Abstract
A previous study found that intracerebroventricular (ICV) infusion of 25 μg of α-MSH reduced the passive responses (crouched stance, eye-closing, piloerection) of guinea pig pups during a 3-hr isolation in a novel environment. Because α-MSH has broad anti-inflammatory properties, the results suggested that proinflammatory factors play a role in mediating the behavior of isolated infants. The present study further investigated this possibility. In Experiment 1, injection of lipopolysacchride (LPS) increased the number of 60-s intervals in which pups expressed the same three responses during a 1-hr test, and ICV infusion of α-MSH significantly reduced the effect of LPS on crouching and piloerection. In Experiment 2, the prostaglandin synthesis inhibitor indomethacin (10mg/kg) reduced the number of 60-s intervals in which pups exhibited both crouching and the full suite of passive responses during a 3-hr isolation in a novel environment. Together these results provide further support for the hypothesis that the passive behaviors exhibited during prolonged isolation are “stress-induced sickness behaviors” mediated by proinflammatory factors.
Keywords: sickness behaviors, acute phase response, inflammation, maternal separation, isolation, guinea pig
Stimulation of the innate immune system results in a systemic physiological and behavioral reaction known as the acute phase response or sickness (Baumann & Gauldie, 1994). Components of the acute phase response include: fever, shifts in the synthesis of liver proteins, activation of the hypothalamic-pituitary-adrenal system, sleepiness, assumption of a curled or hunched posture, piloerection, shivering, and reductions in various activities. Though the behavioral components may appear to be simply the result of debilitation, they are adaptive responses that contribute to recuperation by, for instance, supporting the production of fever (e.g., hunched posture, piloerection, shivering), which in turn, can inhibit further proliferation of pathogens (Aubert, 1999; Hart, 1988). The acute phase response is triggered by the release of proinflammatory cytokines (e.g., IL-1, IL-6, TNF-α) in the periphery and CNS (Dantzer, 2004).
In the last decade, it has become apparent that aspects of the acute phase response, including sickness behaviors, do not occur solely during the course of infection, but rather are sometimes observed during times of environmental challenge or duress (Maier & Watkins, 1998). This “stress-induced sickness” also appears to be mediated by proinflammatory factors, notably the action of cytokines in the CNS. For instance, behavioral and physiological signs of sickness induced by stressors such as electric shock or exposure to a novel environment can be blocked by central administration of anti-inflammatory agents (Maier & Watkins, 1995; Milligan, Nguyen Deak, Hinde, Fleshner, Watkins, & Maier, 1998; Pugh, Nguyen, Gonyea, Fleshner, Watkins, Maier, & Rudy, 1999).
Observations such as these prompted us to consider whether certain behaviors that infants of some species exhibit during maternal separation might also constitute stress-induced sickness behaviors. In some primates, including humans, conditions of separation from the mother can produce a two-stage, active/passive response (Bowlby, 1973; Kaufman & Rosenblum, 1967; Mineka & Suomi, 1978; Spitz, 1946). During the initial so-called “protest” phase, infants vocalize at high rates and often exhibit increased locomotor activity. This reaction is followed within days or weeks in a subgroup of infants by a second, passive stage of responsiveness traditionally referred to as “despair”. This second stage is characterized by inactivity and passive behaviors/postures that suggested sadness or depression to original investigators (Kaufman & Rosenblum, 1967; Spitz, 1946).
When guinea pig pups are isolated in an unfamiliar cage, they too exhibit a two-stage, active/passive response (Hennessy, Long, Nigh, Williams, & Nolan, 1995), though one greatly compressed in time relative to that of primates. Initially the pups repeatedly emit a whistle vocalization and often move about the enclosure, behaviors that seem adapted for re-establishing contact with the mother. However, these behaviors subside after 60 min or so, and the pups exhibit gradually increasing levels of three passive responses: a distinctive crouched stance, prolonged closure of the eyes, and extensive piloerection. These responses become prominent by the third hour of isolation. The term “despair” seems inappropriate for describing the behavior of precocial guinea pig pups during a several-hour exposure to a new cage. Instead, we hypothesized that these passive responses of guinea pigs, and perhaps those of primates in the so-called despair stage, may represent sickness behaviors induced by the stressor of the separation procedures (Hennessy, Deak, & Schiml-Webb, 2001).
In support of this hypothesis, an injection of lipopolysacchride (LPS), which is a product of the cell wall of gram negative bacteria, and which produces a vigorous acute phase response, results in high levels of the same three behaviors. Specifically, when guinea pig pups were injected with 50-250 μg/kg LPS, returned to the home cage for 90 min, and then placed into an empty cage for 60 min, they spent substantial, dose-dependent portions of the isolation engaged in crouching, eye-closing, and piloerection. In contrast, vehicle-treated controls showed little passive behavior during this relatively short isolation (Hennessy, Deak, Schiml-Webb, Wilson, Greenlee, & McCall, 2004).
Further, it was found that intercerebroventricular (ICV) infusion of α-MSH significantly reduced the passive behavior of otherwise untreated pups during a more prolonged (3-hr) isolation (Schiml-Webb, Deak, Greenlee, Maken, & Hennessy, 2006). Because α-MSH is a peptide with a broad range of anti-inflammatory effects (Lipton, Zhao, Ichiyama, Barsh, & Catania, 1999; Luger, Scholzen, Brzoska, & Bohm, 2003), this finding supports the notion that the passive responses are sickness behaviors mediated by proinflammatory factors. Nonetheless, it remains possible that the ICV α-MSH treatment reduced the passive responses by some means other than its anti-inflammatory activity. α-MSH is an endogenous peptide that plays a role in appetite, anxiety, and other motivated responses (Gonzalez, Vaziri, & Wilson, 1996; Rao, Kokare, Sarkar, Khisti, Chopde, & Subhedar, 2003). While what is known about these other actions of α-MSH are not easily reconciled with the behavioral effects we observed, we cannot rule out an influence of our ICV infusion of α-MSH on motivational systems independent of inflammatory action. Therefore, the goal of the first experiment was to determine if the dose of ICV α-MSH used by Schiml-Webb et al (2006) would reduce the passive responses when they were unequivocal sickness behaviors (i.e., produced by injection of LPS) in our guinea pig model, thereby substantiating the conclusions drawn in our previous work. The goal of Experiment 2 was to further test the hypothesis that passive behaviors observed during maternal separation of guinea pig pups represent stress-induced sickness behaviors by examining whether another anti-inflammatory agent, the cyclooxygenase inhibitor indomethacin, would also attenuate the passive responses of pups during protracted isolation.
General Methods
Subjects
Albino guinea pigs (Cavia porcellus) of the Hartley strain were bred in our laboratory. Each lactating female and her litter were housed in a plastic cage (73 × 54 × 24 cm) with sawdust bedding. Water and guinea pig chow were freely available. The colony room was maintained on a 12-hr light-dark cycle (lights on at 0700). The day of birth was designated Day 0. All procedures were approved by the Wright State University Laboratory Animal Care and Use Committee.
Test procedures
For testing, the guinea pig was transported (< 10 s) in a carrying cage from the colony room to the adjoining test room. Here the subject was placed into a clear, empty, uncovered plastic cage (47 × 24 × 20 cm) located on a table under full room lighting. The cage was cleaned with detergent before each use. A trained observer (inter-observer reliability of at least 85%) behind one-way glass scored the number of 60-s intervals in which the pup displayed the crouched stance (body hunched down with head lowered and feet tucked beneath), eye-closing (more than 1 s of sustained complete or near complete closure of one or both eyes), and extensive piloerection (occurring over most of the visible body surface). In addition to these passive responses, the active behaviors of vocalizing and locomotor activity were assessed by recording the number of “whistle” vocalizations (Berryman, 1976) and the number of times that pups crossed lines dividing the cage floor into four, equal-sized segments. Vocalizations were recorded with a hand counter; the other measures were scored with paper and pencil.
Data analysis
For passive behaviors, we analyzed the number of 60-s intervals in which each separate response was observed as well as a “full passive response” score, which consisted of the number of intervals in which pups displayed the full suite of all three passive responses. For many measures there were large numbers of zero scores. Therefore, normality was tested using the Shapiro-Wilks test. When measures within a set of comparisons (e.g., effects of LPS on passive behaviors) violated normality (p < 0.01), the set was analyzed using nonparametric tests (Mann-Whitney U tests for between-group comparisons and Wilcoxon matched-pairs, signed-ranks tests for within-group comparisons). When assumptions were met, data were analyzed with parametric analysis (t-tests for comparison of pairs of means). In the case of parametric analyses, data are depicted as means and standard errors. When nonparametric tests were required, data are presented as medians and semi-interquartile ranges. In each experiment, the number of males and females assigned to different groups was balanced. Preliminary Mann-Whitney U tests for each behavioral measure within each condition yielded no significant differences for either experiment. Therefore, sex differences will not be considered further. A significance value of 0.05 (2-tailed) was used to assess differences between groups.
Experiment 1
Experiment 1 examined whether ICV administration of 25 μg of α-MSH would reduce the sickness responses induced by injection of LPS. Rapid adaptation to repeated LPS administration is a concern (e.g., Soszynski, 2002); therefore, the experiment was designed so that all pups were injected once with LPS and once with vehicle, and separate groups were infused ICV on both occasions with either α-MSH or vehicle.
Method
Twelve pups were assigned to each of two groups: an α-MSH group and an artificial cerebrospinal fluid (a-CSF) vehicle control group. Ten and 11 separate litters contributed pups to these groups, respectively. Following the procedures of Schiml-Webb et al (2006), pups 16-19 days of age underwent surgery for placement of a cannula aimed at the right lateral ventricle under isoflurane anesthesia, following pretreatment with atropine (0.05 mg/kg). Bupivicaine (0.05 mg/kg) was administered subcutaneously to the scalp prior to surgery. A 26-gauge guide cannula was implanted into the right lateral ventricle (AP: −3.0, L: −3.0, DV: −4.0 mm, relative to Bregma) and secured to the skull with cranioplastic cement. A threaded obturator was inserted into the guide cannula. Buprenorphine (0.015 mg) was given intramuscularly 2 and 12 hr post surgery.
Pups were tested twice: once on Day 21-23 and a second time on Day 24-26, with a minimum of 3 days between tests. For each test, the pup received an ICV infusion and was returned to the home cage for 60 min. At this time, it was administered an intraperitoneal (IP) injection and returned to the home cage for another 90 min. The pup was then tested for 60 min, with behavior observed for the full test period as in our earlier study of LPS (Hennessy et al., 2004). For one group, 25 μg of α-MSH (Sigma) in a volume of 5 μl was infused at a rate of 2 μl/min on both test days. For the other group, infusion on both days was of the same volume of a-CSF. For one test, each pup received an IP injection of 200 μg/kg LPS ( E. coli, serotype 0111:B4; Sigma) at a concentration of 200 μg/ml. For the other test, the pup received an equal volume/bw dose of the saline vehicle. The order in which the LPS or vehicle were administered was counterbalanced across pups in each of the two experimental groups. All behavior tests began between 1000 and 1300 hr.
Results
Passive behaviors
As expected, LPS significantly increased the number of 1-min intervals in which each of the four measures of passive response was observed (all Wilcoxon Ts ≤ 11.0, non-tied ns = 21-24, all ps < 0.001; Fig. 1). Low levels of passive responses were observed in the control condition in which pups were injected with saline vehicle. α-MSH had no effect on the passive responses in the control condition. To determine if the effect of LPS specifically was mitigated by α-MSH, the level of each passive behavior measure obtained for each pup when injected with saline was subtracted from the level for that pup when injected with LPS. Comparison of groups infused with α-MSH and a-CSF vehicle showed that α-MSH significantly reduced the effect of LPS on crouching and piloerection [crouch: t (22) = 2.40, p < 0.05; piloerection, t (22) = 2.52, p < 0.05; Fig. 2).
Figure 1.
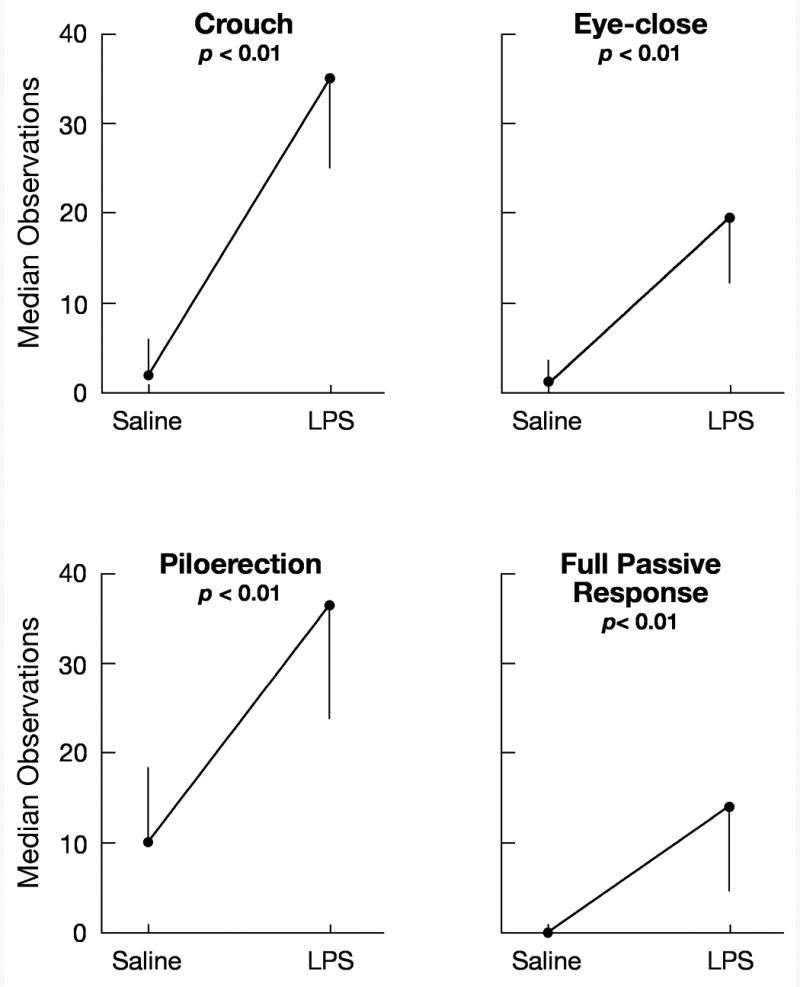
Median number of 60-s intervals in which pups exhibited the four measures of passive response when injected with either saline vehicle or LPS in Experiment 1. Behavior was scored for Min 0-60. The vertical lines indicate the semi-interquartile ranges.
Figure 2.
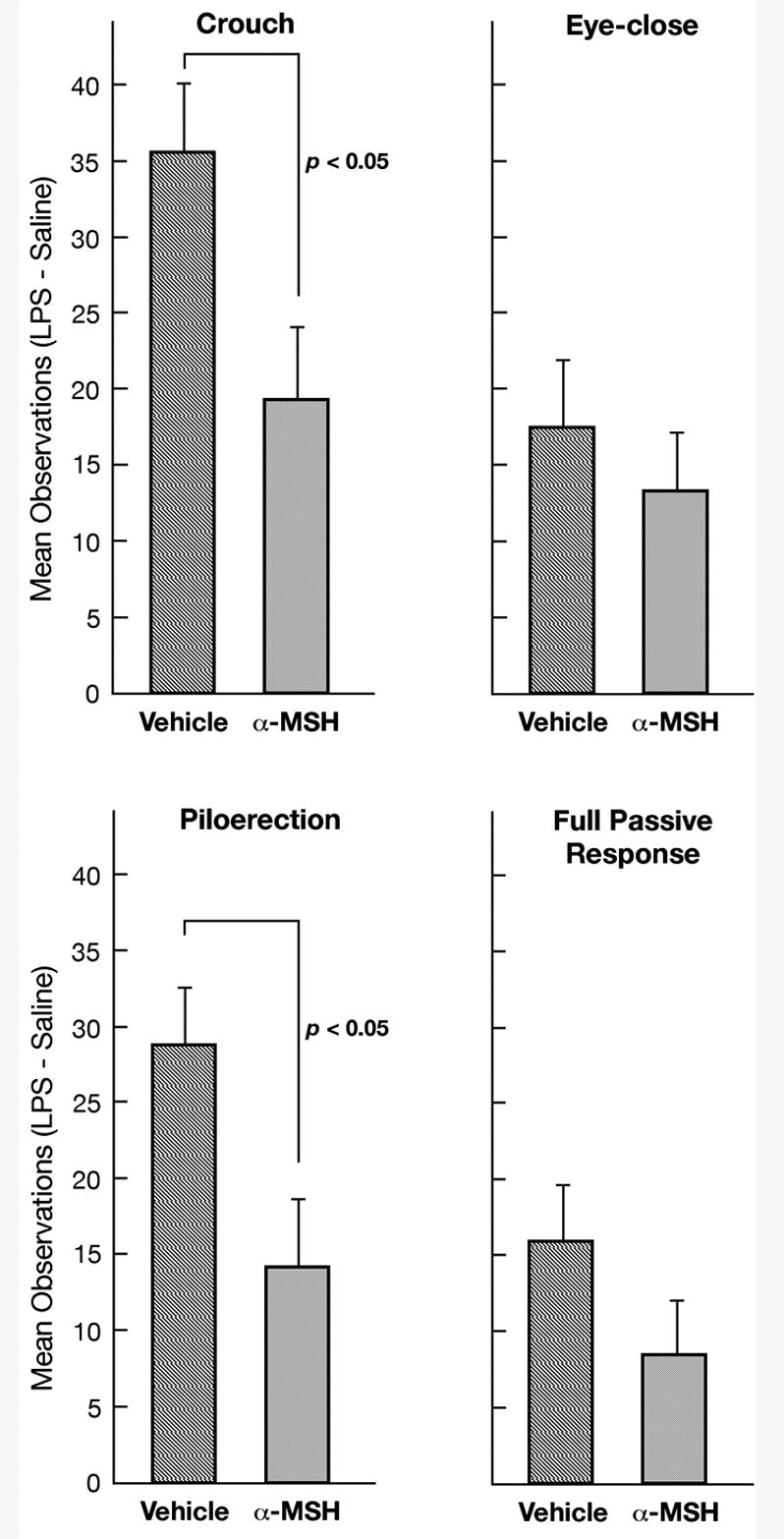
The effect of α-MSH on the change in passive behavior produced by LPS injection in Experiment 1. Shown are the mean number of 60-s intervals in which pups exhibited each of the four measures of passive response when injected with LPS minus the number when injected with saline both for pups infused with a-CSF vehicle and for pups infused with α-MSH. Behavior was scored for Min 0-60. The vertical lines indicate the standard errors of the means.
Active behaviors
LPS greatly reduced the number of vocalizations and line-crossings exhibited by pups (vocalizations: T = 10.0, n = 22, p < 0.001; line-crossings: T = 26, n = 23, p = 0.001). During tests with IP saline, relatively high levels of active behaviors were observed in the a-CSF group. Infusion with α-MSH significantly reduced vocalizing (U = 34, p < 0.05) and, to a marginally significant degree, reduced line-crossing (U = 39, p = 0.06) during the IP saline tests, though the effect was quite variable among pups. For instance, five of the α-MSH-infused pups emitted between 1,495 and 4,000 vocalizations in the 1-hr period, while another four pups emitted between 0 and 4 such calls (Table 1). To evaluate whether the effect of LPS on active behaviors was influenced by α-MSH, the difference in behavior when injected with LPS and saline was determined for each pup. Comparisons of groups infused with α-MSH and those infused with a-CSF fell short of an acceptable level of significance for both active behaviors (vocalizations: U = 42, p > 0.08; line-crossings: U = 41, p > 0.07), though as indicated in Table 1, there was a clear statistical tendency for the difference scores to be smaller among α-MSH-infused pups.
Table 1.
Median number of vocalizations and line-crossings in the control condition (saline) and median change in vocalizations and line-crossings due to LPS (saline - LPS) for pups infused with a-CSF vehicle and those infused with α-MSH. Numbers in parentheses are the semi-interquartile ranges.
| a-CSF | α-MSH | |
|---|---|---|
| Saline | ||
| Vocalization | 2,399.0 (1343.7)* | 198.5 (1,352.0) |
| Line-crossing | 152.0 (92.1) # | 15.5 (54.4) |
| Saline-LPS | ||
| Vocalization | 2,279.0 (1,218.4)# | 198.5 (1,341.5) |
| Line-crossing | 137.5 (72.2) # | 13.5 (56.9) |
p < 0.05 vs α-MSH.
p < 0.10 vs α-MSH
Experiment 2
The primary result of Experiment 1 was that the ICV dose of α-MSH used by Schiml-Webb et al (2006) to reduce the passive responses of guinea pig pups during prolonged isolation also attenuated unequivocal sickness behavior in guinea pig pups. This finding corroborates the conclusion of the earlier study that the passive behaviors observed during prolonged isolation are mediated by proinflammatory factors. To further evaluate this possibility, Experiment 2 examined whether the anti-inflammatory compound indomethacin, which disrupts production of prostaglandins, would reduce the passive responses of guinea pig pups during prolonged isolation. A variety of sickness behaviors are known to be mediated by prostaglandins, and indomethacin has been found to inhibit fever in guinea pigs and sickness behaviors in other species (Blatteis, Quan, Xin, & Ungar, 1990; Johnson et al., 1993; Yirmiya et al., 1997). Unlike α-MSH, indomethacin is not an endogenous compound and, short of toxic effect, is not known to produce behavioral change by means other than its anti-inflammatory action.
Method
Twelve pups, 20-23 days of age, were assigned to each of three conditions: non-treated, vehicle, and indomethacin. No more than one pup from a litter was assigned to a single condition. Pups in the non-treated condition received no injection prior to behavioral testing. Those in the vehicle condition were injected with sodium bicarbonate vehicle (5 ml/kg). Pups in the indomethacin condition received a 10mg/kg dose of indomethacin (Sigma) in an equal volume of vehicle. This dose is in the range frequently found to reverse physiological and behavioral signs of sickness in laboratory rodents, including guinea pigs (Blatteis et al., 1990; Buller, Xu, & Day, 1998; Crestani, Seguy, & Dantzer, 1991; Dunn & Swiergiel, 2000; Sehic, Szekely, Ungar, Oladehin, & Blatteis, 1996; Wieczorek & Dunn, 2005). All injections were made subcutaneously in the nape of the neck 30 min prior to the behavioral test, which consisted of a 3-hr isolation in the standard novel test cage. Due to the longer isolation period in Experiment 2, behavior was sampled for three, 30-min periods at 60-min intervals (i.e., observations at Min 0-30, 60-90, and 150-180), as in previous studies (Hennessy et al., 2004; Schiml-Webb et al., 2006). All tests began between 1200 and 1500 hr.
Results
Passive behaviors
Preliminary Mann-Whitney U tests showed that the non-treated and vehicle control groups did not differ for any measure of passive behavior. Their data, therefore, were combined into a single control group for comparison with pups injected with indomethacin. Significant effects were found for two measures: crouch and full passive response. Injection of indomethacin reduced both behaviors (crouch: U = 33.5, p < 0.05; full response: U = 36.5, p < 0.05, Fig. 3).
Figure 3.

Median number of 60-s intervals in which control (CON) pups and pups injected with 10mg/kg indomethacin (INDO) exhibited the four measures of passive response in Experiment 2. Observations were for Min 0-30, 60-90, and 150-180. SIR indicates the semi-interquartile ranges.
Active behaviors
As for the passive behaviors, the level of active behavior in the nontreated and vehicle groups did not differ, so they were combined into a single control group. Mann-Whitney U tests revealed no significant difference between controls and indomethacin-treated pups for either vocalizing or line-crossing (Table 2).
Table 2.
Median number of vocalizations and line-crossings of pups in the combined control condition (N + V conditions) and for pups receiving 10mg/kg indomethacin (I). Numbers in parentheses are the semi-interquartile ranges.
| Control | Indomethacin | |
|---|---|---|
| Vocalization | 529.5 (908.0) | 1,548.5 (993.9) |
| Line-crossing | 9.0 (12.5) | 9.5 (13.5) |
General Discussion
In Experiment 1, LPS injection resulted in high levels of crouching, eye-closing, and piloerection, the same behaviors seen in guinea pig pups during protracted (3 hr) periods of isolation. This finding replicates previous results (Hennessy et al., 2004), and confirms that the passive behaviors characteristic of prolonged isolation are the same behaviors associated with unequivocal sickness. In Experiment 1, it was also found that ICV infusion of 25 μg of α-MSH significantly reduced the effect of LPS on two of the passive responses, crouching and piloerection. Because this is the same dose and route of administration previously used to reduce passive behaviors during protracted isolation (Schiml-Webb et al., 2006), the results support the conclusion that α-MSH's effect in the previous study was due to it anti-inflammatory properties, rather than some other potential mode of action.
The most unexpected result was the suppressive effect of α-MSH on active behavior in Experiment 1. Under saline-injected control conditions, vocalizing was significantly reduced, and line-crossing was reduced to a marginally significant level. Based on past results (Schiml-Webb et al., 2006, and unpublished findings), we had not predicted a significant effect of α-MSH on the active behaviors, though if we had, we would have predicted an enhancement rather than a suppression of these behaviors for either of two reasons. First, a reduction in active behavior is a commonly observed sickness response (Hart, 1988) and so an anti-inflammatory might be expected to oppose this effect and increase activity. Second, the vocalizing of guinea pig pups during isolation procedures is considered to reflect an anxiety-like state (Borsini, Podhorna, & Marazziti, 2002). Since α-MSH has been found to produce anxiogenic effects in some species (Panksepp & Abbott, 1990; Rao et al., 2003) there would be reason to expect α-MSH would increase vocalizing. It may be worth noting in this regard that the subjective impression of the primary observer during Experiment 1 was that α-MSH had a calming effect on the pups. At present, we see no satisfying explanation for the effects of α-MSH on active behavior in the saline-inject pups of Experiment 1. Clearly, this aspect of the findings will require more investigation to fully understand. We can say, however, that the present results argue against ICV α-MSH having an anxiogenic action in the young guinea pig.
For active behaviors, there also was a marginally significant tendency for α-MSH to reduce the effect of LPS in Experiment 1. However, this tendency may have been an artifact. As just noted, a-CSF and α-MSH-infused pups exhibited rather different levels of active behaviors when injected with saline. Further, LPS greatly suppressed behavior in both groups. (Note the similarity of median values of behavior for the “saline” and “saline minus LPS” scores in Table 1.) Thus, the tendency for a-CSF and α-MSH groups to differ on the “saline minus LPS” measure was likely due to the differential values in the saline-injected control condition, perhaps combined with a “floor” effect in the suppressive action of LPS.
In Experiment 2, indomethacin significantly reduced the crouching and full passive response of guinea pig pups over the course of a prolonged (i.e., 3-hr) isolation in a novel cage. Active behaviors were not affected. Since indomethacin disrupts synthesis of prostaglandins, a final common mediator of many inflammatory effects, but has no obvious other means of reducing passive behavioral responses, the results of Experiment 2 further implicate proinflammatory factors in the expression of the passive responses during protracted isolation.
α-MSH and indomethacin impair inflammation through distinct processes. The effects of α-MSH are extremely broad and involve multiple modes of action. This peptide acts on an assortment of immune cells (monocytes, macrophages, dendritic cells, glia) in both the periphery and CNS to downregulate expression of various proinflammatory cytokines and increase production of the anti-inflammatory cytokine IL-10, as well as on CNS receptors to produce other anti-inflammatory neural effects (Lipton et al., 1999; Lugar et al., 2004). Indomethacin's effects are more circumscribed, inhibiting synthesis of cyclooxygenase, the rate limiting factor in the production of prostaglandins. In the brain, prostaglandins are necessary mediators of some actions of proinflammatory cytokines (Rivest, 1999), including a subset (Avitsur, Weidenfeld, & Yirmiya, 1999; Dunn & Swiergiel, 2000; Johnson, Curtis, Dantzer, & Kelley, 1993; Yirmiya, Barak, Avitsur, Gallily, & Weidenfeld, 1997), though not all (Deak, D'Agonstino, Bellamy, Rosanoff, McElderry, & Bordner, 2005) behavioral components of sickness. Thus, the effect of indomethacin in Experiment 2 indicates a contribution of prostaglandins to the passive behaviors of guinea pig pups. Moreover, the combined results of the two experiments provide convergent evidence supporting the hypothesis (Hennessy et al., 2001) that the passive responses of guinea pig pups during a several hour period of isolation in a novel environment are mediated by proinflammatory factors, and therefore, examples of stress-induced sickness behaviors.
The stress-responsive peptide corticotropin-releasing factor (CRF) provides one potential means by which the isolation procedure may increase proinflammatory activity. CRF is known to stimulate various proinflammatory responses (Leu & Singh, 1992; Singh, Pang, Alexacos, Letourneau, & Theoharides, 1999; Webster et al., 1996). Further, injection of CRF will increase passive responses of isolated guinea pig pups during a brief isolation much as was observed for LPS in Experiment 1 of the present report (Hennessy et al., 1995). Since a CRF antagonist can delay the onset of the passive responses in isolated pups (McInturf & Hennessy, 1996), it appears that endogenous CRF may be in part responsible for the transition from the initial active, to the subsequent passive, phase of responsiveness. Recently we found that α-MSH attenuates the passive responses of guinea pig pups injected with CRF (Schiml-Webb, Miller, Deak, & Hennessy, in preparation), indicating that CRF acts through a proinflammatory mechanism. Together, these findings suggest that CRF release during the stress of isolation in a novel environment stimulates proinflammatory activity which promotes the passive responses (Hennessy, Deak, Schiml-Webb, & Barnum, in press). Moreover, evidence that proinflammatory cytokines can, in turn, stimulate CRF release (Dunn, 2005), suggests the possibility of positive feedback from cytokines to CRF to further amplify behavioral effects.
Recently, proinflammatory factors have been implicated in human depression. The so-called “cytokine hypothesis” holds that increased proinflammatory cytokines underlie some forms of depressive illness, and that in these cases, sickness responses contribute to behavioral symptoms (Schiepers, Wichers, & Maes, 2005). We believe this hypothesis may be of particular interest in the context of maternal separation studies. In human infants, the “despair” stage was at the outset considered a form of depression (Spitz, 1946), and this stage in nonhuman primates traditionally has been viewed as an animal model for depression (McKinney, Moran, & Kraemer, 1984; Willner, 1991). While it remains unclear if the passive responses of separated primates can be regarded as sickness behaviors, and though caution is required in generalizing from studies of sickness in laboratory animals to depression in humans (Dunn, Swiergiel, & de Beaurepaire, 2005), the possibility that prolonged separation can lead to depressive-like symptoms through proinflammatory processes suggests a new framework in which these early findings might be considered.
Acknowledgments
The authors thank Erin O'Brien and Christine Crumbacher for assistance in data collection. The work was supported by grant MH068228 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aubert A. Sickness and behaviour in animals: a motivational perspective. Neurosci Biobehav Rev. 1999;23:1029–1036. doi: 10.1016/s0149-7634(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Avistur R, Weidenfeld J, Yirmiya R. Cytokines inhibit sexual behavior in female rats: II Prostaglandins mediate the suppressive effects of interleukin-1β. Brain Behav Immun. 1999;13:33–45. doi: 10.1006/brbi.1999.0556. [DOI] [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Berryman JC. Guinea-pig vocalizations: their structure, causation, and function. Z Tierpsychol. 1976;41:80–106. doi: 10.1111/j.1439-0310.1976.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Blatteis CM, Quan N, Xin L, Ungar AL. Neuromodulation of acute-phase responses to interleukin-6 in guinea pigs. Brain Res Bull. 1990;25:895–901. doi: 10.1016/0361-9230(90)90185-3. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacol. 2002;163:121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Separation. Vol. 2. New York: Basic Books; 1973. Attachment and loss. [Google Scholar]
- Buller KM, Xu Y, Day TA. Indomethacin attenuates oxytocin and hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1β. J Neuroendocrinol. 1998;10:519–528. doi: 10.1046/j.1365-2826.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. doi: 10.1016/0006-8993(91)91587-q. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Deak T, D'Agonstino LG, Bellamy C, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–134. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Cytokine activation of the hypothalamo-pituitary-adrenal axis. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain. Vol. 15. Elsevier; 2005. pp. 157–174. [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of cyclooxgenases in endotoxin- and interleukin-1-induced hypophagia. Brain Behav Immun. 2000;14:141–152. doi: 10.1006/brbi.1999.0580. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: What can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Vaziri S, Wilson CA. Behavioral effects of α-MSH and MCH after central administration in the female rat. Peptides. 1996;17:171–177. doi: 10.1016/0196-9781(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA. Stress-induced sickness behaviors: An alternative hypothesis for responses during maternal separation. Dev Psychobiol. 2001;39:76–83. doi: 10.1002/dev.1031. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Barnum CJ. Immune influences on behavior and endocrine activity in early experience and maternal separation paradigms. In: Columbus F, editor. Psychneuroendocrinology Research Frontiers. Hauppauge NY: Nova Scientific Publishers; in press. [Google Scholar]
- Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiol Behav. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Long SJ, Nigh CK, Williams MT, Nolan DJ. Effects of peripherally administered corticotropin-releasing factor (CRF) and a CRF antagonist: Does peripheral CRF mediate the behavior of isolated guinea pig pups? Behav Neurosci. 1995;109:1137–1145. doi: 10.1037//0735-7044.109.6.1137. [DOI] [PubMed] [Google Scholar]
- Johnson RW, Curtis SE, Dantzer R, Kelley KW. Central and peripheral prostaglandins are involved in sickness behavior in birds. Physiol Behav. 1993;53:127–131. doi: 10.1016/0031-9384(93)90020-g. [DOI] [PubMed] [Google Scholar]
- Kaufman IC, Rosenblum LA. The reaction to separation in infant monkeys: Anaclitic depression and conservation withdrawal. Psychosom Med. 1967;29:648–675. doi: 10.1097/00006842-196711000-00010. [DOI] [PubMed] [Google Scholar]
- Leu SJ, Singh LK. Stimulation of interleukin-6 production by corticotrophin-releasing hormone. Cell Immunol. 1992;143:220–227. doi: 10.1016/0008-8749(92)90018-k. [DOI] [PubMed] [Google Scholar]
- Lipton JM, Zhao H, Ichiyama T, Barsh GS, Catania A. Mechanisms of antiinflammatory action of alpha-MSH peptides. In vivo and in vitro evidence. Ann NY Acad Sci. 1999;885:173–182. doi: 10.1111/j.1749-6632.1999.tb08674.x. [DOI] [PubMed] [Google Scholar]
- Luger TA, Scholzen TE, Brzoska T, Bohm M. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann NY Acad Sci. 2003;994:133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks enhancement of fear conditioning and interference with escape responding produced by inescapable shock. Brain Res. 1995;695:279–282. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- McInturf SM, Hennessy MB. Peripheral administration of a corticotropin-releasing factor antagonist increases the vocalizing and locomotor activity of isolated guinea pig pups. Physiol Behav. 1996;60:707–710. doi: 10.1016/0031-9384(96)00091-1. [DOI] [PubMed] [Google Scholar]
- McKinney WT, Moran E, Kraemer GW. Separation in nonhuman primates as a model for human depression: Neurobiological implications. In: Post RM, Ballenger J, editors. Neurobiology of Mood Disorders. Baltimore: Williams and Wilkins; 1984. pp. 393–406. [Google Scholar]
- Milligan ED, Nguyen KT, Deak T, Hinde JL, Fleshner M, Watkins LR, Maier SF. The long term acute phase-like responses that follow acute stressor exposure are blocked by alpha-melanocyte stimulating hormone. Brain Res. 1998;810:48–58. doi: 10.1016/s0006-8993(98)00869-5. [DOI] [PubMed] [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psychol Bull. 1978;85:1376–1400. [PubMed] [Google Scholar]
- Panksepp J, Abbott BB. Modulation of separation distress by α-MSH. Peptides. 1990;11:647–653. doi: 10.1016/0196-9781(90)90174-4. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Watkins LR, Maier SF, Rudy JW. Role of interleuken-1 beta in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106:109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- Rao TL, Kokare DM, Sarkar S, Khisti RT, Chopde CT, Subhedar N. GABAergic agents prevent alpha-melanocyte stimulating hormone induced anxiety and anorexia in rats. Pharmacol Biochem Behav. 2003;76:417–423. doi: 10.1016/j.pbb.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Rivest S. What is the cellular source of prostaglandins in the brain in response to systemic inflammation? Facts and controversies. Mol Psychiat. 1999;4:501–507. [PubMed] [Google Scholar]
- Schiml-Webb PA, Deak T, Greenlee TM, Maken DS, Hennessy MB. Alpha-melanocyte stimulating hormone reduces putative stress-induced sickness behaviors in isolated guinea pig pups. Behav Brain Res. 2006;168:326–330. doi: 10.1016/j.bbr.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Schiml-Webb PA, Miller EE, Deak T, Hennessy MB. Alpha-melanocyte stimulating hormone attenuates behavioral effects of corticotropin-releasing factor. (in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Sehic E, Szekely M, Ungar AL, Oladehin A, Blatteis CM. Hypothalamic prostaglandin E2 during lipopolysaccharide-induced fever in guinea pigs. Brain Res Bull. 1996;39:391–399. doi: 10.1016/0361-9230(96)00037-8. [DOI] [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin-releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–239. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Soszynski D. Inhibition of nitric oxide synthase delays the development of tolerance to LPS in rats. Physiol Behav. 2002;76:159–169. doi: 10.1016/s0031-9384(02)00693-5. [DOI] [PubMed] [Google Scholar]
- Spitz RA. Anaclitic depression: An inquiry into the genesis of psychiatric conditions of early childhood: II. Psychoanal Study Child. 1946;2:313–342. [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: Suppression of pituitary ACTH release and peripheral inflammation. Endocrinol. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Wieczorek M, Dunn AJ. Relationships among the behavioral, noradrenergic, and pituitary-adrenal responses to interleukin-1 and the effects of indomethacin. Brain Behav Immun. 2005 doi: 10.1016/j.bbi.2005.10.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Animal models as simulations of depression. Trends Pharmacol Sci. 1991;12:131–136. doi: 10.1016/0165-6147(91)90529-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Barak O, Avitsur R, Gallily R, Weidenfeld J. Intracerebral administration of Mycoplasma fermentans produces sickness behavior: role of prostaglandins. Brain Res. 1997;749:7–81. doi: 10.1016/s0006-8993(96)01295-4. [DOI] [PubMed] [Google Scholar]


