17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–474. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sakai K, Jouvet M. Responses of presumed cholinergic mesopontine tegmental neurons to carbachol microinjections in freely moving cats. Exp Brain Res. 1990;83:115–123. doi: 10.1007/BF00232199. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G. GABA-mediated inhibition of locus coeruleus from the dorsomedial rostral medulla. J Neurosci. 1989;9:2973–2981. doi: 10.1523/JNEUROSCI.09-08-02973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Espana RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MH. Vasoactive sites in the diencephalon of the rabbit. Brain Res. 1980;183:329–340. doi: 10.1016/0006-8993(80)90468-0. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am J Physiol. 1998;274:R655–660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984;306:125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience. 2000;100:741–748. doi: 10.1016/s0306-4522(00)00319-5. [DOI] [PubMed] [Google Scholar]
- Felten DL, Sladek JR., Jr Monoamine distribution in primate brain V. Monoaminergic nuclei: anatomy, pathways and local organization. Brain Res Bull. 1983;10:171–284. doi: 10.1016/0361-9230(83)90045-x. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. Cholinergic mechanisms in learning, memory and dementia: a review of recent evidence. Trends Neurosci. 1991;14:220–223. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- Findlay AL, Hayward JN. Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J Physiol. 1969;201:237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Buchwald NA, Hull CD, Levine MS. GABAergic basal forebrain neurons project to the neocortex: the localization of glutamic acid decarboxylase and choline acetyltransferase in feline corticopetal neurons. J Comp Neurol. 1988;272:489–502. doi: 10.1002/cne.902720404. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. Neuroreport. 1997;8:915–918. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Fornal C, Auerbach S, Jacobs BL. Activity of serotonin-containing neurons in nucleus raphe magnus in freely moving cats. Exp Neurol. 1985;88:590–608. doi: 10.1016/0014-4886(85)90074-3. [DOI] [PubMed] [Google Scholar]
- Freeman A, Ciliax B, Bakay R, Daley J, Miller RD, Keating G, Levey A, Rye D. Nigrostriatal collaterals to thalamus degenerate in parkinsonian animal models. Ann Neurol. 2001;50:321–329. doi: 10.1002/ana.1119. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Boxer PA, Morales FR, Chase MH. Hyperpolarizing membrane responses induced in lumbar motoneurons by stimulation of the nucleus reticularis pontis oralis during active sleep. Brain Res. 1982;248:267–273. doi: 10.1016/0006-8993(82)90584-4. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gacek RR. Location of abducens afferent neurons in the cat. Exp Neurol. 1979;64:342–353. doi: 10.1016/0014-4886(79)90274-7. [DOI] [PubMed] [Google Scholar]
- Gao B, Fritschy JM, Benke D, Mohler H. Neuron-specific expression of GABAA-receptor subtypes: differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881–892. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E. Mechaninsm of Sleep and Wakefulness. In: Lee-Chiong TL Jr, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Hanley & Belfus, Inc; Phildelphia, PA: 2002. pp. 31–39. [Google Scholar]
- Garcia-Rill E, Skinner RD, Conrad C, Mosley D, Campbell C. Projections of the mesencephalic locomotor region in the rat. Brain Res Bull. 1986;17:33–40. doi: 10.1016/0361-9230(86)90158-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD, Fitzgerald JA. Activity in the mesencephalic locomotor region during locomotion. Exp Neurol. 1983;82:609–622. doi: 10.1016/0014-4886(83)90084-5. [DOI] [PubMed] [Google Scholar]
- Gardi J, Obal F, Jr, Fang J, Zhang J, Krueger JM. Diurnal variations and sleep deprivation-induced changes in rat hypothalamic GHRH and somatostatin contents. Am J Physiol. 1999;277:R1339–1344. doi: 10.1152/ajpregu.1999.277.5.R1339. [DOI] [PubMed] [Google Scholar]
- Gassel MM, Ghelarducci B, Marchiafava PL, Pompeiano O. Phasic Changes in Blood Pressure and Heart Rate During the Rapid Eye Movement Episodes of Desynchronized Sleep in Unrestrained Cats. Arch Ital Biol. 1964;102:530–544. [PubMed] [Google Scholar]
- Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Gauthier P, Arnaud C, Gandolfo G, Gottesmann C. Influence of a GABA(B) receptor antagonist on the sleep-waking cycle in the rat. Brain Res. 1997;773:8–14. doi: 10.1016/s0006-8993(97)00643-4. [DOI] [PubMed] [Google Scholar]
- George R, Haslett WL, Jenden DJ. A Cholinergic Mechanism in the Brainstem Reticular Formation: Induction of Paradoxical Sleep. Int J Neuropharmacol. 1964;72:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long-Evans rats. Neuroscience. 2003;116:223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Effects of inflammation produced by chronic lipopolysaccharide administration on the survival of hypocretin neurons and sleep. Brain Res. 2004;1019:162–169. doi: 10.1016/j.brainres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Darracq L, Fort P, Souliere F, Chouvet G, Luppi PH. Electrophysiological evidence that noradrenergic neurons of the rat locus coeruleus are tonically inhibited by GABA during sleep. Eur J Neurosci. 1998;10:964–970. doi: 10.1046/j.1460-9568.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- Gervasoni D, Peyron C, Rampon C, Barbagli B, Chouvet G, Urbain N, Fort P, Luppi PH. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J Neurosci. 2000;20:4217–4225. doi: 10.1523/JNEUROSCI.20-11-04217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Tokuno H, Kuo CC. Developmentally transient expression of acetylcholinesterase within cortical pyramidal neurons of the rat brain. Brain Res Dev Brain Res. 1993;76:23–31. doi: 10.1016/0165-3806(93)90119-u. [DOI] [PubMed] [Google Scholar]
- Gillette MU, McArthur AJ. Circadian actions of melatonin at the suprachiasmatic nucleus. Behav Brain Res. 1996;73:135–139. doi: 10.1016/0166-4328(96)00085-x. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Sejnowski TJ. Physiology. Biological clocks coordinately keep life on time. Science. 2005;309:1196–1198. doi: 10.1126/science.1111420. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner JA. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–17458. [PubMed] [Google Scholar]
- Giovannini MG, Bartolini L, Kopf SR, Pepeu G. Acetylcholine release from the frontal cortex during exploratory activity. Brain Res. 1998;784:218–227. doi: 10.1016/s0006-8993(97)01161-x. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Cornett CM, Heller HC. Activity of suprachiasmatic and hypothalamic neurons during sleep and wakefulness in the rat. Brain Res. 1987;419:279–286. doi: 10.1016/0006-8993(87)90594-4. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Central nervous regulation of body temperature during sleep. Science. 1976;194:537–539. doi: 10.1126/science.973138. [DOI] [PubMed] [Google Scholar]
- Glotzbach SF, Heller HC. Changes in the thermal characteristics of hypothalamic neurons during sleep and wakefulness. Brain Res. 1984;309:17–26. doi: 10.1016/0006-8993(84)91006-0. [DOI] [PubMed] [Google Scholar]
- Gnadt JW, Pegram GV. Cholinergic brainstem mechanisms of REM sleep in the rat. Brain Res. 1986;384:29–41. doi: 10.1016/0006-8993(86)91216-3. [DOI] [PubMed] [Google Scholar]
- Goebel HH, Komatsuzaki A, Bender MB, Cohen B. Lesions of the pontine tegmentum and conjugate gaze paralysis. Arch Neurol. 1971;24:431–440. doi: 10.1001/archneur.1971.00480350065007. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Circuitry of the frontal association cortex and its relevance to dementia. Arch Gerontol Geriatr. 1987;6:299–309. doi: 10.1016/0167-4943(87)90029-x. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Szymusiak R, King J, Steininger T, McGinty D. Sleep-related c-Fos protein expression in the preoptic hypothalamus: effects of ambient warming. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2079–2088. doi: 10.1152/ajpregu.2000.279.6.R2079. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. [Amygdaloid complex and vigilance in the rat] J Physiol (Paris) 1969;61(Suppl 1):135. [PubMed] [Google Scholar]
- Gottesmann C. Introduction to the neurophysiological study of sleep: central regulation of skeletal and ocular activities. Arch Ital Biol. 1997;135:279–314. [PubMed] [Google Scholar]
- Gottesmann C. Neurophysiological support of consciousness during waking and sleep. Prog Neurobiol. 1999;59:469–508. doi: 10.1016/s0301-0082(99)00014-3. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Gray BG, Dostrovsky JO. Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. I. Effects on lumbar spinal cord nociceptive and nonnociceptive neurons. J Neurophysiol. 1983;49:932–947. doi: 10.1152/jn.1983.49.4.932. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol. 1977;175:37–78. doi: 10.1002/cne.901750105. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Greene RW, Carpenter DO. Actions of neurotransmitters on pontine medical reticular formation neurons of the cat. J Neurophysiol. 1985;54:520–531. doi: 10.1152/jn.1985.54.3.520. [DOI] [PubMed] [Google Scholar]
- Greene RW, Gerber U, McCarley RW. Cholinergic activation of medial pontine reticular formation neurons in vitro. Brain Res. 1989;476:154–159. doi: 10.1016/0006-8993(89)91549-7. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J Comp Neurol. 1994;339:251–268. doi: 10.1002/cne.903390206. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006;26:3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak LL, Heller HC, van den Pol AN. Metabotropic glutamate receptor activation modulates kainate and serotonin calcium response in astrocytes. J Neurosci. 1997;17:1825–1837. doi: 10.1523/JNEUROSCI.17-05-01825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harper RM, Sieck GC. Discharge correlations between neurons in the nucleus parabrachialis medialis during sleep-waking states. Brain Res. 1980;199:343–358. doi: 10.1016/0006-8993(80)90694-0. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56:61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Hass H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O. Molecular mechanisms of sleep-wake regulation: roles of prostaglandins D2 and E2. Faseb J. 1991;5:2575–2581. [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Morrison AR, Mann GL. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res. 1982;239:81–105. doi: 10.1016/0006-8993(82)90835-6. [DOI] [PubMed] [Google Scholar]
- Henley K, Morrison AR. A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol Exp (Wars) 1974;34:215–232. [PubMed] [Google Scholar]
- Henn V, Cohen B. Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res. 1976;108:307–325. doi: 10.1016/0006-8993(76)90188-8. [DOI] [PubMed] [Google Scholar]
- Hernandez-Peon R, Chavez-Ibarra G, Morgane P, Timo-Iaria C. Cholinergic pathways for sleep, alertness and rage in the limbic midbrain circuit. Acta Neurologica Latinoamericana. 1962;8:93–96. [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hess R, Jr, Koella WP, Akert K. Cortical and subcortical recordings in natural and artificially induced sleep in cats. Electroencephalogr Clin Neurophysiol Suppl. 1953;5:75–90. doi: 10.1016/0013-4694(53)90055-8. [DOI] [PubMed] [Google Scholar]
- Hess WR. The diencephalic sleep centre. In: Delafresnaye JF, editor. Brain Mechanisms and Conciousness. Blackwell; Oxford: 1954. pp. 117–136. [Google Scholar]
- Highstein SM, Maekawa K, Steinacker A, Cohen B. Synaptic input from the pontine reticular nuclei to absucens motoneurons and internuclear neurons in the cat. Brain Res. 1976;112:162–167. doi: 10.1016/0006-8993(76)90344-9. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kawakami T. Inhibitory reticular neurons related to the quick phase of vestibular nystagmus--their location and projection. Exp Brain Res. 1977;27:377–386. doi: 10.1007/BF00235511. [DOI] [PubMed] [Google Scholar]
- Hillarp NA, Fuxe K, Dahlstrom A. Demonstration and mapping of central neurons containing dopamine, noradrenaline, and 5-hydroxytryptamine and their reactions to psychopharmaca. Pharmacol Rev. 1966;18:727–741. [PubMed] [Google Scholar]
- Hobson JA. The Effects of Chronic Brain-Stem Lesions on Cortical and Muscular Activity During Sleep and Waking in the Cat. Electroencephalogr Clin Neurophysiol. 1965;19:41–62. doi: 10.1016/0013-4694(65)90006-4. [DOI] [PubMed] [Google Scholar]
- Hobson JA. Arrest of firing of aminergic neurones during REM sleep: implications for dream theory. Brain Res Bull. 1999;50:333–334. doi: 10.1016/s0361-9230(99)00146-x. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Goldberg M, Vivaldi E, Riew D. Enhancement of desynchronized sleep signs after pontine microinjection of the muscarinic agonist bethanechol. Brain Res. 1983;275:127–136. doi: 10.1016/0006-8993(83)90424-9. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Freedman R, Pivik RT. Time course of discharge rate changes by cat pontine brain stem neurons during sleep cycle. J Neurophysiol. 1974;37:1297–1309. doi: 10.1152/jn.1974.37.6.1297. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hohagen F, Timmer J, Weyerbrock A, Fritsch-Montero R, Ganter U, Krieger S, Berger M, Bauer J. Cytokine production during sleep and wakefulness and its relationship to cortisol in healthy humans. Neuropsychobiology. 1993;28:9–16. doi: 10.1159/000118993. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Hoshino K, Pompeiano O, Magherini PC, Mergner T. The oscillatory system responsible for the oculomotor activity during the bursts of REM. Arch Ital Biol. 1976;114:278–309. [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–526. [PubMed] [Google Scholar]
- Ibuka N, Inouye SI, Kawamura H. Analysis of sleep-wakefulness rhythms in male rats after suprachiasmatic nucleus lesions and ocular enucleation. Brain Res. 1977;122:33–47. doi: 10.1016/0006-8993(77)90660-6. [DOI] [PubMed] [Google Scholar]
- Ibuka N, Kawamura H. Loss of circadian rhythm in sleep-wakefulness cycle in the rat by suprachiasmatic nucleus lesions. Brain Res. 1975;96:76–81. doi: 10.1016/0006-8993(75)90574-0. [DOI] [PubMed] [Google Scholar]
- Igusa Y, Sasaki S, Shimazu H. Excitatory premotor burst neurons in the cat pontine reticular formation related to the quick phase of vestibular nystagmus. Brain Res. 1980;182:451–456. doi: 10.1016/0006-8993(80)91202-0. [DOI] [PubMed] [Google Scholar]
- Iijima K, Ohtomo K. Immunocytochemical study using a GABA antiserum for the demonstration of inhibitory neurons in the rat locus ceruleus. Am J Anat. 1988;181:43–52. doi: 10.1002/aja.1001810106. [DOI] [PubMed] [Google Scholar]
- Imeri L, Bianchi S, Opp MR. Inhibition of caspase-1 in rat brain reduces spontaneous non-rapid eye movement (NREM) sleep and NREM sleep enhancement induced by lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00828.2005. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yamatodani A, Ando-Yamamoto M, Tohyama M, Watanabe T, Wada H. Organization of histaminergic fibers in the rat brain. J Comp Neurol. 1988;273:283–300. doi: 10.1002/cne.902730302. [DOI] [PubMed] [Google Scholar]
- Inoue S, Honda K, Komoda Y, Uchizono K, Ueno R, Hayaishi O. Differential sleep-promoting effects of five sleep substances nocturnally infused in unrestrained rats. Proc Natl Acad Sci U S A. 1984;81:6240–6244. doi: 10.1073/pnas.81.19.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac SO, Berridge CW. Wake-promoting actions of dopamine D1 and D2 receptor stimulation. J Pharmacol Exp Ther. 2003;307:386–394. doi: 10.1124/jpet.103.053918. [DOI] [PubMed] [Google Scholar]
- Ishimura K, Takeuchi Y, Fujiwara K, Tominaga M, Yoshioka H, Sawada T. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci Lett. 1988;91:265–270. doi: 10.1016/0304-3940(88)90691-x. [DOI] [PubMed] [Google Scholar]
- Ito K, Yanagihara M, Imon H, Dauphin L, McCarley RW. Intracellular recordings of pontine medial gigantocellular tegmental field neurons in the naturally sleeping cat: behavioral state-related activity and soma size difference in order of recruitment. Neuroscience. 2002;114:23–37. doi: 10.1016/s0306-4522(02)00253-1. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol. 1986;27:183–194. doi: 10.1016/0301-0082(86)90008-0. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Gannon PJ, Azmitia EC. Atlas of serotonergic cell bodies in the cat brainstem: an immunocytochemical analysis. Brain Res Bull. 1984;13:1–31. doi: 10.1016/0361-9230(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172:601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Jell RM. Responses of hypothalamic neurones to local temperature and to acetylcholine, noradrenaline and 5-hydroxytryptamine. Brain Res. 1973;55:123–134. doi: 10.1016/0006-8993(73)90492-7. [DOI] [PubMed] [Google Scholar]
- Jell RM. Responses of rostral hypothalamic neurones to peripheral temperature and to amines. J Physiol. 1974;240:295–307. doi: 10.1113/jphysiol.1974.sp010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- John J, Kumar VM. Effect of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep. 1998;21:587–598. doi: 10.1093/sleep/21.6.587. [DOI] [PubMed] [Google Scholar]
- John J, Kumar VM, Gopinath G, Ramesh V, Mallick H. Changes in sleep-wakefulness after kainic acid lesion of the preoptic area in rats. Jpn J Physiol. 1994;44:231–242. doi: 10.2170/jjphysiol.44.231. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–586. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Jones TA, Bickford RG. Sleep: Control of the paradoxical episode? Neuroscience Letters. 1977;4:85–92. doi: 10.1016/0304-3940(77)90149-5. [DOI] [PubMed] [Google Scholar]
- Jouvet M. [Research on the neural structures and responsible mechanisms in different phases of physiological sleep.] Arch Ital Biol. 1962;100:125–206. [PubMed] [Google Scholar]
- Jouvet M. Paradoxical Sleep--a Study of Its Nature and Mechanisms. Prog Brain Res. 1965;18:20–62. doi: 10.1016/s0079-6123(08)63582-7. [DOI] [PubMed] [Google Scholar]
- Jouvet M, Delorme F. Locus coeruleus et sommeil paradoxal. C R Soc Biol. 1965;159:895–899. [Google Scholar]
- Jouvet M, Michel F. Corrélations électromyographiques du sommeil chez le Chat décortiqué et mésencéphalique chronique. C R Soc Biol. 1959;153:422–425. [PubMed] [Google Scholar]
- Jouvet M, Michel F. [On the neural tract responsible for the rapid cortical activity during the course of physiological sleep in the cat (paradoxal phase).] C R Seances Soc Biol Fil. 1960;154:995–998. [PubMed] [Google Scholar]
- Jouvet M, Michel F, Courjon J. Sur la mise en jeu de deux mécaismes à expression électro-encéphalographique différente au cours du sommeil physiologique chez le Chat. C R Hebd Seances Acad Sci. 1959a;248:3043–3045. [PubMed] [Google Scholar]
- Jouvet M, Michel F, Courjon J. Sur un stade d’activité électrique rapide au cours du sommeil physiologique. C R Seances Soc Biol Fil. 1959b;153:1024–1028. [PubMed] [Google Scholar]
- Jouvet M, Mounier D. [Effect of lesions of the pontile reticular formation on sleep in the cat.] C R Seances Soc Biol Fil. 1960;154:2301–2305. [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kaitin KI. Preoptic area unit activity during sleep and wakefulness in the cat. Exp Neurol. 1984;83:347–357. doi: 10.1016/S0014-4886(84)90103-1. [DOI] [PubMed] [Google Scholar]
- Kajimura N, Uchiyama M, Takayama Y, Uchida S, Uema T, Kato M, Sekimoto M, Watanabe T, Nakajima T, Horikoshi S, Ogawa K, Nishikawa M, Hiroki M, Kudo Y, Matsuda H, Okawa M, Takahashi K. Activity of midbrain reticular formation and neocortex during the progression of human non-rapid eye movement sleep. J Neurosci. 1999;19:10065–10073. doi: 10.1523/JNEUROSCI.19-22-10065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinchuk AV, Urrila AS, Alanko L, Heiskanen S, Wigren HK, Suomela M, Stenberg D, Porkka-Heiskanen T. Local energy depletion in the basal forebrain increases sleep. Eur J Neurosci. 2003;17:863–869. doi: 10.1046/j.1460-9568.2003.02532.x. [DOI] [PubMed] [Google Scholar]
- Kaneko CR, Evinger C, Fuchs AF. Role of cat pontine burst neurons in generation of saccadic eye movements. J Neurophysiol. 1981;46:387–408. doi: 10.1152/jn.1981.46.3.387. [DOI] [PubMed] [Google Scholar]
- Karadzic V, Micic D, Rakic L. Alterations of free amino acids concentrations in cat brain induced by rapid eye movement sleep deprivation. Experientia. 1971;27:509–511. doi: 10.1007/BF02147566. [DOI] [PubMed] [Google Scholar]
- Karashima A, Nakamura K, Horiuchi M, Nakao M, Katayama N, Yamamoto M. Elicited ponto-geniculo-occipital waves by auditory stimuli are synchronized with hippocampal theta-waves. Psychiatry Clin Neurosci. 2002;56:343–344. doi: 10.1046/j.1440-1819.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- Karashima A, Nakao M, Honda K, Iwasaki N, Katayama N, Yamamoto M. Theta wave amplitude and frequency are differentially correlated with pontine waves and rapid eye movements during REM sleep in rats. Neurosci Res. 2004;50:283–289. doi: 10.1016/j.neures.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T. Maintained and evoked unit activity in the mesencephalic reticular formation of the freely behaving cat. Exp Neurol. 1970;28:450–470. doi: 10.1016/0014-4886(70)90182-2. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–48. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Mamoru O, Ito S. Ascending cholinergic and monoaminergic systems in the brainstem: do they constitute a reticular activating system? In: Nagatsu T, et al., editors. Alzheimer’s and Parkinson’s Diseases II: Basic and Therapeutic Strategies. Plenum Publishing; New York: 1990. [Google Scholar]
- Kayama Y, Negi T, Sugitani M, Iwama K. Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience. 1982;7:655–666. doi: 10.1016/0306-4522(82)90071-9. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ogawa T. Electrophysiology of ascending, possibly cholinergic neurons in the rat laterodorsal tegmental nucleus: comparison with monoamine neurons. Neurosci Lett. 1987;77:277–282. doi: 10.1016/0304-3940(87)90512-x. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ohta M, Jodo E. Firing of ‘possibly’ cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–220. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- Keller EL. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974;37:316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Kerkhofs M, Van Cauter E, Van Onderbergen A, Caufriez A, Thorner MO, Copinschi G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol. 1993;264:E594–598. doi: 10.1152/ajpendo.1993.264.4.E594. [DOI] [PubMed] [Google Scholar]
- Kerr DI, Ong J. GABAB receptors. Pharmacol Ther. 1995;67:187–246. doi: 10.1016/0163-7258(95)00016-a. [DOI] [PubMed] [Google Scholar]
- Khateb A, Fort P, Alonso A, Jones BE, Muhlethaler M. Pharmacological and immunohistochemical evidence for serotonergic modulation of cholinergic nucleus basalis neurons. Eur J Neurosci. 1993;5:541–547. doi: 10.1111/j.1460-9568.1993.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Khubchandani M, Jagannathan NR, Mallick HN, Mohan Kumar V. Functional MRI shows activation of the medial preoptic area during sleep. Neuroimage. 2005;26:29–35. doi: 10.1016/j.neuroimage.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Kimura H, McGeer PL, Peng JH, McGeer EG. The central cholinergic system studied by choline acetyltransferase immunohistochemistry in the cat. J Comp Neurol. 1981;200:151–201. doi: 10.1002/cne.902000202. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Kitsikis A, Steriade M. Immediate behavioral effects of kainic acid injections into the midbrain reticular core. Behav Brain Res. 1981;3:361–380. doi: 10.1016/0166-4328(81)90005-x. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaue R. Die bioelektrische Tätigkeit der Grosshirnrinde im normalen Schlaf und in der Narkose durch Schlafmittel. Journal Psych Neuro. 1937;47:510–531. [Google Scholar]
- Knox CK, King GW. Changes in the Breuer-Hering reflexes following rostral pontine lesion. Respir Physiol. 1976;28:189–206. doi: 10.1016/0034-5687(76)90038-4. [DOI] [PubMed] [Google Scholar]
- Knox GV, Campbell C, Lomax P. The effects of acetylcholine and nicotine on unit activity in the hypothalamic thermoregulatory centers of the rat. Brain Res. 1973;51:215–223. doi: 10.1016/0006-8993(73)90374-0. [DOI] [PubMed] [Google Scholar]
- Ko EM, Estabrooke IV, McCarthy M, Scammell TE. Wake-related activity of tuberomammillary neurons in rats. Brain Res. 2003;992:220–226. doi: 10.1016/j.brainres.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci U S A. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Vertes RP. Dorsal raphe neurons: synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J Neurophysiol. 1992;68:1463–1467. doi: 10.1152/jn.1992.68.4.1463. [DOI] [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Res. 1998;780:176–179. [PubMed] [Google Scholar]
- Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114:277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- Kostowski W. Effects of some cholinergic and anticholinergic drugs injected intracerebrally to the midline pontine area. Neuropharmacology. 1971;10:595–605. doi: 10.1016/0028-3908(71)90025-6. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Cebers G, Liljequist S. Kainate receptor-mediated activation of the AP-1 transcription factor complex in cultured rat cerebellar granule cells. Brain Res Bull. 2000;52:127–133. doi: 10.1016/s0361-9230(00)00247-1. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Hayaishi O. Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep. Neurosci Res. 1994;19:31–38. doi: 10.1016/0168-0102(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Kodama T, Takahashi K, Okai K, Kayama Y. Firing properties of neurones in the laterodorsal hypothalamic area during sleep and wakefulness. Psychiatry Clin Neurosci. 2002;56:339–340. doi: 10.1046/j.1440-1819.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): an anterograde and retrograde analysis. J Comp Neurol. 2004;468:361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Obal F. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal F., Jr Sleep function. Front Biosci. 2003;8:d511–519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1) Am J Physiol. 1984;246:R994–999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Datta S, Chhina GS, Singh B. Alpha-adrenergic system in medial preoptic area involved in sleep-wakefulness in rats. Brain Res Bull. 1986;16:463–468. doi: 10.1016/0361-9230(86)90174-7. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Datta S, Chhina NG, Singh B. Sleep-awake responses elicited from medial preoptic area on application of norepinephrine and phenoxybenzamine in free moving rats. Brain Res. 1984;322:322–325. doi: 10.1016/0006-8993(84)90125-2. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Datta S, Singh B. The role of reticular activating system in altering medial preoptic neuronal activity in anesthetized rats. Brain Res Bull. 1989;22:1031–1037. doi: 10.1016/0361-9230(89)90016-6. [DOI] [PubMed] [Google Scholar]
- Kumar VM, Khan NA, John J. Male sexual behaviour not abolished after medial preoptic lesion in adult rats. Neuroreport. 1996;7:1481–1484. doi: 10.1097/00001756-199606170-00007. [DOI] [PubMed] [Google Scholar]
- Kurosawa M, Okada K, Sato A, Uchida S. Extracellular release of acetylcholine, noradrenaline and serotonin increases in the cerebral cortex during walking in conscious rats. Neurosci Lett. 1993;161:73–76. doi: 10.1016/0304-3940(93)90143-9. [DOI] [PubMed] [Google Scholar]
- Lai YY, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol. 1993;336:321–330. doi: 10.1002/cne.903360302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;12:229–238. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395–409. doi: 10.1093/sleep/28.4.395. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz GR, Carlton SM, Katayama Y, Gonzalo-Ruiz A, Holstege G, DeSalles AA, Hayes RL. Afferent and efferent connections of the cholinoceptive medial pontine reticular formation (region of the ventral tegmental nucleus) in the cat. Brain Res Bull. 1989;22:665–688. doi: 10.1016/0361-9230(89)90087-7. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci U S A. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MN, Martin PJ. The cardiovascular system: The Heart. sect 2. Vol. 1. American Physiological Society; Bethesda, MD: 1979. Neural control of the heart. Handbook of Physiology; pp. 581–620. [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Varga V, Sik A, Kocsis B. GABAergic control of the ascending input from the median raphe nucleus to the limbic system. J Neurophysiol. 2005;94:2561–2574. doi: 10.1152/jn.00379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Lin JS, Luppi PH, Salvert D, Sakai K, Jouvet M. [Histamine-immunoreactive neurons in the hypothalamus of cats] C R Acad Sci III. 1986;303:371–376. [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Lincoln DW. Correlation of unit activity in the hypothalamus with EEG patterns associated with the sleep cycle. Exp Neurol. 1969;24:1–18. doi: 10.1016/0014-4886(69)90002-8. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT(2) receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Liu RH, Fung SJ, Reddy VK, Barnes CD. Localization of glutamatergic neurons in the dorsolateral pontine tegmentum projecting to the spinal cord of the cat with a proposed role of glutamate on lumbar motoneuron activity. Neuroscience. 1995;64:193–208. doi: 10.1016/0306-4522(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Liu ZW, Gao XB. Adenosine inhibits activity of hypocretin/orexin neurons by the A1 receptor in the lateral hypopthalamus: A possible sleep-promoting effect. J Neurophysiol. 2007;97:837–848. doi: 10.1152/jn.00873.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533–538. doi: 10.1016/0006-8993(79)90584-5. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935a;81:597–598. doi: 10.1126/science.81.2111.597. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart G. Further observations on the potential rhythms of the cerebral cortex during sleep. Science. 1935b;82:198–200. doi: 10.1126/science.82.2122.198. [DOI] [PubMed] [Google Scholar]
- Loomis AL, Harvey EN, Hobart GAI. Cerebral states during sleep, as studied by human brain potentials. J Exp Psych. 1937;21:127–144. [Google Scholar]
- Loomis AL, Harvey EN, Hobart GAI. Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. J of Neurophys. 1938;1:413–430. [Google Scholar]
- Lowie AD, Spyer KM. Central Regulation of Autonomic Functions. Oxford University Press; New York: 1990. [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576. doi: 10.1523/JNEUROSCI.22-11-04568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW, Reiner PB. Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro. Proc Natl Acad Sci U S A. 1992;89:743–747. doi: 10.1073/pnas.89.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque JM, Malherbe P, Richards JG. Localization of GABAA receptor subunit mRNAs in the rat locus coeruleus. Brain Res Mol Brain Res. 1994;24:219–226. doi: 10.1016/0169-328x(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972;35:445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Cholinergic pontine mechanisms causing state-dependent respiratory depression. NIPS. 1992;7:220–224. [Google Scholar]
- Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:R544–554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA, Lorinc Z. Microdialysis of cat pons reveals enhanced acetylcholine release during state-dependent respiratory depression. Am J Physiol. 1991;261:R766–770. doi: 10.1152/ajpregu.1991.261.3.R766. [DOI] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. The time-course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep cycles. Brain Res. 1983;274:365–370. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- Lydic R, McCarley RW, Hobson JA. Timing function of the dorsal raphe nucleus and the temporal organization of the ultradian sleep cycle. Exp Brain Res Suppl. 1985;12:125–144. [Google Scholar]
- Lydic R, Orem J. Respiratory neurons of the pneumotaxic center during sleep and wakefulness. Neurosci Lett. 1979;15:187–192. doi: 10.1016/0304-3940(79)96111-1. [DOI] [PubMed] [Google Scholar]
- Macadar AW, Chalupa LM, Lindsley DB. Differentiation of brain stem loci which affect hippocampal and neocortical electrical activity. Exp Neurol. 1974;43:499–514. doi: 10.1016/0014-4886(74)90190-3. [DOI] [PubMed] [Google Scholar]
- Maciewicz RJ, Kaneko CR, Highstein SM, Eagen K. Vestibular and medullary brain stem afferents to the abducens nucleus in the cat. Brain Res. 1977;123:229–240. doi: 10.1016/0006-8993(77)90476-0. [DOI] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L, Jones BE. c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci. 2002;15:774–778. doi: 10.1046/j.1460-9568.2002.01907.x. [DOI] [PubMed] [Google Scholar]
- Mancia G, Zanchetti A. Cardiovascular regulation during sleep. In: Orem J, Barnes CD, editors. Physiology In Sleep. Academic Press; New York: 1980. pp. 1–55. [Google Scholar]
- Manohar S, Noda H, Adey WR. Behavior of mesencephalic reticular neurons in sleep and wakefulness. Exp Neurol. 1972;34:140–157. doi: 10.1016/0014-4886(72)90195-1. [DOI] [PubMed] [Google Scholar]
- Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, von Frenckell R, Franck G. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–143. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- Maquet P, Lejeune H, Pouthas V, Bonnet M, Casini L, Macar F, Timsit-Berthier M, Vidal F, Ferrara A, Degueldre C, Quaglia L, Delfiore G, Luxen A, Woods R, Mazziotta JC, Comar D. Brain activation induced by estimation of duration: a PET study. Neuroimage. 1996;3:119–126. doi: 10.1006/nimg.1996.0014. [DOI] [PubMed] [Google Scholar]
- Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- Marks GA, Birabil CG. Enhancement of rapid eye movement sleep in the rat by cholinergic and adenosinergic agonists infused into the pontine reticular formation. Neuroscience. 1998;86:29–37. doi: 10.1016/s0306-4522(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Marshall L, Derad I, Strasburger CJ, Fehm HL, Born J. A determinant factor in the efficacy of GHRH administration in promoting sleep: high peak concentration versus recurrent increasing slopes. Psychoneuroendocrinology. 1999;24:363–370. doi: 10.1016/s0306-4530(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- Mason P. Ventromedial medulla: pain modulation and beyond. J Comp Neurol. 2005;493:2–8. doi: 10.1002/cne.20751. [DOI] [PubMed] [Google Scholar]
- Matsumura H, Nakajima T, Osaka T, Satoh S, Kawase K, Kubo E, Kantha SS, Kasahara K, Hayaishi O. Prostaglandin D2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci U S A. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Takahata R, Hayaishi O. Inhibition of sleep in rats by inorganic selenium compounds, inhibitors of prostaglandin D synthase. Proc Natl Acad Sci U S A. 1991;88:9046–9050. doi: 10.1073/pnas.88.20.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M. Differential effects of sodium butyrate and physostigmine upon the activities of para-sleep in acute brain stem preparations. Brain Res. 1969;13:247–265. doi: 10.1016/0006-8993(69)90285-6. [DOI] [PubMed] [Google Scholar]
- Matthias H, Munk J, Roelfsema PR, Koing P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS. Axoaxonic synapses on terminals of group II muscle spindle afferent axons in the spinal cord of the cat. Eur J Neurosci. 1999;11:2151–2159. doi: 10.1046/j.1460-9568.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Mayo KE, Godfrey PA, Suhr ST, Kulik DJ, Rahal JO. Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- McBride RL, Sutin J. Projections of the locus coeruleus and adjacent pontine tegmentum in the cat. J Comp Neurol. 1976;165:265–284. doi: 10.1002/cne.901650302. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Mechanisms and models of REM sleep control. Arch Ital Biol. 2004;142:429–467. [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Single neuron activity in cat gigantocellular tegmental field: selectivity of discharge in desynchronized sleep. Science. 1971;174:1250–1252. doi: 10.1126/science.174.4015.1250. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975a;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Discharge patterns of cat pontine brain stem neurons during desynchronized sleep. J Neurophysiol. 1975b;38:751–766. doi: 10.1152/jn.1975.38.4.751. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Nelson JP, Hobson JA. Ponto-geniculo-occipital (PGO) burst neurons: correlative evidence for neuronal generators of PGO waves. Science. 1978;201:269–272. doi: 10.1126/science.663656. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13:480–487. doi: 10.1016/0166-2236(90)90081-k. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. The sleep-wake switch: A neuronal alarm clock. Nat Med. 2000;6:510–511. doi: 10.1038/74988. [DOI] [PubMed] [Google Scholar]
- McGinty DJ. Somnolence, recovery and hyposomnia following ventro-medial diencephalic lesions in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:70–79. doi: 10.1016/0013-4694(69)90035-2. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- Mendelson WB. The sleep-inducing effect of ethanol microinjection into the medial preoptic area is blocked by flumazenil. Brain Res. 2001;892:118–121. doi: 10.1016/s0006-8993(00)03243-1. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Bergmann BM, Tung A. Baseline and post-deprivation recovery sleep in SCN-lesioned rats. Brain Res. 2003;980:185–190. doi: 10.1016/s0006-8993(03)02896-8. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV. Characterization of the hypnotic effects of triazolam microinjections into the medial preoptic area. Life Sci. 1992;50:1117–1128. doi: 10.1016/0024-3205(92)90349-t. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Schally AV, Petrusz P. Immunocytochemical localization of growth hormone-releasing factor in the rat hypothalamus. Endocrinology. 1984;114:1082–1085. doi: 10.1210/endo-114-4-1082. [DOI] [PubMed] [Google Scholar]
- Mergner T, Pompeiano O. Single unit firing patterns in the vestibular nuclei related to saccadic eye movement in the decerebrate cat. Arch Ital Biol. 1978;116:91–119. [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–633. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Atlas of cholinergic neurons in the forebrain and upper brainstem of the macaque based on monoclonal choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Neuroscience. 1984;12:669–686. doi: 10.1016/0306-4522(84)90163-5. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11:3423–3426. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- Methippara MM, Alam MN, Szymusiak R, McGinty D. Preoptic area warming inhibits wake-active neurons in the perifornical lateral hypothalamus. Brain Res. 2003;960:165–173. doi: 10.1016/s0006-8993(02)03808-8. [DOI] [PubMed] [Google Scholar]
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–451. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian regulation of sleep in mammals: role of the suprachiasmatic nucleus. Brain Res Brain Res Rev. 2005;49:429–454. doi: 10.1016/j.brainresrev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Bergmann BM, Waldenar W, Rechtschaffen A. Recovery sleep following sleep deprivation in intact and suprachiasmatic nuclei-lesioned rats. Sleep. 1983;6:217–233. doi: 10.1093/sleep/6.3.217. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K, McCarley RW. Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res. 1988;451:397–402. doi: 10.1016/0006-8993(88)90792-5. [DOI] [PubMed] [Google Scholar]
- Mitler M, Dement W. Cataplectic-like behavior in cats after microinjections of carbochol in pontine reticular formation. Brain Res. 1964;68:335–343. doi: 10.1016/0006-8993(74)90402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Reis DJ. Cerebellum: a pressor response elicited from the fastigial nucleus and its efferent pathway in brainstem. Brain Res. 1969;13:595–599. doi: 10.1016/0006-8993(69)90269-8. [DOI] [PubMed] [Google Scholar]
- Mizukawa K, McGeer PL, Tago H, Peng JH, McGeer EG, Kimura H. The cholinergic system of the human hindbrain studied by choline acetyltransferase immunohistochemistry and acetylcholinesterase histochemistry. Brain Res. 1986;379:39–55. doi: 10.1016/0006-8993(86)90253-2. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992;51:391–394. doi: 10.1016/0031-9384(92)90157-w. [DOI] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Lue FA, Eisen J, Keystone E, Gorczynski RM. The relationship of interleukin-1 and immune functions to sleep in humans. Psychosom Med. 1986;48:309–318. doi: 10.1097/00006842-198605000-00001. [DOI] [PubMed] [Google Scholar]
- Monti JM, Fernandez M, Jantos H. Sleep during acute dopamine D1 agonist SKF 38393 or D1 antagonist SCH 23390 administration in rats. Neuropsychopharmacology. 1990;3:153–162. [PubMed] [Google Scholar]
- Monti JM, Jantos H. Dose-dependent effects of the 5-HT1A receptor agonist 8-OH-DPAT on sleep and wakefulness in the rat. J Sleep Res. 1992;1:169–175. doi: 10.1111/j.1365-2869.1992.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Monti JM, Pellejero T, Jantos H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J Neural Transm. 1986;66:1–11. doi: 10.1007/BF01262953. [DOI] [PubMed] [Google Scholar]
- Moon-Edley S, Graybiel AM. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983;217:187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morgane PJ. Historical and modern concepts of hypothalamic organization and function. In: Morgane PJ, editor. Anatomy of the Hypothalamus. Vol. 1. Marcel Dekker, Inc; New York: 1979. pp. 1–64. [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Panksepp J. Handbook of the Hypothalamus. 3A. Marcel Dekker, Inc; New York: 1980. Behavioral studies of the hypothalamus. [Google Scholar]
- Morgane PJ, Stern WC. Chemical anatomy of brain: circuits in relation to sleep and wakefulness. In: Weitzman ED, editor. Advances in Sleep Research. Vol. 1. Spectrum Publications, Flushing; New York: 1974. pp. 1–131. [Google Scholar]
- Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61:391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Morrison AR. Relationships between phenomena of paradoxical sleep and their counterparts in wakefulness. Acta Neurobiol Exp (Wars) 1979;39:567–583. [PubMed] [Google Scholar]
- Morrison AR, Pompeiano O. Vestibular influences during sleep. IV. Functional relations between vestibular nuclei and lateral geniculate nucleus during desynchronized sleep. Arch Ital Biol. 1966;104:425–458. [PubMed] [Google Scholar]
- Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 1986;243:117–138. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- Moruzzi G. Reticular Influences on the Eeg. Electroencephalogr Clin Neurophysiol. 1964;16:2–17. doi: 10.1016/0013-4694(64)90021-5. [DOI] [PubMed] [Google Scholar]
- Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroenceph Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Mosko S, Moore RY. Retinohypothalamic tract development: alteration by suprachiasmatic lesions in the neonatal rat. Brain Res. 1979;164:1–15. doi: 10.1016/0006-8993(79)90002-7. [DOI] [PubMed] [Google Scholar]
- Mraovitch S, Kumada M, Reis DJ. Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res. 1982;232:57–75. doi: 10.1016/0006-8993(82)90610-2. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribtution of GABAergic neurons and terminals in the rat CNS. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy: GABA and Neuropeptides in the CNS. Vol. 4. Elsevier; Amsterdam: 1985. pp. 436–608. [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci. 1994;14:2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N. Effects of iontophoretic application of 5-hydroxytryptamine, noradrenaline and acetylcholine upon hypothalamic temperature-sensitive neurones in rats. Jpn J Physiol. 1973;23:435–446. doi: 10.2170/jjphysiol.23.435. [DOI] [PubMed] [Google Scholar]
- Murck H, Struttmann T, Czisch M, Wetter T, Steiger A, Auer DP. Increase in amino acids in the pons after sleep deprivation: a pilot study using proton magnetic resonance spectroscopy. Neuropsychobiology. 2002;45:120–123. doi: 10.1159/000054949. [DOI] [PubMed] [Google Scholar]
- Nagai T, McGeer PL, McGeer EG. Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol. 1983;218:220–238. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- Naito K, Osama H, Ueno R, Hayaishi O, Honda K, Inoue S. Suppression of sleep by prostaglandin synthesis inhibitors in unrestrained rats. Brain Res. 1988;453:329–336. doi: 10.1016/0006-8993(88)90173-4. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Nanopoulos D, Belin MF, Maitre M, Vincendon G, Pujol JF. Immunocytochemical evidence for the existence of GABAergic neurons in the nucleus raphe dorsalis. Possible existence of neurons containing serotonin and GABA. Brain Res. 1982;232:375–389. doi: 10.1016/0006-8993(82)90281-5. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. Neural associations of the frontal cortex. Acta Neurobiol Exp (Wars) 1972;32:125–140. [PubMed] [Google Scholar]
- Nauta WJH. Hypothalamic regulation of sleep in rats. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, Turek FW. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JP, McCarley RW, Hobson JA. REM sleep burst neurons, PGO waves, and eye movement information. J Neurophysiol. 1983;50:784–797. doi: 10.1152/jn.1983.50.4.784. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cereb Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Nistico G, De Sarro GB, Langer SZ. Behavioural and electrocortical power spectrum effects of 5-methoxytryptoline and other analogs after intraventricular administration in rats. Eur J Pharmacol. 1987;142:121–128. doi: 10.1016/0014-2999(87)90660-1. [DOI] [PubMed] [Google Scholar]
- Nistico G, DeSarro G, Rotiroti D, editors. Behavioral and electrocortical spectrum power changes of interleukins and tumor necrosis factor after microinjection into different areas of the brain. Masson; Milan, Italy: 1992. [Google Scholar]
- Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997b;273:R451–455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997a;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez A. Unit activity of rat basal forebrain neurons: relationship to cortical activity. Neuroscience. 1996;72:757–766. doi: 10.1016/0306-4522(95)00582-x. [DOI] [PubMed] [Google Scholar]
- O’Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Alfoldi P, Cady AB, Johannsen L, Sary G, Krueger JM. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol. 1988;255:R310–316. doi: 10.1152/ajpregu.1988.255.2.R310. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Alt J, Taishi P, Gardi J, Krueger JM. Sleep in mice with nonfunctional growth hormone-releasing hormone receptors. Am J Physiol Regul Integr Comp Physiol. 2003;284:R131–139. doi: 10.1152/ajpregu.00361.2002. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Fang J, Taishi P, Kacsoh B, Gardi J, Krueger JM. Deficiency of growth hormone-releasing hormone signaling is associated with sleep alterations in the dwarf rat. J Neurosci. 2001;21:2912–2918. doi: 10.1523/JNEUROSCI.21-08-02912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal F, Jr, Floyd R, Kapas L, Bodosi B, Krueger JM. Effects of systemic GHRH on sleep in intact and hypophysectomized rats. Am J Physiol. 1996;270:E230–237. doi: 10.1152/ajpendo.1996.270.2.E230. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Krueger JM. GHRH and sleep. Sleep Med Rev. 2004;8:367–377. doi: 10.1016/j.smrv.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Payne L, Kapas L, Opp M, Krueger JM. Inhibition of growth hormone-releasing factor suppresses both sleep and growth hormone secretion in the rat. Brain Res. 1991;557:149–153. doi: 10.1016/0006-8993(91)90128-i. [DOI] [PubMed] [Google Scholar]
- Obal F, Jr, Payne L, Opp M, Alfoldi P, Kapas L, Krueger JM. Growth hormone-releasing hormone antibodies suppress sleep and prevent enhancement of sleep after sleep deprivation. Am J Physiol. 1992;263:R1078–1085. doi: 10.1152/ajpregu.1992.263.5.R1078. [DOI] [PubMed] [Google Scholar]
- Onoe H, Ueno R, Fujita I, Nishino H, Oomura Y, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in monkeys. Proc Natl Acad Sci U S A. 1988;85:4082–4086. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomura Y. Input-output organization in the hypothalamus relating to food intake behavior. In: Morgane PJ, Panksepp J, editors. Handbook of the Hypothalamus. Vol. 2. Marcel Dekker, Inc; New York: 1980. [Google Scholar]
- Oomura Y, Takigawa M. Input-output organization between the frontal cortex and the lateral hypothalamus. In: Desiraju T, editor. Mechanisms in Transmission of Signals for Conscious Behaviour. Elsevier; Amsterdam: 1976. pp. 163–192. [Google Scholar]
- Opp MR, Krueger JM. Anti-interleukin-1 beta reduces sleep and sleep rebound after sleep deprivation in rats. Am J Physiol. 1994a;266:R688–695. doi: 10.1152/ajpregu.1994.266.3.R688. [DOI] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain Res. 1994b;639:57–65. doi: 10.1016/0006-8993(94)91764-7. [DOI] [PubMed] [Google Scholar]
- Opp MR, Obal F, Jr, Krueger JM. Interleukin 1 alters rat sleep: temporal and dose-related effects. Am J Physiol. 1991;260:R52–58. doi: 10.1152/ajpregu.1991.260.1.R52. [DOI] [PubMed] [Google Scholar]
- Orem J, Netick A, Dement WC. Breathing during sleep and wakefulness in the cat. Respir Physiol. 1977;30:265–289. doi: 10.1016/0034-5687(77)90035-4. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL. Behavioral phenomenology of sleep (somatic and vegetative) Experientia. 1980;36:6–11. doi: 10.1007/BF02003941. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL, Rabini C. Shivering and panting during sleep. Brain Res. 1967;6:789–791. doi: 10.1016/0006-8993(67)90139-4. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL, Rabini C. Sleep and environmental temperature. Arch Ital Biol. 1970;108:369–387. [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knockout mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. GABA and Glycine. In: Bloom F, Kupfer D, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press, Ltd; New York: 1995. pp. 87–94. [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, Luxen A, Cleeremans A, Maquet P. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20:125–134. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- Petitjean F, Buda C, Janin M, Touret M, Salvert D, Jouvet M, Bobillier P. [Sleep and monoamines: differential radioautography of central neurons after systematic injection of tritiated 5-hydroxytryptophane (5 HTP 3H) or of dihydroxyphenylalanine (DOPA 3H)] C R Acad Sci Hebd Seances Acad Sci D. 1978;287:1135–1139. [PubMed] [Google Scholar]
- Petitjean F, Sakai K, Blondaux C, Jouvet M. Hypersomnia by isthmic lesion in cat. II. Neurophysiological and pharmacological study. Brain Res. 1975;88:439–453. doi: 10.1016/0006-8993(75)90656-3. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol. 2006;498:227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipson EA. Control of breathing during sleep. Am Rev Respir Dis. 1978;118:909–939. doi: 10.1164/arrd.1978.118.5.909. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Acetylcholine release from the cerebral cortex: its role in cortical arousal. Brain Res. 1968;7:378–389. doi: 10.1016/0006-8993(68)90004-8. [DOI] [PubMed] [Google Scholar]
- Pierce ET, Foote WE, Hobson JA. The efferent connection of the nucleus raphe dorsalis. Brain Res. 1976;107:137–144. doi: 10.1016/0006-8993(76)90102-5. [DOI] [PubMed] [Google Scholar]
- Pirch JH. Basal forebrain and frontal cortex neuron responses during visual discrimination in the rat. Brain Res Bull. 1993;31:73–83. doi: 10.1016/0361-9230(93)90013-2. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, Morrison AR. Vestibular influences during sleep. I. Abolition of the rapid eye movements of desynchronized sleep following vestibular lesions. Arch Ital Biol. 1965;103:569–595. [PubMed] [Google Scholar]
- Ponzoni A, Monti JM, Jantos H. The effects of selective activation of the 5-HT3 receptor with m-chlorophenylbiguanide on sleep and wakefulness in the rat. Eur J Pharmacol. 1993;249:259–264. doi: 10.1016/0014-2999(93)90520-r. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Gillette MU. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrochi JJ, Mamelak AN, Binder D, Williams J, Hobson JA. Dose-related suppression of REM sleep and PGO waves by the serotonin-1 agonist eltoprazine. Neuropsychopharmacology. 1993;8:7–13. doi: 10.1038/npp.1993.2. [DOI] [PubMed] [Google Scholar]
- Quattrochi JJ, Mamelak AN, Madison RD, Macklis JD, Hobson JA. Mapping neuronal inputs to REM sleep induction sites with carbachol-fluorescent microspheres. Science. 1989;245:984–986. doi: 10.1126/science.2475910. [DOI] [PubMed] [Google Scholar]
- Radulovacki M. Role of adenosine in sleep in rats. Rev Clin Basic Pharm. 1985;5:327–339. [PubMed] [Google Scholar]
- Radulovacki M, Carley DW. The laboratory rat as a model of sleep-related breathing disorders. In: Carley DW, Radulovacki M, editors. Sleep-Related Breathing Disorders. Basel, Marcel Dekker; New York: 2003. pp. 265–295. [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ram A, Pandey HP, Matsumura H, Kasahara-Orita K, Nakajima T, Takahata R, Satoh S, Terao A, Hayaishi O. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–89. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Ranson SW. Somnolence caused by hypothalamic lesions in the monkey. Arch Neurol Psychiat. 1939;41:1–23. [Google Scholar]
- Rasmussen K, Heym J, Jacobs BL. Activity of serotonin-containing neurons in nucleus centralis superior of freely moving cats. Exp Neurol. 1984;83:302–317. doi: 10.1016/S0014-4886(84)90100-6. [DOI] [PubMed] [Google Scholar]
- Reader TA, Masse P, de Champlain J. The intracortical distribution of norepinephrine, dopamine and serotonin in the cerebral cortex of the cat. Brain Res. 1979;177:499–513. doi: 10.1016/0006-8993(79)90467-0. [DOI] [PubMed] [Google Scholar]
- Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Bartlett D, Jr, Putnam MD. Changes in the respiratory cycle associated with sleep. Respir Physiol. 1976;28:227–238. doi: 10.1016/0034-5687(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. J Neurochem. 2002;82:1561–1565. doi: 10.1046/j.1471-4159.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- Richardson RT, DeLong MR. Context-dependent responses of primate nucleus basalis neurons in a go/no-go task. J Neurosci. 1990;10:2528–2540. doi: 10.1523/JNEUROSCI.10-08-02528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MJ, Calver AR, Filippov AK, Hirst WD, Russell RB, Wood MD, Nasir S, Couve A, Brown DA, Moss SJ, Pangalos MN. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. GABA: the road to neurotransmitter status. In: Olsen R, Venter C, editors. Benzodiazepine/GABA receptors and chloride channels: structural and functional properties. Alan R Liss; New York: 1986. pp. 1–39. [Google Scholar]
- Robinson TE, Vanderwolf CH. Electrical stimulation of the brain stem in freely moving rats: II. Effects on hippocampal and neocortical electrical activity, and relations to behavior. Exp Neurol. 1978;61:485–515. doi: 10.1016/0014-4886(78)90018-3. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Vanderwolf CH, Pappas BA. Are the dorsal noradrenergic bundle projections from the locus coeruleus important for neocortical or hippocampal activation? Brain Res. 1977;138:75–98. doi: 10.1016/0006-8993(77)90785-5. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Caruncho HJ, Lopez-Real A, Wojcik S, Guerra MJ, Labandeira-Garcia JL. Rat brain cholinergic, dopaminergic, noradrenergic and serotonergic neurons express GABAA receptors derived from the alpha3 subunit. Receptors Channels. 2001;7:471–478. [PubMed] [Google Scholar]
- Ropert N, Steriade M. Input-output organization of midbrain reticular core. J Neurophysiol. 1981;46:17–31. doi: 10.1152/jn.1981.46.1.17. [DOI] [PubMed] [Google Scholar]
- Rusak B. Neural mechanisms for entrainment and generation of mammalian circadian rhythms. Fed Proc. 1979;38:2589–2595. [PubMed] [Google Scholar]
- Russier M, Kopysova IL, Ankri N, Ferrand N, Debanne D. GABA and glycine co-release optimizes functional inhibition in rat brainstem motoneurons in vitro. J Physiol. 2002;541:123–137. doi: 10.1113/jphysiol.2001.016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- Rye DB, Saper CB, Lee HJ, Wainer BH. Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol. 1987;259:483–528. doi: 10.1002/cne.902590403. [DOI] [PubMed] [Google Scholar]
- Saito H, Sakai K, Jouvet M. Discharge patterns of the nucleus parabrachialis lateralis neurons of the cat during sleep and waking. Brain Res. 1977;134:59–72. doi: 10.1016/0006-8993(77)90925-8. [DOI] [PubMed] [Google Scholar]
- Sakai K. Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In: Hobson JA, Brazier MB, editors. The Reticular Formation Revisited. Raven Press; New York: 1980. pp. 427–447. [Google Scholar]
- Sakai K, Crochet S. Serotonergic dorsal raphe neurons cease firing by disfacilitation during paradoxical sleep. Neuroreport. 2000;11:3237–3241. doi: 10.1097/00001756-200009280-00037. [DOI] [PubMed] [Google Scholar]
- Sakai K, Crochet S. Role of dorsal raphe neurons in paradoxical sleep generation in the cat: no evidence for a serotonergic mechanism. Eur J Neurosci. 2001;13:103–112. [PubMed] [Google Scholar]
- Sakai K, Crochet S. Role of noradrenergic locus coeruleus neurons in behavioral state control: no evidence for an inhibitory control of paradoxical sleep generation in the cat. J Sleep Res. 2002;11:199. [Google Scholar]
- Sakai K, Crochet S. A neural mechanism of sleep and wakefulness. Sleep and Biological Rhythms. 2003;1:29–42. [Google Scholar]
- Sakai K, Jouvet M. Brain stem PGO-on cells projecting directly to the cat dorsal lateral geniculate nucleus. Brain Res. 1980;194:500–505. doi: 10.1016/0006-8993(80)91231-7. [DOI] [PubMed] [Google Scholar]
- Sakai K, Sastre JP, Kanamori N, Jouvet M. State-specific neurons in the ponto-medullary reticular formation with special reference to the postural atonia during paradoxical sleep in the cat. In: Pompeiano O, Ajmone-Marsan C, editors. Brain Mechanisms and Perceptual Awareness. Raven Press; New York: 1981. pp. 405–429. [Google Scholar]
- Sakai K, Vanni-Mercier G, Jouvet M. Evidence for the presence of PS-OFF neurons in the ventromedial medulla oblongata of freely moving cats. Exp Brain Res. 1983;49:311–314. doi: 10.1007/BF00238591. [DOI] [PubMed] [Google Scholar]
- Sakai K, Yoshimoto Y, Luppi PH, Fort P, El Mansari M, Salvert D, Jouvet M. Lower brainstem afferents to the cat posterior hypothalamus: a double-labeling study. Brain Res Bull. 1990;24:437–455. doi: 10.1016/0361-9230(90)90098-k. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Morrison AR, Mann GL, Harris JS, Yoo L, Ross RJ. Sleep patterning and behaviour in cats with pontine lesions creating REM without atonia. J Sleep Res. 1994;3:233–240. doi: 10.1111/j.1365-2869.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: toward a unifying hypothesis. Brain Res Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Sastre JP, Buda C, Kitahama K, Jouvet M. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience. 1996;74:415–426. doi: 10.1016/0306-4522(96)00190-x. [DOI] [PubMed] [Google Scholar]
- Sastre JP, Jouvet M. [Oneiric behavior in cats] Physiol Behav. 1979;22:979–989. doi: 10.1016/0031-9384(79)90344-5. [DOI] [PubMed] [Google Scholar]
- Sastre JP, Sakai K, Jouvet M. Bilateral lesions of the dorsolateral pontine tegmentum, II. Effects on muscle atonia. Sleep Res. 1978;7:44. [Google Scholar]
- Sastre JP, Sakai K, Jouvet M. Are the gigantocellular tegmental field neurons responsible for paradoxical sleep? Brain Res. 1981;229:147–161. doi: 10.1016/0006-8993(81)90752-6. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Distribution of central cholinergic neurons in the baboon (Papio papio). I. General morphology. J Comp Neurol. 1985a;236:197–214. doi: 10.1002/cne.902360205. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Distribution of central cholinergic neurons in the baboon (Papio papio). II. A topographic atlas correlated with catecholamine neurons. J Comp Neurol. 1985b;236:215–233. doi: 10.1002/cne.902360206. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- Schussler P, Yassouridis A, Uhr M, Kluge M, Weikel J, Holsboer F, Steiger A. Growth hormone-releasing hormone and corticotropin-releasing hormone enhance non-rapid-eye-movement sleep after sleep deprivation. Am J Physiol Endocrinol Metab. 2006;291:E549–556. doi: 10.1152/ajpendo.00641.2005. [DOI] [PubMed] [Google Scholar]
- Schwierin B, Borbely AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Semba K. Aminergic and cholinergic afferents to REM sleep induction regions of the pontine reticular formation in the rat. J Comp Neurol. 1993;330:543–556. doi: 10.1002/cne.903300410. [DOI] [PubMed] [Google Scholar]
- Semba K, Fibiger HC. Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 1992;323:387–410. doi: 10.1002/cne.903230307. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Armstrong DM, Gillin JC. Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study. Neurosci Lett. 1988;95:19–23. doi: 10.1016/0304-3940(88)90625-8. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Fishbein W. Continuous pontine cholinergic microinfusion via mini-pump induces sustained alterations in rapid eye movement (REM) sleep. Pharmacol Biochem Behav. 1986;25:1253–1261. doi: 10.1016/0091-3057(86)90120-6. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Kilduff TS, Bloom FE, McCarley RW. Cholinergically induced REM sleep triggers Fos-like immunoreactivity in dorsolateral pontine regions associated with REM sleep. Brain Res. 1992;580:351–357. doi: 10.1016/0006-8993(92)90968-f. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, McGinty DJ. Pontine neuronal response to local cholinergic infusion: relation to REM sleep. Brain Res. 1986;386:20–31. doi: 10.1016/0006-8993(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253:R142–149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Shute CC, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Harper RM. Discharge of neurons in the parabrachial pons related to the cardiac cycle: changes during different sleep-waking states. Brain Res. 1980;199:385–399. doi: 10.1016/0006-8993(80)90696-4. [DOI] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS. Behavioral organization of reticular formation: studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements. J Neurophysiol. 1983;50:696–716. doi: 10.1152/jn.1983.50.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RP, Gershon MD, Brooks DC. The role of the raphe nuclei in the regulation of ponto-geniculo-occipital wave activity. Brain Res. 1973;58:313–330. doi: 10.1016/0006-8993(73)90004-8. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Nistri A. GABA receptor mechanisms in the central nervous system. Prog Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- Siwek DF, Pandya DN. Prefrontal projections to the mediodorsal nucleus of the thalamus in the rhesus monkey. J Comp Neurol. 1991;312:509–524. doi: 10.1002/cne.903120403. [DOI] [PubMed] [Google Scholar]
- Skinner JE. Electrocortical desynchronization during functional blockade of the mesencephalic reticular formation. Brain Res. 1970;22:254–258. doi: 10.1016/0006-8993(70)90011-9. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Garcia-Rill E. The mesencephalic locomotor region (MLR) in the rat. Brain Res. 1984;323:385–389. doi: 10.1016/0006-8993(84)90319-6. [DOI] [PubMed] [Google Scholar]
- Smith AD, Olson RJ, Justice JB., Jr Quantitative microdialysis of dopamine in the striatum: effect of circadian variation. J Neurosci Methods. 1992;44:33–41. doi: 10.1016/0165-0270(92)90111-p. [DOI] [PubMed] [Google Scholar]
- Snyder F, Hobson JA, Morrison DF, Goldfrank F. Changes in Respiration, Heart Rate, and Systolic Blood Pressure in Human Sleep. J Appl Physiol. 1964;19:417–422. doi: 10.1152/jappl.1964.19.3.417. [DOI] [PubMed] [Google Scholar]
- Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RF, Wenthold RJ, Baker R. Evidence for glycine as an inhibitory neurotransmitter of vestibular, reticular, and prepositus hypoglossi neurons that project to the cat abducens nucleus. J Neurosci. 1989;9:2718–2736. doi: 10.1523/JNEUROSCI.09-08-02718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng LF, Johnson LC, Lubin A. Autonomic correlates of eye movement bursts during stage REM sleep. Psychophysiology. 1968;4:311–323. doi: 10.1111/j.1469-8986.1968.tb02773.x. [DOI] [PubMed] [Google Scholar]
- Spriggs DR, Deutsch S, Kufe DW. Genomic structure, induction, and production of TNF-alpha. Immunol Ser. 1992;56:3–34. [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Srividya R, Mallick HN, Kumar VM. Differences in the effects of medial and lateral preoptic lesions on thermoregulation and sleep in rats. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F. Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology. 1992;56:566–573. doi: 10.1159/000126275. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW, Nieuwenhuys R. The raohe nuclei of the rat brainstem: A cytoarchitectonic and immuohistochemical study. In: PC E, editor. Chemical Neuroanatomy. Raven Press; New York: 1983. pp. 131–207. [Google Scholar]
- Steinbusch HW, van der Kooy D, Verhofstad AA, Pellegrino A. Serotonergic and non-serotonergic projections from the nucleus raphe dorsalis to the caudate-putamen complex in the rat, studied by a combined immunofluorescence and fluorescent retrograde axonal labeling technique. Neurosci Lett. 1980;19:137–142. doi: 10.1016/0304-3940(80)90184-6. [DOI] [PubMed] [Google Scholar]
- Steinfels GF, Heym J, Strecker RE, Jacobs BL. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983;258:217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- Steininger TL, Rye DB, Wainer BH. Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol. 1992;321:515–543. doi: 10.1002/cne.903210403. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6:2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brainstem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990b;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. Plenum Press; New York: 1990. [Google Scholar]
- Steriade M, Oakson G, Ropert N. Firing rates and patterns of midbrain reticular neurons during steady and transitional states of the sleep-waking cycle. Exp Brain Res. 1982;46:37–51. doi: 10.1007/BF00238096. [DOI] [PubMed] [Google Scholar]
- Steriade M, Pare D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990a;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Sakai K, Jouvet M. Bulbo-thalamic neurons related to thalamocortical activation processes during paradoxical sleep. Exp Brain Res. 1984;54:463–475. doi: 10.1007/BF00235472. [DOI] [PubMed] [Google Scholar]
- Stern WC, Forbes WB, Morgane PJ. Absence of ponto-geniculo-occipital (PGO) spikes in rats. Physiol Behav. 1974;12:293–295. doi: 10.1016/0031-9384(74)90186-3. [DOI] [PubMed] [Google Scholar]
- Stern WC, Jalowiec JE, Shabshelowitz H, Morgane PJ. Effects of growth hormone on sleep-waking patterns in cats. Horm Behav. 1975;6:189–196. doi: 10.1016/0018-506x(75)90035-5. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, MacFabe DF, Vanderwolf CH. Cholinergic activation of the electrocorticogram: role of the substantia innominata and effects of atropine and quinuclidinyl benzilate. Brain Res. 1984;322:219–232. doi: 10.1016/0006-8993(84)90112-4. [DOI] [PubMed] [Google Scholar]
- Stipanuk M. Biochemical and physiological aspects of human nutrition. W.B. Saunders Comp; Philidelphia: 2000. [Google Scholar]
- Strecker RE, Morairty S, Thakkar MM, Porkka-Heiskanen T, Basheer R, Dauphin LJ, Rainnie DG, Portas CM, Greene RW, McCarley RW. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav Brain Res. 2000;115:183–204. doi: 10.1016/s0166-4328(00)00258-8. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT. Principles of frontal lobe function. Oxford University Press; New York: 2002. [Google Scholar]
- Sullivan CE. Bilateral carotid body resection in asthma: vulnerability to hypoxic death in sleep. Chest. 1980;78:354. doi: 10.1378/chest.78.2.354. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swett CP, Hobson JA. The effects of posterior hypothalamic lesions on behavioral and electrographic manifestations of sleep and waking in cats. Arch Ital Biol. 1968;106:283–293. [PubMed] [Google Scholar]
- Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-waking discharge of basal forebrain projection neurons in cats. Brain Res Bull. 1989;22:423–430. doi: 10.1016/0361-9230(89)90069-5. [DOI] [PubMed] [Google Scholar]
- Taal W, Holstege JC. GABA and glycine frequently colocalize in terminals on cat spinal motoneurons. Neuroreport. 1994;5:2225–2228. doi: 10.1097/00001756-199411000-00005. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk N, McErlane SA, Soja PJ. State-related inhibition by GABA and glycine of transmission in Clarke’s column. J Neurosci. 2002;22:5777–5788. doi: 10.1523/JNEUROSCI.22-13-05777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett. 1999;457:157–161. doi: 10.1016/s0014-5793(99)01030-3. [DOI] [PubMed] [Google Scholar]
- Tahishi P, Gardi J, Chen Z, Fang J, Krueger JM. Sleep deprivation increases the expression of TNF alpha mRNA and TNF 55kD receptor mRNA in the rat brain. The Physiologist. 1999:42. [Google Scholar]
- Taishi P, Bredow S, Guha-Thakurta N, Obal F, Jr, Krueger JM. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Koyama Y, Kayama Y, Yamamoto M. Effects of orexin on the laterodorsal tegmental neurones. Psychiatry Clin Neurosci. 2002;56:335–336. doi: 10.1046/j.1440-1819.2002.00967.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Sasaki T, Mammoto A, Hotta I, Takaishi K, Imamura H, Nakano K, Kodama A, Takai Y. Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl. Oncogene. 1998;16:3279–3284. doi: 10.1038/sj.onc.1201874. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Fang J, Krueger JM. An anti-tumor necrosis factor antibody suppresses sleep in rats and rabbits. Brain Res. 1995;690:241–244. doi: 10.1016/0006-8993(95)00609-t. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Fang J, Seyer JM, Wang Y, Krueger JM. An interleukin-1 receptor fragment inhibits spontaneous sleep and muramyl dipeptide-induced sleep in rabbits. Am J Physiol. 1996a;271:R101–108. doi: 10.1152/ajpregu.1996.271.1.R101. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Seyer JM, Wang Y, Krueger JM. Inhibition of tumor necrosis factor attenuates physiological sleep in rabbits. Neuroreport. 1996b;7:642–646. doi: 10.1097/00001756-199601310-00063. [DOI] [PubMed] [Google Scholar]
- Terao A, Matsumura H, Yoneda H, Saito M. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport. 1998;9:3791–3796. doi: 10.1097/00001756-199812010-00005. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch Ital Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Phasic but not tonic REM-selective discharge of periaqueductal gray neurons in freely behaving animals: relevance to postulates of GABAergic inhibition of monoaminergic neurons. Brain Res. 2002;945:276–280. doi: 10.1016/s0006-8993(02)02914-1. [DOI] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thompson SM. Modulation of inhibitory synaptic transmission in the hippocampus. Prog Neurobiol. 1994;42:575–609. doi: 10.1016/0301-0082(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacol Biochem Behav. 1991;40:33–40. doi: 10.1016/0091-3057(91)90317-u. [DOI] [PubMed] [Google Scholar]
- Tobler I, Borbely AA, Groos G. The effect of sleep deprivation on sleep in rats with suprachiasmatic lesions. Neurosci Lett. 1983;42:49–54. doi: 10.1016/0304-3940(83)90420-2. [DOI] [PubMed] [Google Scholar]
- Tobler I, Borbely AA, Schwyzer M, Fontana A. Interleukin-1 derived from astrocytes enhances slow wave activity in sleep EEG of the rat. Eur J Pharmacol. 1984;104:191–192. doi: 10.1016/0014-2999(84)90391-1. [DOI] [PubMed] [Google Scholar]
- Toppila J, Alanko L, Asikainen M, Tobler I, Stenberg D, Porkka-Heiskanen T. Sleep deprivation increases somatostatin and growth hormone-releasing hormone messenger RNA in the rat hypothalamus. J Sleep Res. 1997;6:171–178. doi: 10.1046/j.1365-2869.1997.00049.x. [DOI] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- Trampus M, Ferri N, Monopoli A, Ongini E. The dopamine D1 receptor is involved in the regulation of REM sleep in the rat. Eur J Pharmacol. 1991;194:189–194. doi: 10.1016/0014-2999(91)90104-x. [DOI] [PubMed] [Google Scholar]
- Trulson ME. Simultaneous recording of substantia nigra neurons and voltammetric release of dopamine in the caudate of behaving cats. Brain Res Bull. 1985;15:221–223. doi: 10.1016/0361-9230(85)90140-6. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Preussler DW. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83:367–377. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Preussler DW, Howell GA. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci Lett. 1981;26:183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- Tung A, Bluhm B, Mendelson WB. The hypnotic effect of propofol in the medial preoptic area of the rat. Life Sci. 2001;69:855–862. doi: 10.1016/s0024-3205(01)01179-1. [DOI] [PubMed] [Google Scholar]
- Tung A, Mendelson WB. Anesthesia and sleep. Sleep Med Rev. 2004;8:213–225. doi: 10.1016/j.smrv.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- Urade Y, Fujimoto N, Hayaishi O. Purification and characterization of rat brain prostaglandin D synthetase. J Biol Chem. 1985;260:12410–12415. [PubMed] [Google Scholar]
- Urade Y, Fujimoto N, Kaneko T, Konishi A, Mizuno N, Hayaishi O. Postnatal changes in the localization of prostaglandin D synthetase from neurons to oligodendrocytes in the rat brain. J Biol Chem. 1987;262:15132–15136. [PubMed] [Google Scholar]
- Urade Y, Kitahama K, Ohishi H, Kaneko T, Mizuno N, Hayaishi O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci U S A. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Creamer K, Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J Physiol. 2006;573:679–695. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Vautrelle N, Dahan L, Savasta M, Chouvet G. Glutamatergic-receptors blockade does not regularize the slow wave sleep bursty pattern of subthalamic neurons. Eur J Neurosci. 2004;20:392–402. doi: 10.1111/j.1460-9568.2004.03488.x. [DOI] [PubMed] [Google Scholar]
- Ursin R. The effects of 5-hydroxytryptophan and L-tryptophan on wakefulness and sleep patterns in the cat. Brain Res. 1976;106:105–115. doi: 10.1016/0006-8993(76)90076-7. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Jouvet M. Neurones spécifiques de l’éveil dans l’hypothalamus postérieur du Chat. C R Acad Sci III. 1984;298:195–200. [PubMed] [Google Scholar]
- Vanni-Mercier G, Sakai K, Lin JS, Jouvet M. Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol. 1989;127:133–164. [PubMed] [Google Scholar]
- Varga V, Szekely AD, Csillag A, Sharp T, Hajos M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Velazquez-Moctezuma J, Gillin JC, Shiromani PJ. Effect of specific M1, M2 muscarinic receptor agonists on REM sleep generation. Brain Res. 1989;503:128–131. doi: 10.1016/0006-8993(89)91712-5. [DOI] [PubMed] [Google Scholar]
- Velazquez-Moctezuma J, Shalauta M, Gillin JC, Shiromani PJ. Cholinergic antagonists and REM sleep generation. Brain Res. 1991;543:175–179. doi: 10.1016/0006-8993(91)91064-8. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brain stem activation of the hippocampus: a role for the magnocellular reticular formation and the MLF. Electroencephalogr Clin Neurophysiol. 1980;50:48–58. doi: 10.1016/0013-4694(80)90322-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP. An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J Neurophysiol. 1981;46:1140–1159. doi: 10.1152/jn.1981.46.5.1140. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brain stem generation of the hippocampal EEG. Prog Neurobiol. 1982;19:159–186. doi: 10.1016/0301-0082(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brainstem control of the events of REM sleep. Prog Neurobiol. 1984;22:241–288. doi: 10.1016/0301-0082(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Vertes RP. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol. 1991;313:643–668. doi: 10.1002/cne.903130409. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Hippocampal theta rhythm: a tag for short-term memory. Hippocampus. 2005;15:923–935. doi: 10.1002/hipo.20118. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Colom LV, Fortin WJ, Bland BH. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res. 1993;96:419–429. doi: 10.1007/BF00234110. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM. Distribution, quantification, and morphological characteristics of serotonin-immunoreactive cells of the supralemniscal nucleus (B9) and pontomesencephalic reticular formation in the rat. J Comp Neurol. 1997;378:411–424. doi: 10.1002/(sici)1096-9861(19970217)378:3<411::aid-cne8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Viana Di Prisco G. Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cogn Neurosci Rev. 2004;3:173–200. doi: 10.1177/1534582304273594. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Martin GF. Autoradiographic analysis of ascending projections from the pontine and mesencephalic reticular formation and the median raphe nucleus in the rat. J Comp Neurol. 1988;275:511–541. doi: 10.1002/cne.902750404. [DOI] [PubMed] [Google Scholar]
- Vigers GP, Caffes P, Evans RJ, Thompson RC, Eisenberg SP, Brandhuber BJ. X-ray structure of interleukin-1 receptor antagonist at 2.0-A resolution. J Biol Chem. 1994;269:12874–12879. [PubMed] [Google Scholar]
- Villablanca J. Behavioral and polygraphic study of “sleep” and “wakefulness” in chronic decerebrate cats. Electroencephalogr Clin Neurophysiol. 1966;21:562–577. doi: 10.1016/0013-4694(66)90175-1. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Reiner PB. The immunohistochemical localization of choline acetyltransferase in the cat brain. Brain Res Bull. 1987;18:371–415. doi: 10.1016/0361-9230(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Satoh K, Armstrong DM, Fibiger HC. NADPH-diaphorase: a selective histochemical marker for the cholinergic neurons of the pontine reticular formation. Neurosci Lett. 1983;43:31–36. doi: 10.1016/0304-3940(83)90124-6. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Satoh K, Armstrong DM, Panula P, Vale W, Fibiger HC. Neuropeptides and NADPH-diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience. 1986;17:167–182. doi: 10.1016/0306-4522(86)90234-4. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–259. [Google Scholar]
- Walmsley B, Wieniawa-Narkiewicz E, Nicol MJ. Ultrastructural evidence related to presynaptic inhibition of primary muscle afferents in Clarke’s column of the cat. J Neurosci. 1987;7:236–243. doi: 10.1523/JNEUROSCI.07-01-00236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Taverna FA, Huang XP, MacDonald JF, Hampson DR. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993;259:1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- Ward DG, Gunn CG. Locus coeruleus complex: differential modulation of depressor mechanisms. Brain Res. 1976;107:407–411. doi: 10.1016/0006-8993(76)90237-7. [DOI] [PubMed] [Google Scholar]
- Watson AH, Bazzaz AA. GABA and glycine-like immunoreactivity at axoaxonic synapses on 1a muscle afferent terminals in the spinal cord of the rat. J Comp Neurol. 2001;433:335–348. doi: 10.1002/cne.1143. [DOI] [PubMed] [Google Scholar]
- Watts AG. The efferent projections of the suprachiasmatic nucleus: anatomical insights into the control of circadian rhythms. In: Klein DC, et al., editors. Suprachiasmatic Nucleus: the Mind’s Clock. Oxford University Press; New York: 1991. pp. 77–106. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS, Pascoe JP. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. J Neurosci. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Reiner PB. Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro. J Neurosci. 1993;13:3878–3883. doi: 10.1523/JNEUROSCI.13-09-03878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Res. 1990;509:213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]
- Winson J. Interspecies differences in the occurrence of theta. Behav Biol. 1972;7:479–487. doi: 10.1016/s0091-6773(72)80210-4. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Sheppard AC. Localization of GABA(B) receptors in midbrain monoamine containing neurons in the rat. Brain Res Bull. 2001;56:1–5. doi: 10.1016/s0361-9230(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Wisor JP, O’Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik WJ, Fornal C, Radulovacki M. Effect of tryptophan on sleep in the rat. Neuropharmacology. 1980;19:163–167. doi: 10.1016/0028-3908(80)90133-1. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull. 1989;23:519–540. doi: 10.1016/0361-9230(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Harrison JB, Buchwald JS. Cholinergic neurons of the feline pontomesencephalon. II. Ascending anatomical projections. Brain Res. 1990;520:55–72. doi: 10.1016/0006-8993(90)91691-9. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Datta S, Wu M, Alreja M. Hippocampal theta rhythm is reduced by suppression of the H-current in septohippocampal GABAergic neurons. Eur J Neurosci. 2004;19:2299–2309. doi: 10.1111/j.0953-816X.2004.03316.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Ling GM, Marczynski TJ. The Effects of Chemical Stimulation of the Preoptic Region, Nucleus Centralis Medialis, or Brain Stem Reticular Formation with Regard to Sleep and Wakefulness. Recent Adv Biol Psychiatry. 1963;84:9–20. [PubMed] [Google Scholar]
- Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: locus of the sensitive region. Neuroscience. 1990a;39:279–293. doi: 10.1016/0306-4522(90)90267-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA. A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neuroscience. 1990b;39:295–304. doi: 10.1016/0306-4522(90)90268-9. [DOI] [PubMed] [Google Scholar]
- Yamasu K, Shimada Y, Sakaizumi M, Soma G, Mizuno D. Activation of the systemic production of tumor necrosis factor after exposure to acute stress. Eur Cytokine Netw. 1992;3:391–398. [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Mancillas JR, Morales FR, Chase MH. C-fos expression in the pons and medulla of the cat during carbachol-induced active sleep. J Neurosci. 1993;13:2703–2718. doi: 10.1523/JNEUROSCI.13-06-02703.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Peterfi Z, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNFalpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCrea R, Berthoz A, Vidal PP. Morphological and physiological characteristics of inhibitory burst neurons controlling horizontal rapid eye movements in the alert cat. J Neurophysiol. 1982;48:761–784. doi: 10.1152/jn.1982.48.3.761. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986a;243:488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE, Braun A. Afferents to basal forebrain cholinergic projection neurons: an update. Adv Exp Med Biol. 1991;295:43–100. doi: 10.1007/978-1-4757-0145-6_2. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986b;250:282–295. doi: 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Zanchetti A. Brain stem mechanisms of sleep. Anesthesiology. 1967;28:81–99. doi: 10.1097/00000542-196701000-00009. [DOI] [PubMed] [Google Scholar]
- Zardetto-Smith AM, Johnson AK. Chemical topography of efferent projections from the median preoptic nucleus to pontine monoaminergic cell groups in the rat. Neurosci Lett. 1995;199:215–219. doi: 10.1016/0304-3940(95)12003-m. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemlan FP, Behbehani MM, Beckstead RM. Ascending and descending projections from nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis: an autoradiographic and horseradish peroxidase study in the rat. Brain Res. 1984;292:207–220. doi: 10.1016/0006-8993(84)90757-1. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen Z, Taishi P, Obal F, Fang J, Krueger JM. Sleep deprivation increases rat hypothalamic growth hormone-releasing hormone messenger RNA in the rat hypothalamus. American Journal of Physiology. 1999;275:R1755–R1761. doi: 10.1152/ajpregu.1998.275.6.R1755. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


