Abstract
During winter, increased thermoregulatory demands coincide with limited food availability necessitating physiological trade offs among expensive physiological processes resulting in seasonal breeding among small mammals. In the laboratory, short winter-like day lengths induce regression of the reproductive tract, but also enhance many aspects of immune function. It remains unspecified the extent to which bolstered immune responses in short days represent enhanced immune function per se compared to long days or represents energetic disinhibition mediated by the regression of the reproductive tract. Cohabitation of male Siberian hamsters with intact female conspecifics can block short-day reproductive regression. We sought to determine whether female cohabitation could also block the enhanced immune function associated with short days. Adult male Siberian hamsters were housed in long or short day lengths in one of three housing conditions: (1) single-housed, (2) housed with a same sex littermate, or (3) housed with an ovariectomized female. Delayed-type hypersensitivity (DTH) responses were assessed after 8 wk of photoperiod treatment. Housing with an ovariectomized female was not sufficient to block short-day reproductive regression, but prevented short-day enhancement of DTH responses. Housing with a male littermate did not alter reproductive or immune responses in either photoperiod. These data suggest that short day enhancement of immune function is independent of photoperiod-mediated changes in the reproductive system.
Keywords: Seasonality, photoperiodism, social-housing, immune function, Siberian hamsters, delayed-type hypersensitivity, steroid hormones
Introduction
In nontropical habitats, environmental conditions vary in a continuous, but predictable manner across each year (Bronson, 1989). During winter, low ambient temperatures coincide with reduced food availability; this can create an energetic bottleneck for small animals necessitating trade-offs among expensive physiological processes (Goldman and Nelson, 1993). Some small mammals cope with the energetic bottleneck by restricting breeding during spring and early summer when environmental conditions are mild and food availability is high. Additionally, many physiological adaptations associated with the changing seasons require significant time to develop (e.g., reproductive regression or molt). Mechanisms have evolved in animals that monitor photoperiod (day length) to anticipate and prepare for future changes in environmental conditions. In the laboratory, exposure to short day lengths for weeks or months induces involution of the reproductive tract in small mammals, among many other non-reproductive adaptations including enhancement of several components of immune function (Nelson, 2004; Nelson and Demas, 1996).
Short-day enhancement of immune function has been reported for several species of birds, reptiles, as well as large and small mammals. In general, there are two competing explanations for these phenomena: (1) The winter ‘immunoenhancement hypothesis’; animals enhance some aspects of their immune systems as a prophylactic measure to maintain immune defenses at a steady-state despite the heightened stressors and reduced energy availability associated with winter (Sinclair and Lochmiller, 2000), and (2) An alternative hypothesis predicts that maintaining fully functional reproductive and immune systems contemporaneously is impossible due to energetic constraints (Greenman, et al., 2005). This hypothesis predicts that the immune system is suppressed during the breeding season, or energetic investment is shunted away from immunological processes, or both processes occur. The question arises, do stronger short-day immune responses represent an enhancement compared to long-day immune responses; alternatively, is elevated short-day immune activity the result of a release from energetic or neuroendocrine constraints imposed by the reproductive system. The difficulty in testing these two hypotheses is that photoperiod alterations in the reproductive and immune systems occur over similar time scales. Additionally, melatonin the physiological cue that mediates short-day regression of the reproductive system also increases various aspects of immune activity (Demas and Nelson, 1998a).
Our strategy to address this issue is to dissociate the reproductive and immune responses to day length in the same animal. For instance, the addition of melatonin in vitro to cultured lymphocytes increases mitogen-stimulated proliferation (Drazen, et al., 2000a), an effect that cannot be mediated by a dynamic reproductive system as it is no longer present. Additionally, photoperiod-induced alterations in the immune system are independent of sex steroids. Castrated Siberian hamsters (Phodopus sungorus) and deer mice (Peromyscus maniculatus) bearing testosterone implants exhibit immune responses comparable to intact or castrated animals with empty implants (Demas and Nelson, 1998b; Prendergast, et al., 2005). The use of intermediate or perinatal photoperiods can also dissociate the reproductive and immune responses to day length (Prendergast, et al., 2004; Weil, et al., 2006). Also, some individual hamsters do not regress their reproductive tracts in response to short day lengths (Prendergast, et al., 2001), but these individuals display the short-day pattern of immune responses (Drazen, et al., 2000b). Artificially elevating circulating androgens, however, certainly does not fully recapitulate the effects of short day exposure in terms of negative feedback or receptor distribution (Bittman, et al., 2003; Prendergast, et al., 2006; Tetel, et al., 2004), and only alters one component of the short-day phenotype. Further, short-day reproductive non-responders have aberrant circadian properties which could potentially interfere with the reception of a signal imposed by the reproductive system (Prendergast et al., 2001). Taken together, these results suggest that short-day enhancement of immune responses is independent of reproductive state, but these results are not sufficient to test the hypothesis fully. Indeed, photoinduction of reproductive state decreases splenocyte proliferation in castrated European starlings (Sturnus vulgaris,), an effect that is blocked by exogenous melatonin (Bentley, et al., 1998). Additionally, suppression of prolactin release in long days is sufficient to induce the short day pattern of tumor-resistance in deer mice (Nelson and Blom, 1994) and lymphocyte proliferation in domestic steer (Auchtung and Dahl, 2004). These results could suggest that long-day provoked elevated prolactin concentrations could mediate a suppression of certain immune responses in long days (Cincotta, et al., 1995). Thus, although suggestive, these studies do not definitively indicate whether greater immune responses in short days indicate enhancement or disinhibition.
Cohabitation of male Siberian hamsters with intact female conspecifics can override the inhibitory effects of short day lengths on the reproductive system (Hegstrom and Breedlove, 1999). This phenomenon represents an opportunity to directly test the hypothesis that maintaining a ‘reproductive state’ in short days abrogates the short day pattern of immune responses. Male white-footed mice (Peromyscus leucopus) housed with ovariectomized females (or males) attenuate short-day enhancement of delayed-type hypersensitivity reactions, despite no alteration in reproductive morphology or testosterone concentrations (Pyter, et al., 2005). In the present experiment, male Siberian hamsters were housed with ovariectomized females to determine whether overriding reproductive regression in short days would also block short day-increases in DTH responses. The goals of this experiment were: (1) to test the hypothesis that reproductive regression would be blocked by housing with ovariectomized female hamsters, and (2) to determine whether elevated immune responses would occur in short-day hamsters in which suppression of the reproductive tract was blocked by social cues.
Methods
Animals
All procedures were approved by the Ohio State University (OSU) Institutional Laboratory Animal Care and Use Committee and conducted in accordance with National Institutes of Health guidelines. Siberian hamsters (Phodopus sungorus) used in this study were bred in our colony at Ohio State University (OSU) and were derived from a wild caught stock originally trapped by Dr. Katherine Wynne-Edwards, Queens University, Kingston, Ontario, Canada. Animals were housed in standard conditions with ad libitum access to food (Harlan Teklad 8640, Indianapolis, IN) and filtered tap water. Colony rooms were maintained at constant temperature (21° ± 4°C) and humidity (50 ± 10%).
All hamsters were housed with same sex siblings from weaning until ~50 days of age in long-day conditions (16h light/day). Male hamsters at ~50 days of age were randomly assigned to one of three social housing conditions: (1) single-housed (Single[LD n=10; SD n=8]), (2) housed with a male sibling (+ Male [LD n=4; SD n=12]), or (3) housed with a non-sibling ovariectomized female (+ Female [LD n=7; SD n=5]). Hamsters from each group were then either transferred to short-day conditions (8h light/day) or maintained in long days throughout the experiment. All experimental procedures were conducted after 8 weeks in photoperiod treatment. Lights-off occurred at 15:00 h Eastern Standard Time (EST) in both photoperiods. Animals from both photoperiod treatments were run concurrently in separate colony rooms.
Experimental Procedure
Induction of DTH
Hamsters were lightly anesthetized with isoflurane vapors and then a 2 × 3 cm patch was shaved on the dorsum. On consecutive days 25μl of 2,4,dinitro-1-flurobenzene (DNFB; Sigma) in a 0.5% solution (wt/volume) of 4:1 acetone to olive oil was applied to the dorsal skin in the same location. Following sensitization the thickness of both pinnae was assessed with a constant loading dial micrometer (Long Island Indicator Service, Hauppauge, New York). Seven days later hamsters were again anesthetized, pinnae thickness measured, then challenged with 20μl of 0.2% (wt/volume) DNFB in 4:1 acetone to olive oil on the surface of the right pinna. The left pinna was treated with the vehicle solution. Both pinnae were measured every 24h for the next six days by the same investigator (Z.M. Weil). All measurements were made between 13:00 and 15:00 h EST and animals were brought into the procedure room one at a time to minimize any potential stressors.
Following the conclusion of DTH measurements hamsters were weighed and their pelage assessed on a scale of 1 to 5 (1= dark summer coat color, 5= light winter color) (Duncan and Goldman, 1985). Hamsters were then deeply anesthetized with isoflurane vapors, rapidly decapitated, and trunk blood was collected. Reproductive tissues including paired testes, epididymides, and seminal vesicles were excised and weighed. Hamsters that were housed either singly or with a male sibling with testes masses >2 standard deviations higher than the average body mass corrected mass of the singly-housed short day males were considered nonresponsive and were removed from subsequent analyses. One single-housed and one hamster in the + Male group were nonresponsive and excluded. Because previous reports indicate that social housing with a female conspecific can block reproductive response to short days, we did not remove “nonresponders” from the group housed with a female (Hegstrom and Breedlove, 1999; Pyter et al., 2005). Spleens were also removed and weighed.
RIA Procedures
Trunk blood samples were allowed to clot for 30 min; and then centrifuged for 30 min at 3000 rpm, the sera collected and stored in polypropylene microcentrifuge tubes at −70°C (Bilbo, et al., 2002). Serum testosterone concentrations were determined in a single assay using a Diagnostic Systems Laboratories (Webster, TX) 125I double-antibody kit. The intra-assay coefficient of variation was <5%. Serum cortisol concentrations were determined in a single assay using a Diagnostic Systems Laboratories 125I double antibody kit. The intra-assay coefficient of variation was <5%. Both kits have been validated previously for use in this species (Reburn and Wynne-Edwards, 1999).
Statistical Analysis
Body and tissue masses, hormone concentrations and pelage colors were analyzed with a 2-factor ANOVA (photoperiod x housing condition). Tissue masses are corrected for body mass and the estimated marginal means are presented throughout. Following a significant F score multiple comparisons were conducted with Tukey tests. Because one of the goals of this study was to determine if social housing with an ovariectomized female could block the suppressive effects of short days on the reproductive system we also analyzed the effects of housing condition within the short day group (i.e. testing for simple main effects). DTH reactions were analyzed with a 2-factor repeated measures ANOVA with photoperiod and housing condition as within-subject factors and time as a between subject variable. All statistical differences were considered significant if p<0.05.
Results
Somatic and Reproductive Responses
At the conclusion of the study, short day hamsters weighed less than those housed in long days (F1,41=10.66, p<0.01, unpublished data) regardless of housing condition. Additionally, short day hamsters had lighter more winter-like pelage than hamsters housed in long days (F1,45=34.278, p<0.0001, unpublished data), regardless of housing condition.
Exposure to short day lengths reduced testes mass (F1,41=30.64, p<0.0001; fig 1a). Additionally, housing condition altered final testes masses (F2,41=4.496, p<0.05).However, there was no significant interaction between the two variables. Testes masses were not different among the short day hamsters in different housing conditions (p>0.05 (fig 1a). Paired epididymides mass was lower in short-day hamsters as compared to long-day hamsters (F1,41=31.175, p<0.0001; fig 1b); housing condition also altered epididymal mass (F1,41=6.258, p<0.0005). Post hoc analyses indicated that hamsters housed with males had smaller epididymides than hamsters in any other group (p<0.05 for both) The interaction between the two variables was not significant (F1,41=.945, p=0.39). There was also a simple main effect of housing condition in short day hamsters (F2,21=24.58, p<0.0001) such that males housed with females had larger epididymides than hamsters in any other group. Similarly, seminal vesicle mass was also decreased by short day lengths (F1,41=4.08, p<0.05; fig 1c) and altered by housing condition (F2, 41=5.705, p<0.01) such that hamsters housed with females had larger seminal vesicles than any other group (p<0.05 for both). The interaction between photoperiod and housing condition was not significant (F2,41=0.438, p=0.649). Spleen mass was not altered by day length (F1,40=0.231, p=0.634) or housing condition (F2,40=0.017, p=.984).
Figure 1.
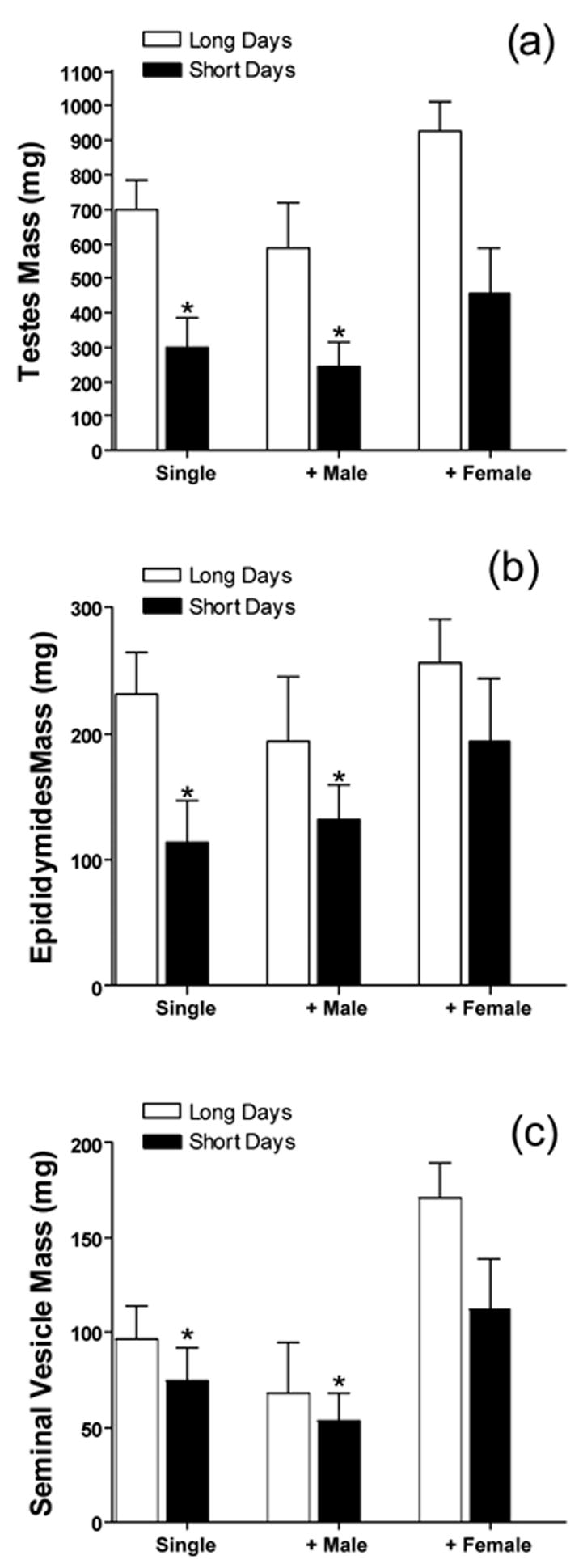
Short days reduce reproductive tissue masses and circulating androgens. Social housing alters these parameters in long, but not short, day lengths. (a) Paired testes mass (mg); (b) paired epididymides mass (mg); (c) seminal vesicle mass (mg) Data are presented as mean (±SEM) and differences are considered statistically significant if p<0.05. * significantly lower than long day hamsters in the same condition.
Hormones
Circulating testosterone was higher in hamsters housed in long day lengths (F1,39=25.53, p<0.0001; fig 2a). Housing conditions also altered testosterone concentrations (F2,39=4.39, p<0.05). Additionally, there was a significant interaction between photoperiod and housing conditions (F2,39=5.14, p<0.05) as housing with a female increased circulating testosterone concentrations in long days (p<0.05), but not short days (ns). Circulating cortisol was not altered by photoperiod (F1,40=0.02, p=0.88; fig 2). However, housing condition did alter cortisol concentrations (F2,40=7.83, p<0.01). The effects of housing on cortisol concentrations did not differ across photoperiod (F2,40=0.63, p=0.53).
Figure 2.
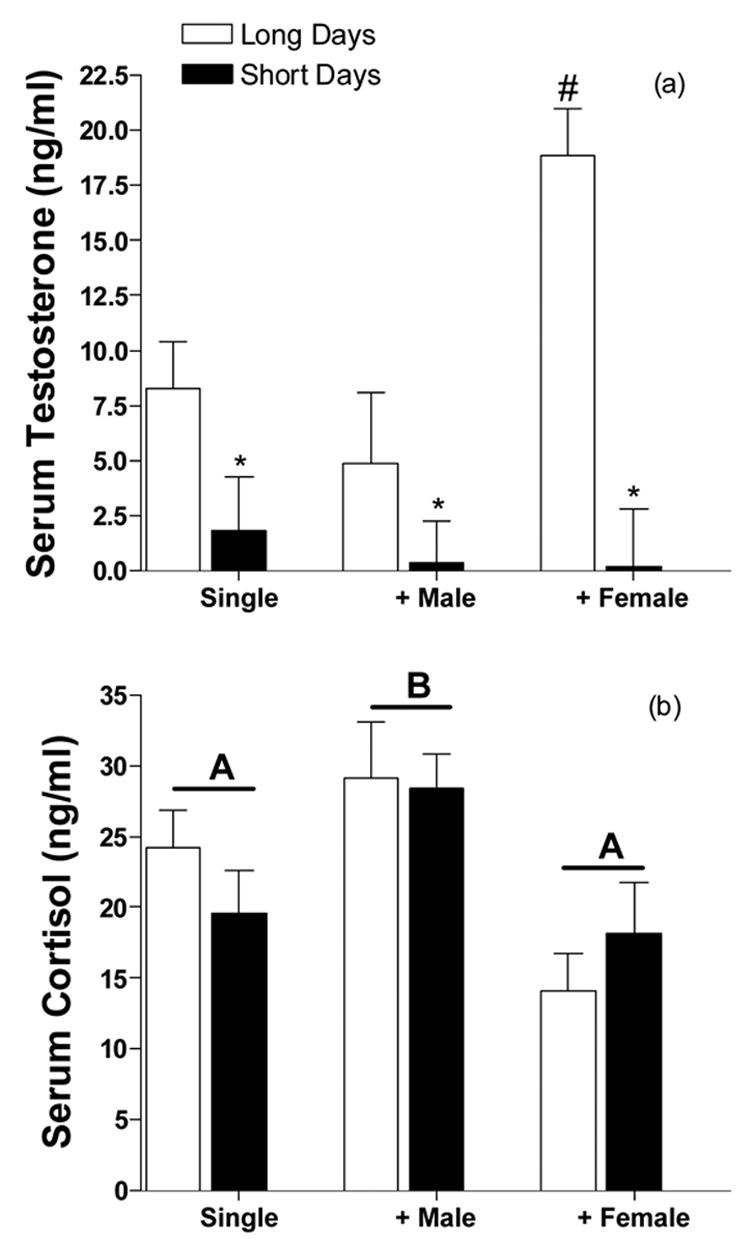
Social housing alters circulating testosterone concentrations (a) and housing with a male conspecific increases circulating cortisol concentrations (b). Data are presented as mean (±SEM) in ng/ml. Groups with different letters are statistically different (p<0.05) from one another. * Significantly lower than long day hamsters in the same condition. # Significantly higher than single-housed hamsters.
Immune responses
DNFB elicited strong swelling responses that peaked 48 h after challenge and declined by 5 days post-challenge (F5,42=21.26, p<0.0001; fig 3). DTH responses were higher in short-day hamsters than hamsters housed in long days (F5, 42=3.26, p<0.05). Housing condition also altered DTH responses (F10,42=2.017, p<0.05). The interaction between the two variables was not significant (F10,42=0.90, p=0.54). Female cohabitation among short-day male hamsters significantly reduced DTH responses relative to all other groups (p<0.05). A similar effect was not apparent in long-day hamsters.
Figure 3.
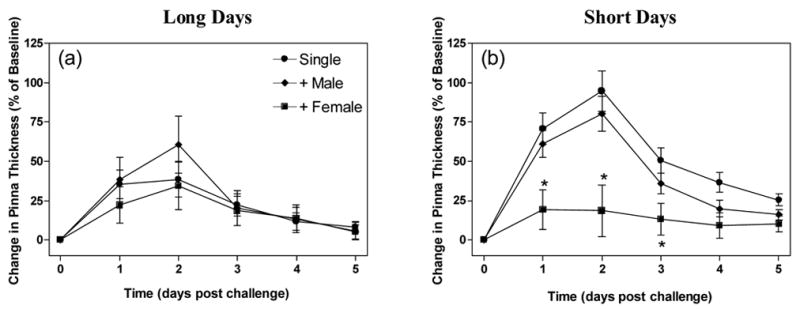
Photoperiod and social housing condition alter delayed-type hypersensitivity responses. Short day hamsters had enhanced DTH responses if housed singly or with a same sex littermate. Data are presented as a percentage of baseline values (mean ±SEM) for long (a) and short day (b) hamsters. * Significantly lower than all other groups in the same photoperiod.
Discussion
Social housing interacted with photoperiod to regulate reproductive and immune responses. Housing with an ovariectomized female increased reproductive tissue masses and circulating androgen concentrations in long-day hamsters. In short days, female cohabitation increased the mass of some male accessory reproductive tissues, but neither paired testes mass nor circulating testosterone concentrations differed from short-day animals in other housing conditions. Despite not overriding the suppressive effects of short photoperiods on the reproductive tract, cohabitation with an ovariectomized female blunted DTH responses in short-day male hamsters.
The goals of this experiment were: (1) to determine whether reproductive regression could be blocked by housing with an ovariectomized female, and (2) to determine whether maintaining reproductive competence was incompatible with the higher DTH responses typically observed in short-day males (Bilbo et al., 2002). Short-day enhancement of cutaneuous immune function was blocked by social housing with an ovariectomized female. Importantly, this effect was not apparent in males housed with same-sex siblings, suggesting that heterosexual cues are necessary for the blocking effect. Further, variation in basal glucocorticoid concentrations cannot explain the attenuated DTH responses in short-day hamsters housed with ovariectomized females as cortisol concentrations were equivalent among single-housed and female-housed males. Finally, short-day males housed with a female had equivalent circulating testosterone and testes size to individuals in other treatment groups. It should be noted that our experimental design only assessed steroid hormone concentrations once at the conclusion of the experiment and so it remains possible that social housing may have altered steroid signaling early in photoperiod treatment.
Taken together, these data indicate that the immune system is responsive to the social environment; however, these results do not support the hypothesis that maintaining a fully functional reproductive system diverts energetic investment from the immune system when food is available ad libitum. In the current experiment, short-day male hamsters housed with females had both fully regressed reproductive systems and blunted immune responses. If the short day pattern of immune responses was simply the result of an energetic disinhibition mediated by regression of the reproductive tract, then social housing should not be sufficient to block the short-day pattern of immune responses in males with regressed gonads. Rather, two alternative hypotheses must be considered. First, the costs of reproductive state could be paid independently of the reproductive tract and steroid hormones. Second, and more likely, the short-day pattern of immune responses represents an enhancement that occurs independently of photoperiodic effects on the reproductive system.
The original Hamilton-Zuk hypothesis (Hamilton and Zuk, 1982) and the subsequent mechanistic version (Folstad and Karter, 1992), termed the immunocompetence handicap hypothesis (ICHH), stated that the maintenance of androgen-dependent ornamentation represented an energetic handicap that impaired host defense. Housing male rodents with females increases circulating androgens. In the current experiment, however, heterosexual cues blocked enhanced immune activity in the absence of effects on the reproductive system and circulating testosterone. Our results are consistent with an androgen-independent reduction in humoral immune function in pair-housed voles (Klein and Nelson, 1999). Additionally, photostimulation into reproductive state altered wing-web swelling responses in house sparrows (Greenman et al., 2005). In our experiment, housing with female, but not male, conspecifics altered short-day patterns of DTH responses, suggesting that this phenomenon is linked to reproductive cues. Additionally, long-day female cohabitation was sufficient to alter reproductive morphology and circulating androgen concentrations, but not immune responses. This is consistent with previous reports in Siberian hamsters (Demas, et al., 2004) and could reflect a floor effect in the DTH response. In any case, if enhanced immune responses represent a disinhibition mediated by regression of the reproductive tract, then increasing the size of that tract should adversely affect immune responses, a result not observed in the present study. Taken together, these results suggest that the ICHH may need to be updated to include the growing list of androgen-independent effects of reproductive cues on the immune system.
In this experiment, housing with an ovariectomized female was not sufficient to block reproductive regression in short days as previously reported with intact females (Hegstrom and Breedlove, 1999). Importantly, the females in the previous study were impregnated and bore litters even in short days (Hegstrom and Breedlove, 1999), a phenomenon we have observed in our lab (Pyter and Nelson, 2006). Intact females were not used in the present experiment to avoid the potential confound of differential rates of pregnancies or litter size variability between photoperiods. Our data would suggest that the salient cue responsible for blocking short day-induced regression in Siberian hamsters is ovary-dependent. However, it is not possible to determine whether the cue is related to the behavior of the stimulus female (e.g., sex behavior) or chemical in nature. Long-day hamsters displayed the expected increase in testis mass and androgen concentrations, indicating that short days block the ability of male hamsters to perceive or respond to the cues associated with the presence of an ovariectomized female.
The suppressive effect of housing with a receptive female on the DTH response appears to be ovary-independent, although the nature of this cue also remains unspecified. Future studies are necessary to investigate the nature of these signals that evoke differential responses in the reproductive and immune systems. These data suggest that stronger immune responses in short days represent an enhancement over those that occur in long days and not an energetic disinhibition mediated by regression of the reproductive tract. Our results are consistent with previous studies that have reported enhanced immune responses in castrated or testosterone-implanted rodents housed in short days (Demas and Nelson, 1998b; Prendergast et al., 2005). The adaptive significance of suppression of the immune system by exposure to a female conspecific remains unspecified and will be the target of future research.
Acknowledgments
We thank Lynn Martin and Stephanie Kidder for helpful comments and discussion of earlier versions of this manuscript. This research was supported by NIH grants MH 57535 and MH 66144 and NSF grant IBN 04-16897.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auchtung TL, Dahl GE. Prolactin mediates photoperiodic immune enhancement: effects of administration of exogenous prolactin on circulating concentrations, receptor expression, and immune function in steers. Biol Reprod. 2004;71(6):1913–8. doi: 10.1095/biolreprod.104.031005. [DOI] [PubMed] [Google Scholar]
- Bentley GE, Demas GE, Nelson RJ, Ball GF. Melatonin, immunity and cost of reproductive state in male European starlings. Proc Biol Sci. 1998;265(1402):1191–5. doi: 10.1098/rspb.1998.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci USA. 2002;99(6):4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL, Ehrlich DA, Ogdahl JL, Jetton AE. Photoperiod and testosterone regulate androgen receptor immunostaining in the Siberian hamster brain. Biol Reprod. 2003;69(3):876–84. doi: 10.1095/biolreprod.102.010900. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- Cincotta AH, Knisely TL, Landry RJ, Miers WR, Gutierrez PJ, Esperanza P, Meier AH. The immunoregulatory effects of prolactin in mice are time of day dependent. Endocrinology. 1995;136(5):2163–71. doi: 10.1210/endo.136.5.7720666. [DOI] [PubMed] [Google Scholar]
- Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of Siberian hamsters. Physiol Behav. 2004;83(1):73–9. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Exogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (Peromyscus maniculatus) J Biol Rhythms. 1998a;13(3):245–52. doi: 10.1177/074873098129000084. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Short-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus) J Comp Physiol [B] 1998b;168(6):419–26. doi: 10.1007/s003600050161. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Klein SL, Yellon SM, Nelson RJ. In vitro melatonin treatment enhances splenocyte proliferation in prairie voles. J Pineal Res. 2000a;28(1):34–40. doi: 10.1034/j.1600-079x.2000.280105.x. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Kriegsfeld LJ, Schneider JE, Nelson RJ. Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am J Physiol Regul Integr Comp Physiol. 2000b;278(6):R1401–7. doi: 10.1152/ajpregu.2000.278.6.R1401. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Physiological doses of prolactin stimulate pelage pigmentation in Djungarian hamster. Am J Physiol. 1985;248(6 Pt 2):R664–7. doi: 10.1152/ajpregu.1985.248.6.R664. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males and the immunocompetence handicap hypothesis. Am Nat. 1992;139(3):603–622. [Google Scholar]
- Goldman BD, Nelson RJ. Melatonin and seasonality in mammals. In: Yu HS, Reiter RJ, editors. Melatonin: Biosynthesis, Physiological Effects, and Clinical Applications. CRC; Boca Raton: 1993. pp. 225–252. [Google Scholar]
- Greenman CG, Martin LB, Hau M. Reproductive state, but not testosterone, reduces immune function in male house sparrows (Passer domesticus) Physiol Biochem Zool. 2005;78(1):60–68. doi: 10.1086/425194. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218(4570):384–7. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- Hegstrom C, Breedlove S. Social cues attenuate photoresponsiveness of the male reproductive system in Siberian hamsters (Phodopus sungorus) J Biol Rhythms. 1999;14(1):54. doi: 10.1177/074873099129000443. [DOI] [PubMed] [Google Scholar]
- Klein SL, Nelson RJ. Social interactions unmask sex differences in humoral immunity in voles. Anim Behav. 1999;57(3):603–610. doi: 10.1006/anbe.1998.1038. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25(4):187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Blom JM. Photoperiodic effects on tumor development and immune function. J Biol Rhythms. 1994;9(3–4):233–49. doi: 10.1177/074873049400900305. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71(4):511. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Dhabhar FS, Nelson RJ. Effects of photoperiod history on immune responses to intermediate day lengths in Siberian hamsters (Phodopus sungorus) J Neuroimmunol. 2004;149(1–2):31–9. doi: 10.1016/j.jneuroim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ. Short day lengths enhance skin immune responses in gonadectomised Siberian hamsters. J Neuroendocrinol. 2005;17(1):18–21. doi: 10.1111/j.1365-2826.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Wen J, Horton TH, Nelson RJ. Refractoriness to short day lengths augments tonic and gonadotrophin-releasing hormone-stimulated lutenising hormone secretion. J Neuroendocrinol. 2006;18(5):339–48. doi: 10.1111/j.1365-2826.2006.01419.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76(3):293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Neigh GN, Nelson RJ. Social environment modulates photoperiodic immune and reproductive responses in adult male white-footed mice (Peromyscus leucopus) Am J Physiol Regul Integr Comp Physiol. 2005;288(4):R891–6. doi: 10.1152/ajpregu.00680.2004. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Nelson RJ. Enduring effects of photoperiod on affective behaviors in Siberian hamsters (Phodopus sungorus) Behav Neurosci. 2006;120(1):125–34. doi: 10.1037/0735-7044.120.1.125. [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35(2):163–76. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Sinclair JA, Lochmiller RL. The winter immunoenhancement hypothesis: associations among immunity, density, and survival in prairie vole (Microtus ochrogaster) populations. Can J Zool. 2000;78(2):254–264. [Google Scholar]
- Tetel MJ, Ungar TC, Hassan B, Bittman EL. Photoperiodic regulation of androgen receptor and steroid receptor coactivator-1 in Siberian hamster brain. Brain Res Mol Brain Res. 2004;131(1–2):79–87. doi: 10.1016/j.molbrainres.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Pyter LM, Martin LB, 2nd, Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am J Physiol. 2006;290(6):R1714–9. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]


