Abstract
Inheritance of a defect in a neuronal mechanism that regulates response to auditory stimuli was studied in nine families with multiple cases of schizophrenia. The defect, a decrease in the normal inhibition of the P50 auditory-evoked response to the second of paired stimuli, is associated with attentional disturbances in schizophrenia. Decreased P50 inhibition occurs not only in most schizophrenics, but also in many of their nonschizophrenic relatives, in a distribution consistent with inherited vulnerability for the illness. Neurobiological investigations in both humans and animal models indicated that decreased function of the α7-nicotinic cholinergic receptor could underlie the physiological defect. In the present study, a genome-wide linkage analysis, assuming autosomal dominant transmission, showed that the defect is linked [maximum logarithm of the odds (lod) score = 5.3 with zero recombination] to a dinucleotide polymorphism at chromosome 15q13-14, the site of the α7-nicotinic receptor. Despite many schizophrenics’ extremely heavy nicotine use, nicotinic receptors were not previously thought to be involved in schizophrenia. The linkage data thus provide unique new evidence that the α7-nicotinic receptor gene may be responsible for the inheritance of a pathophysiological aspect of the illness.
Keywords: human chromosome 15, nicotinic receptors, genetic linkage
Although family, twin, and adoption studies indicate that schizophrenia has a significant genetic component, these studies also show that the inheritance of schizophrenia is complex, involving an uncertain mode of transmission, incomplete penetrance, and probable genetic heterogeneity (1, 2). Linkage studies using schizophrenia and related psychiatric cases as phenotypes have found possible loci for schizophrenia at various chromosomal sites in subsets of families (3–6). However, the findings do not account completely for the inheritance of schizophrenia, nor do they delineate which aspects of this multifactorial illness might be influenced by a specific locus. An alternative strategy advocated for genetic studies of complex diseases involves the use of a specific neurobiological characteristic of the illness, as an additional phenotype that might reflect more closely the effect of a single genetic alteration (7–9). Such a trait could be part of the inherited diathesis of the illness, which produces schizophrenia in combination with other pathogenic elements (10).
Various psychophysiological paradigms demonstrate altered brain functions in schizophrenic patients and their relatives that might reflect such inherited traits (9, 11–13). Many findings indicate basic deficits in the regulation of response to sensory stimuli that may underlie patients’ more apparent symptoms, such as hallucinations and delusions (14). In addition to hearing voices, patients often attend to apparently extraneous stimuli in their surroundings that normal individuals generally ignore. Such symptoms suggest that neuronal mechanisms responsible for the filtering or gating of sensory input to higher brain centers are deficient. One method developed for examining such neuronal mechanisms compares the responses to the first and second of paired stimuli. The first stimulus elicits an excitatory response that also activates inhibitory mechanisms, which then diminish the excitatory response to the second stimulus. The ratio of the amplitude of the second response to the first is inversely related to the strength of inhibition (15). This method was used to study the response to auditory stimuli in schizophrenia, using an electrically positive evoked potential occurring 50 ms after an auditory stimulus (P50). Inhibition of the P50 response to a second identical stimulus, presented 500 ms after the first, is diminished in schizophrenics (16–19). This diminished inhibition, measured as an elevation in the ratio of P50 amplitudes, is correlated with schizophrenics’ decreased performance in a neuropsychological measure of sustained attention, as well as diminished performance in a word recognition task (20, 21). Inhibition of the P50 response was used to identify possible genetic mechanisms in two different ways: first, the measure was studied in animal and related clinical investigations to identify neurobiological mechanisms that can be related to candidate genes; second, the measure was used as a phenotype for linkage analysis to identify any chromosomal areas that contain genes responsible for the abnormality in schizophrenics.
The neurobiological mechanism of inhibition of human P50 to repeated auditory stimuli was initially investigated using an auditory-evoked potential recorded from the rat as an analog. Both the human and rat potentials show similar decreased responses to repeated auditory stimuli (22). Neuronal recordings identified the pyramidal neurons of the hippocampus as a major source of the rat evoked potential. These pyramidal neurons have a decremented response to repeated auditory stimuli that parallels the decrement in the evoked potential (23). The decrement is lost after transection of the fimbria-fornix, a fiber tract that includes afferents to the hippocampus from cholinergic neurons in the basal forebrain (24). Nicotine normalizes inhibition of response in the fimbria-fornix-lesioned animals (25). Studies with pharmacological antagonists in unlesioned animals indicated that a specific subset of nicotinic cholinergic receptors is involved in the inhibitory mechanism. The inhibition is selectively blocked by the snake toxin α-bungarotoxin (26), which suggests that the receptor contains the α7-nicotinic cholinergic receptor subunit; it is the only known nicotinic receptor subunit in the mammalian brain sensitive to this toxin (27, 28). Neither scopolamine, mecamylamine, nor κ-bungarotoxin, which are antagonists of other types of cholinergic receptors, blocked the inhibition. Receptor autoradiography using 125I-labeled α-bungarotoxin showed the most intense binding to nonpyramidal hippocampal neurons that contain the inhibitory neurotransmitter γ-aminobutyric acid (29). This labeling is consistent with physiological evidence that cholinergic synapses activate interneurons, which inhibit the pyramidal neuron response to the second stimulus (30, 31).
There are several areas of apparent concordance between these findings in rats and P50 inhibition in humans. P50 has been recorded from the human hippocampus (32, 33), and human hippocampal neurons have rapidly decreasing responses to auditory stimuli, similar to those observed with rat hippocampal neurons (34). Nicotine in high doses transiently normalizes the abnormality in P50 inhibition in schizophrenics and in their relatives, much as it normalizes inhibition in rats after fimbria-fornix lesions (25, 35, 36). The effect of nicotine on P50 inhibition in relatives of schizophrenics is not blocked by mecamylamine, which blocks all known nicotinic receptors in human brain, except the α7-nicotinic receptor (37). In situ hybridization showed that α7-nicotinic receptor mRNA is expressed in human hippocampal neurons (37). Some of the nonpyramidal neurons of the human hippocampus are intensely labeled by α-bungarotoxin, as was the case in rats. A preliminary study showed that α-bungarotoxin labeling was decreased in postmortem hippocampus from eight schizophrenics (38). Finally, schizophrenic patients are particularly heavy tobacco smokers, even when compared with other psychiatric patients (39, 40). This heavy nicotine use may reflect an attempt at self-medication of an endogenous neuronal deficit (41).
In parallel to these biological studies in human and animals, the P50-evoked potential abnormality was also investigated as a phenotype for genetic linkage analysis. A genome-wide scan was initiated, independent of any candidate gene hypothesis, in nine multiplex schizophrenic pedigrees, which were also phenotyped with P50 recordings (Figs. 1 and 2). The deficit in inhibition of the P50 response in these and other schizophrenic families is generally found in one of the parents and half the siblings, including the schizophrenic probands (44). Although elevated P50 ratios are significantly associated with the apparent genetic risk for schizophrenia, many individuals in the pedigrees who have the deficit are clinically unaffected (45). In that respect, the distribution of the trait resembles several other neurobiological abnormalities in schizophrenics and their relatives, such as deficits in smooth pursuit eye movements and reaction time (11, 12). These traits have been proposed to be alternative expressions of a latent trait or endophenotype (12), which, in combination with other pathogenic elements, gives rise to schizophrenia.
Figure 1.
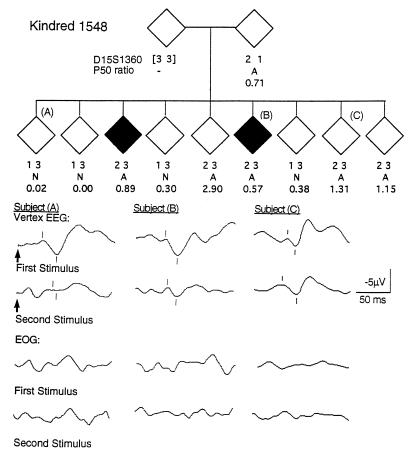
Segregation of alleles of D15S1360 and P50 ratio in a portion of K1548. A indicates abnormal and N indicates normal, based on previously determined distributions of the P50 ratio for schizophrenics and normals (42). The ♦ represents a schizophrenic. The pedigree has been altered in several ways to protect subject confidentiality. Auditory-evoked responses to paired stimuli (arrows) demonstrate normal and abnormal P50 ratios in clinically unaffected siblings (subjects A and C) and an abnormal P50 ratio in a schizophrenic sibling (subject B). A computer algorithm (43) identified the P50 waves in the vertex electroencephalography (marks below the tracings) and measured their amplitudes relative to the preceding negative peaks (marks above the tracings). Simultaneous electrooculographic recordings illustrate that the P50 wave was not generated by eye movement artifact.
Figure 2.
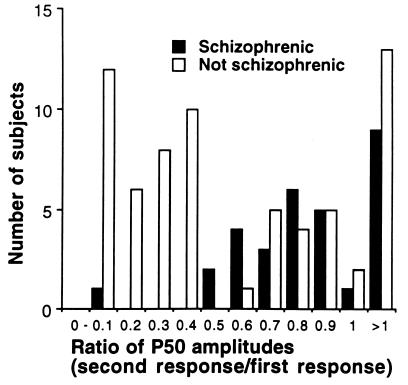
Distribution of P50 ratios in the nine pedigrees. Values ≤0.40 were coded unaffected and values ≥0.50 were coded affected, based on previous studies of unrelated normals and schizophrenics (42).
In 1993 we reported preliminary linkage analyses between the P50 ratio abnormality and 318 restriction fragment length polymorphism and tandem repeat DNA markers in the nine kindreds (46). DNA markers mapping to four chromosomal regions, one of which was 15q14, revealed small positive logarithm of odds ratio (lod) scores, assuming autosomal dominant transmission. Subsequently, the α7-nicotinic receptor gene was localized to the 15q14 region (47, 48). Because of the converging evidence from neurobiological investigations, which implicated α7-receptor function in abnormal P50 inhibition, and the preliminary linkage study, which provided suggestive evidence for heritability of the trait near the α7-receptor gene’s chromosomal location, we undertook a new linkage study with informative markers at the α7-receptor gene locus. Two new DNA polymorphic loci were isolated, D15S1360 from a yeast artificial chromosome (YAC) containing the α7-nicotinic receptor gene and L76630 from an α7-containing clone in a genomic phage library. These markers were used with over 500 highly polymorphic markers in a 10-centimorgan resolution genome-wide scan of the nine pedigrees. The results demonstrate a highly significant linkage between D15S1360 and the abnormality in P50 suppression.
MATERIALS AND METHODS
Recruitment and Diagnoses of Subjects.
Pedigrees were selected for presence of at least two cases of schizophrenia in a nuclear family. Two psychiatrists made clinical diagnoses of schizophrenia, chronic type, blind to pedigree and genetic information, using Research Diagnostic Criteria (49, 50). Nine families with 104 members were studied. All subjects gave written informed consent.
Electrophysiological Recording.
Electroencephalographic activity was recorded at the vertex and electrooculographic activity was recorded from the superior orbital-lateral canthus. Five averages of sixteen responses each to paired clicks were obtained, using previously described techniques (51). The P50 responses are distinguished from prestimulus activity for both normals and schizophrenics at a high level of significance (P < 0.001). The averages were reviewed by two investigators, blind to genetic information, who rejected any average containing excessive electrooculographic activity, drowsiness, startle, or other artifacts; the remainder were combined into a grand average, from which the P50 amplitudes were measured and their ratio (second response/first response) was calculated automatically by a computer algorithm (43). Seven subjects were not used, because artifact-free averages could not be selected from their recordings. Recordings were initially performed in 1991–1992 and repeated in 1994–1995. The earlier recordings were reanalyzed for 2 subjects who are now deceased, for 10 subjects who refused repeat recording, and for 2 patients now on atypical neuroleptics, which can normalize the P50 ratio; other neuroleptic medications do not affect the phenotype (52).
Isolation of Polymorphisms.
The polymorphic marker D15S1360 was isolated from a YAC containing the α7-nicotinic receptor gene. A probe was constructed by PCR from total human hippocampal RNA, using rat α7-primers (GenBank accession no. M85273M85273) for amplification of the cytoplasmic loop between membrane-spanning regions III and IV. The PCR fragment was sequenced and human primers designed to generate a 338-bp product, which was cloned into pBluescript SK(−). This probe was used to isolate a human α7 cDNA (GenBank accession no. U40583U40583). The Washington University human YAC library was screened with the same primers. Two clones were isolated, B132H10 (150 kbp) and B134H10 (300 kbp), on the TAFE (Transverse Alternating Field Electrophoresis, Beckman) gel system. A sublibrary of B134H10 was prepared in the λZAP phagemid vector (Stratagene) by complete MboI digestion of the intact YAC DNA in a low-melt agarose plug. The DNA was extracted and ligated into BamHI-digested and phosphatased vector, transformed into XL1Blue-(MRF′) (Stratagene), and screened with a (CA)16 oligonucleotide. One clone contained a microsatellite [(CA)5T(CA)12TA(CA)5C(CA)3], which mapped to chromosome 15 (Human/Rodent Hybrid Mapping Panel 1; Coriell Cell Repositories, Camden, NJ). Flanking primers amplified four alleles (111, 113, 115, and 117 bp). Allele frequencies (0.006, 0.528, 0.376, and 0.090; heterozygosity 0.57) were estimated from individuals marrying into the pedigrees. PCR was performed using GeneAmp with 2 mM MgCl2 and a Model 9600 thermal cycler (Perkin–Elmer), with the following conditions: 94°C for 4 min; followed by 35 cycles of 94°C for 30 sec, 51°C for 1 min 15 sec, and 72°C for 1 min; and finally 72°C for 10 min. Additionally, genomic P1 artificial chromosome clones for α7 were obtained from Genome Systems (St. Louis). PAC-64-A1 is 120 kbp long and contains both D15S1360 and the 5′ end of the coding region. L76630 was localized in a genomic fragment containing the α7-nicotinic receptor gene (CHRNA7), isolated from a human genomic library (Stratagene), by screening with a human α7-cDNA clone (HP411). A 6-kbp EcoRI genomic fragment was identified, partially sequenced, and found to include a CA dinucleotide repeat 3′ of the last exon (GenBank accession no. L76630). Flanking primers amplified three alleles (180, 178, and 176 bp); allele frequencies were 0.06, 0.62, and 0.32, with heterozygosity 0.51. PCR was performed with 1.5 mM MgCl2 as follows: 94°C for 5 min; 20 cycles of 94°C for 1 min, 56°C for 2 min, and 72°C for 1 min; and 72°C for 5 min. The two polymorphisms were genetically mapped in 96 individuals from six reference families (Centre d’Étude du Polymorphisme Humain).
Linkage Analysis.
Parameters for lod score analyses of P50 ratios were determined from the distribution of values in 43 unrelated normal individuals and 36 unrelated schizophrenic patients (42) and from observations of the segregation of P50 ratios in the nine multiplex schizophrenic families. Elevated P50 ratios were defined as values ≥0.50, which were found in 91% of the unrelated schizophrenics and in 6% of the normals. Of the remaining unrelated schizophrenics, most had values between 0.41 and 0.49, a range therefore coded unknown for the linkage analysis. If this unknown range was extended to include values between 0.40 and 0.60, results were not changed substantially (e.g., lod scores were decreased by an average of 0.54 across the markers in the 15q13-14 region due to the loss in information). For lod score analyses, frequency of a gene for abnormal P50 ratio was fixed at 0.05, penetrance for the normal genotype was fixed at 0.01, and penetrance for the abnormal genotypes was fixed at 0.8 (46). These parameters result in a morbidity for abnormal P50 ratio of 8.7% and a phenocopy rate among abnormal subjects of 10.4%. The fastlink version of the linkage program was used to compute lod scores at various recombination fractions, Θ (53). No significant heterogeneity was found using the homog program (54). The chance of false positive lod score results was determined using slink (55); 1000 replicates of the pedigrees were simulated, assuming no linkage to the marker under analysis. lod score analysis was performed for each replicate under the dominant model; the highest score observed for D15S1360 and P50 under the assumption of no linkage was 1.87.
Nonparametric Methods.
Sibling pair analysis was performed using the sibpal program (56). Marker data were used to estimate the proportion of alleles shared through a common ancestor (i.e., identical by descent) for each possible sibling pairing within the linkage families. A test was performed to determine if the proportion of alleles shared was >0.50 for abnormal/abnormal pairs. To calculate P values, 1000 replicates of the nine families were simulated for each marker to determine empirical distributions. Degrees of freedom were adjusted downward for nonindependence when multiple pairings were used from the same sibship within a family. A newly developed method, nonparametric linkage, uses information from all genotyped members of a pedigree to assess the extent of alleles shared identical by descent among all affected individuals. The resulting statistic is normalized by first subtracting the expected sharing score under the null hypothesis of no linkage from the observed score and then dividing by the score variance under the null hypothesis. Thus the statistic is asymptotically distributed as a standard normal variable (Z score) under the null hypothesis. Calculations of nonparametric linkage statistics were carried out using the genehunter computer programs (57). genehunter also uses an improvement to a previously described algorithm to perform complete multipoint linkage analysis with a large number of highly polymorphic markers in pedigrees of moderate size (58). Due to computational constraints, the three largest pedigrees were each split into two parts.
RESULTS
Characterization of Markers.
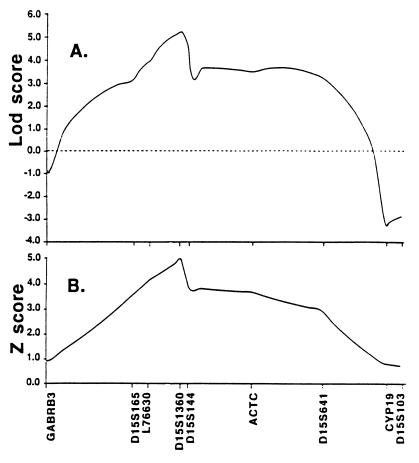
D15S1360, a complex microsatellite with four alleles, was localized in a 300-kbp human YAC, in which presence of the entire α7-gene was confirmed by comparison of Southern blots of BamHI-restricted YAC and human DNA. D15S1360 is <120 kbp from the first exon of the α7-gene, based on their copresence in a P1 artificial chromosome. L76630, also present in the same YAC, is a three-allele CA repeat, which is 3 kbp, 3′ of the last exon of the α7-gene. D15S1360 was localized within a 7-centimorgan region between markers D15S165 and D15S144 on the chromosome 15 map, the region previously found to contain the α7-gene (47, 48). Because of its lower heterozygosity, L76630 could not be definitively placed; possible locations extended from GABRB3 to D15S641. Mapping results also showed recombination between D15S1360 and L76630 (two-point lod score 8.05 at Θ = 0.06). For multipoint mapping of the P50 deficit, the most likely location of each marker was used (Fig. 3).
Figure 3.
(A) Multipoint parametric lod score analysis for P50 ratio abnormality with markers at 15q. (B) Multipoint nonparametric linkage analysis for P50 ratio abnormality with markers at 15q. Results are presented for the most likely map locations for D15S1360 and L76630; the total length of the abscissa is 45 centimorgans.
Linkage Analysis.
A genome-wide scan was performed with 542 highly polymorphic simple sequence repeat DNA loci spaced at ≈10-centimorgan intervals. Pairwise lod score linkage analysis assuming autosomal dominant transmission was used to screen for loci underlying the abnormality measured by the P50 ratio. The lod score is the common logarithm of the likelihood ratio, a ratio determined by dividing the likelihood of linkage for a given value of the recombination frequency (Θ < 0.5) by the likelihood of no linkage. Values over 3.0 (odds ratio 1000:1) generally indicate the presence of linkage (54). Only one marker, D15S1360, yielded a lod score >3.0 (lod score maximum 5.30, Θ = 0.0, P < 0.001; (Table 1). DNA markers flanking D15S1360 also gave positive lod scores (Table 2). Multipoint analysis showed a maximum score at D15S1360 of 5.29 (Fig. 3A).
Table 1.
Two-point lod scores for P50 ratio with D15S1360
| Kindred | Abnormal P50 ratios/total members | Recombination fraction
|
|||||
|---|---|---|---|---|---|---|---|
| 0.00 | 0.05 | 0.10 | 0.20 | 0.30 | 0.40 | ||
| K1480 | 6/7 | 0.51 | 0.49 | 0.46 | 0.38 | 0.27 | 0.14 |
| K1494 | 8/11 | −0.26 | −0.21 | −0.17 | −0.09 | −0.04 | −0.01 |
| K1501 | 7/10 | 1.54 | 1.38 | 1.22 | 0.87 | 0.51 | 0.17 |
| K1546 | 6/13 | −0.85 | −0.65 | −0.49 | −0.26 | −0.12 | −0.03 |
| K1556 | 3/5 | −0.18 | −0.14 | −0.10 | −0.06 | −0.02 | −0.01 |
| K1545 | 4/10 | −0.07 | 0.07 | 0.12 | 0.12 | 0.07 | 0.02 |
| K1524 | 5/10 | 1.53 | 1.39 | 1.24 | 0.92 | 0.57 | 0.20 |
| K1527 | 10/17 | 1.53 | 1.35 | 1.17 | 0.82 | 0.46 | 0.15 |
| K1548 | 8/14 | 1.53 | 1.41 | 1.27 | 0.95 | 0.59 | 0.21 |
| Total | 57/97 | 5.30 | 5.10 | 4.72 | 3.64 | 2.28 | 0.84 |
Table 2.
Linkage results for P50 ratio with chromosome 15q markers
| Marker | Sibling pair analysis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Two-point lod score analysis Recombination fraction
|
Proportion of alleles shared | T | P | Nonparametric linkage
|
||||||
| 0.00 | 0.05 | 0.1 | 0.2 | 0.3 | Z score | P | ||||
| GABRB3 | −2.60 | −1.67 | −1.02 | −0.28 | −0.02 | 0.49 | −0.27 | 0.605 | −0.42 | 0.649 |
| D15S165 | 0.04 | 0.77 | 1.02 | 1.05 | 0.76 | 0.65 | 3.33 | 0.002 | 2.02 | 0.028 |
| L76630 | −0.07 | 0.15 | 0.27 | 0.35 | 0.25 | 0.61 | 3.19 | 0.003 | 1.90 | 0.035 |
| D15S1360 | 5.30 | 5.10 | 4.72 | 3.64 | 2.28 | 0.70 | 4.07 | 0.0005 | 3.95 | 0.0002 |
| D15S144 | 1.21 | 1.42 | 1.50 | 1.34 | 0.88 | 0.56 | 1.82 | 0.044 | 1.44 | 0.081 |
| ACTC | 2.62 | 2.55 | 2.41 | 1.94 | 1.27 | 0.63 | 2.63 | 0.010 | 2.08 | 0.025 |
| D15S641 | 1.70 | 1.76 | 1.71 | 1.40 | 0.91 | 0.59 | 3.05 | 0.004 | 1.00 | 0.160 |
| CYP19 | −2.34 | −0.68 | 0.13 | 0.84 | 0.85 | 0.49 | −0.18 | 0.570 | 0.28 | 0.374 |
| D15S103 | −3.50 | −2.11 | −1.33 | −0.47 | −0.07 | 0.49 | −0.21 | 0.582 | 0.20 | 0.404 |
Linkage analysis between L76630, the other marker at the α7 locus, and the P50 ratio phenotype was inconclusive due to the marker’s low heterozygosity. Heterozygosity, the probability that an individual has different alleles of the marker, is related to the number of alleles of the marker and their frequency in the population. Higher heterozygosity provides more information for linkage analysis. In kindred 1548 (Fig. 1), for example, one parent has an abnormal P50 ratio and two different D15S1360 alleles, termed 1 and 2. The 2 allele has one additional repetition of the CA nucleotides and thus is 2 bp longer than the 1 allele. The 2 allele is inherited by offspring with abnormal P50 ratios, whereas the 1 allele is inherited by those with normal P50 ratios. Thus, abnormal P50 ratio is genetically linked to the 2 allele and the D15S1360 lod score is positive. However, the two L76630 alleles are identical in this parent, so that a relationship between L76630 alleles and P50 ratio cannot be determined in the offspring. Consequently, the L76630 lod score is 0 in this family, as well as in two others with positive D15S1360 linkages.
Because lod scores are influenced by assumptions about the mode of inheritance, the results were verified by two methods that do not require such assumptions (Table 2). Both methods count the frequency that two siblings or other related pairs, both of whom have abnormal P50 ratios, also have the same alleles of a particular DNA marker. Generally, sibling pairs are expected to have only half their alleles identical, based on the random probability of inheriting either one of the two alleles of each parent. Elevations above that value, to a maximum of 0.70 at D15S1360, indicate that sibs who share abnormal P50 ratios have generally also inherited the same D15S1360 allele from at least one parent and thus provide significant evidence for genetic linkage at 15q13-14 (P < 0.0005). Similar analyses were also performed with a model-free pedigree analysis method, nonparametric linkage, that examines the extent of allele identity among all affected individuals in the pedigree. Two point results were again most significant for D15S1360 (Z = 3.95, P < 0.0002). A complete multipoint analysis using all nine chromosome 15q markers simultaneously (Fig. 3B) gave a maximum value at D15S1360 (Z = 5.04, P < 0.000016).
Two kindreds, K1494 and K1546, gave consistently negative lod scores for P50 with the 15q13-14 markers. In K1494, both the mother and the father of the primary sibship had abnormal P50 ratios, and of their nine offspring, six were also abnormal; as a result, genome-wide linkage analysis did not reveal a clearly positive signal for this bilineal family. For K1546, the highest genome-wide pairwise lod score was 1.60 at Θ = 0.0 with D14S125. In addition, D14S298, which maps 4 centimorgans from D14S125, yielded a lod score of 1.05 at Θ = 0.0 for 1546. Overall lod scores in all nine families for these two markers were positive, though nonsignificant (for D14S125, lod score maximum = 0.82, Θ = 0.2; for D14S298, lod score maximum = 0.89, Θ = 0.2). In the genome-wide scan, the highest lod score for all nine families outside the 15q13-14 region with the P50 ratio phenotype was 2.09 at Θ = 0 for D2S43. However, simulations indicate this lod score may have occurred by chance and more informative microsatellite markers flanking D2S43, a two-allele marker, gave significantly negative scores.
To test linkage at 15q13-14 with clinical schizophrenia as a phenotype independent of the P50 ratio abnormality, linkage analysis was performed using only affected cases of schizophrenia in a penetrance-free model. The analyses showed a small positive lod score for D15S1360 (lod score maximum = 1.33 at Θ = 0.07). lod score simulations indicated that this result could be a chance finding; 7 of 1000 simulations produced higher scores. No other lod score for the 15q13-14 markers exceeded 1.0. Sibling pair and nonparametric linkage analyses were similarly positive but did not reach the 0.001 level of significance for D15S1360 (T = 1.99, P = 0.033; and Z = 2.27, P = 0.018, respectively).
DISCUSSION
Our data strongly suggest that the P50 auditory sensory deficit in schizophrenia is genetically linked to the locus of the α7-nicotinic receptor gene on chromosome 15q14. A number of authors have advocated linkage studies of biological elements of schizophrenia to clarify the mechanism of inheritance (8, 9). The significant linkage obtained with the P50 ratio phenotype supports the value of this strategy, but interpretation of such studies must be cautious, especially with regard to the role of a particular deficit in the pathogenesis of schizophrenia itself. Biological phenotypes have been proposed to clarify some of the difficulties inherent in the clinical phenotype, such as low penetrance, heterogeneity, and phenocopies, and thus differences between the two phenotypes, such as those found in the present study, might be expected. It has been suggested that the clinical illness may be less penetrant, because multiple genetic and nongenetic factors are required to produce clinical illness, whereas a specific biological defect may occur as the result of a single gene effect (10). Thus, some gene carriers would be expected to have an abnormal P50 ratio, the more penetrant phenotype, but not schizophrenia, which is less penetrant. The lower lod scores observed in this study with schizophrenia as phenotype support that position; several kindreds had higher lod scores for P50 ratio than for schizophrenia because there were many family members with abnormal P50 ratios who did not have schizophrenia.
Several other studies have raised the possibility that the chromosome 15q13-14 region is involved in psychotic illness. Psychoses resembling schizophrenia have been observed in Prader–Willi syndrome, a mental retardation linked to deletions and abnormal DNA imprinting in the 15q11-13 region (59). The imprinting abnormality affects the expression of many genes in this region. Several families in Sephardic and other populations have coexistent schizophrenia and Marfan syndrome, a disease linked to dominant mutations in the fibrillin gene at 15q21 (60, 61). The cosegregation of the two illnesses may be based on their chromosomal proximity. Psychosis also occurs in a large French Canadian kindred that has a recessive demyelination disease linked to markers at 15q14 (62). An Italian kindred contains two cousins with psychotic illness and a partial trisomy of chromosome 15, derived independently from abnormal meioses involving a balanced familial translocation with a 15q13 breakpoint, that was present in each of their mothers. It was suggested that the new trisomies may have caused the de novo appearance of illness (63). Further studies will be needed to determine to what extent the appearance of psychoses in these families with other genetic abnormalities at 15q13-14 involves the α7-gene.
In addition to a possible insight into the inheritance of the risk for schizophrenia, this linkage study also provides new data about the identity of neuronal mechanisms involved in its pathophysiology. The results are consistent with clinical and neurobiological evidence for the involvement of the α7-nicotinic receptor gene in sensory gating deficits in schizophrenia, although the possibility exists that another gene in the region of linkage underlies the deficit in P50 gating. Therefore, the role of the α7-receptor in the sensory processing defects and other abnormalities in schizophrenia cannot be fully assessed until the mutations responsible for the linkage are identified and replicated and their neurobiological effects throughout the brain are understood. Nevertheless, the finding of a significant linkage to support the role of the α7-nicotinic receptor in the pathophysiology of sensory and attentional disturbance in schizophrenia is unique. Many neurotransmitter systems have been hypothesized to be at least partly responsible for schizophrenia, but direct biological assessment of a specific neuronal receptor function in human subjects is usually not feasible because of the brain’s complexity and inaccessibility. Genetic investigations, including linkage studies, are therefore a critical test of the involvement of a particular mechanism in schizophrenia. Linkage at the α7-nicotinic receptor locus thus supports the neurobiological evidence that this gene has a role in a pathophysiological aspect of schizophrenia, which had not been previously considered, despite schizophrenics’ well known heavy dependence on nicotine.
Acknowledgments
We thank Brith Otterud and Mark Leppert for mapping information and Katheleen Gardiner and Roberta Payne for their assistance. A.O.-U. performed his work in the laboratory of Dr. Arthur L. Beaudet. We appreciate the time and effort given by the families participating in this study. This work was supported by National Institutes of Health Grants MH44212, MH01089, MH10168-F32, MO1RR00064, MH36321, MH42642, DA09457, AG00029, MH00728, HG00017, and RR03655, the National Institute on Drug Abuse Intramural Program, the Scottish Rite Schizophrenia Research Foundation, and the Veterans Affairs Medical Research Service.
Footnotes
Abbreviations: lod, logarithm of odds ratio; YAC, yeast artificial chromosome.
References
- 1.Risch N. Genet Epidemiol. 1990;7:3–16. doi: 10.1002/gepi.1370070103. [DOI] [PubMed] [Google Scholar]
- 2.Tsuang M T. Br J Psychiatry. 1993;163:299–307. doi: 10.1192/bjp.163.3.299. [DOI] [PubMed] [Google Scholar]
- 3.Pulver A E, Karayiorgou M, Lasseter V K, Wolyniec P, Kasch L, Antonarakis S, Housman D, Kazazian H H, Meyers D, Nestadt G. Am J Med Genet. 1994;54:44–50. doi: 10.1002/ajmg.1320540109. [DOI] [PubMed] [Google Scholar]
- 4.Coon H, Sobell J, Heston L, Sommer S, Hoff M, Holik J, Umar F, Robertson M, Reimherr F, Wender P, Byerley W. Am J Med Genet. 1994;54:12–20. doi: 10.1002/ajmg.1320540105. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Sun C E, Walczak C A, Ziegle J S, Kipps B R, Goldion L R, Diehl S R. Nat Genet. 1995;10:41–46. doi: 10.1038/ng0595-41. [DOI] [PubMed] [Google Scholar]
- 6.Silverman J M, Greenberg D A, Altsteil L D, Siever L J, Mohs R C, Smith C J, Zhou G, Hollander T E, Yang X-P, Kedache M, Le G, Zaccario M L, Davis K L. Am J Med Genet. 1996;67:162–171. doi: 10.1002/(SICI)1096-8628(19960409)67:2<162::AID-AJMG6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Lalouel J-M. Am J Hum Genet. 1985;37:700–718. [PMC free article] [PubMed] [Google Scholar]
- 8.Lander E S. Nature (London) 1988;336:105–106. doi: 10.1038/336105a0. [DOI] [PubMed] [Google Scholar]
- 9.Sham P C, Morton N E, Muir W J, Walker M, Collins A, Shields D C, St. Clair D M, Blackwood D M. Psychiatr Genet. 1994;4:29–38. [PubMed] [Google Scholar]
- 10.Meehl P E. Am Psychol. 1962;17:827–838. [Google Scholar]
- 11.De Amicis L E, Wagstaff D A, Cromwell R L. J Nerv Ment Dis. 1986;174:177–179. doi: 10.1097/00005053-198603000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Holzman P S, Kringlen E, Matthysee S, Flanagan S D, Lipton R B, Cramer G, Levin S, Lange K, Levy D L. Arch Gen Psychiatry. 1988;45:641–647. doi: 10.1001/archpsyc.1988.01800310049006. [DOI] [PubMed] [Google Scholar]
- 13.Braff D L, Grillon C, Geyer M A. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 14.Venables P. In: Progress in Experimental Personality Research. Maher B A, editor. New York: Academic; 1964. pp. 1–47. [PubMed] [Google Scholar]
- 15.Eccles J C. The Inhibitory Pathways of the Central Nervous System. Liverpool, U.K.: Liverpool Univ. Press; 1969. p. 76. [Google Scholar]
- 16.Adler L E, Pachtman E, Franks R, Pecevich M, Waldo M C, Freedman R. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- 17.Boutros N N, Zouridakis G, Overall J. Clin Electroencephalogr. 1991;22:20–45. doi: 10.1177/155005949102200109. [DOI] [PubMed] [Google Scholar]
- 18.Erwin R J, Mawhinney H M, Gur R C, Gur R E. Biol Psychiatry. 1991;30:430–442. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- 19.Judd L L, McAdams L, Budnick B, Braff D L. Am J Psychiatry. 1992;149:488–493. doi: 10.1176/ajp.149.4.488. [DOI] [PubMed] [Google Scholar]
- 20.Cullum C M, Harris J G, Waldo M C, Smernoff E, Madison A, Nagamoto H T, Griffith J, Adler L E, Freedman R. Schizophr Res. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- 21.Vinogradova S, Solomon S, Ober B A, Biggins C A, Shenaut G K, Fein G. Biol Psychiatry. 1996;39:821–824. doi: 10.1016/0006-3223(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 22.Adler L E, Rose G, Freedman R. Biol Psychiatry. 1986;21:787–798. doi: 10.1016/0006-3223(86)90244-1. [DOI] [PubMed] [Google Scholar]
- 23.Bickford-Wimer P C, Nagamoto H, Johnson R, Adler L E, Egan M, Rose G M, Freedman R. Biol Psychiatry. 1990;27:183–192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- 24.Vinogradova O. In: The Hippocampus 2: Neurophysiology and Behavior. Issacson R L, Pribram K H, editors. New York: Plenum; 1975. pp. 3–69. [Google Scholar]
- 25.Bickford P C, Wear K. Brain Res. 1995;705:235–240. doi: 10.1016/0006-8993(95)01157-9. [DOI] [PubMed] [Google Scholar]
- 26.Luntz-Leybman V, Bickford P, Freedman R. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- 27.Couturier S, Bertrand D, Matter J M, Hernandez M C, Bertrand S, Millar N, Valera S, Barkas T, Ballivet M. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 28.Schoepfer R, Conroy W G, Whiting P, Gore M, Lindstrom J. Neuron. 1990;5:35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- 29.Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L. J Neurosci. 1993;13:1965–1975. doi: 10.1523/JNEUROSCI.13-05-01965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller C L, Freedman R. Neuroscience. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- 31.Hershman K M, Freedman R, Bickford P C. Neurosci Lett. 1995;190:133–136. doi: 10.1016/0304-3940(95)11523-y. [DOI] [PubMed] [Google Scholar]
- 32.Goff W R, Williamson P D, VanGilder C, Allison R, Fisher T C. Prog Clin Neurophysiol. 1980;7:126–145. [Google Scholar]
- 33.Makela J P, Hamalainen M, Hari R, McEvoy L. Electroencephalogr Clin Neurophysiol. 1994;92:414–424. doi: 10.1016/0168-5597(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 34.Wilson C L, Babb T L, Halgren E, Wang M L, Crandall P H. Exp Neurol. 1984;84:74–97. doi: 10.1016/0014-4886(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Adler L E, Hoffer L D, Griffith J, Waldo M C, Freedman R. Biol Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- 36.Adler L E, Hoffer L D, Wiser A, Freedman R. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 37.Freedman R, Adler L E, Bickford P, Byerley W, Coon H, Cullum M, Griffith J, Harris J G, Leonard S, Miller C, Myles-Worsley M, Nagamoto H T, Rose G M, Waldo M. Harv Rev Psychiatr. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- 38.Freedman R, Hall M, Adler L E, Leonard S. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 39.deLeon J, Dadvand M, Canuso C, White A O, Stanilla J K, Simpson G M. Am J Psychiatry. 1995;152:453–455. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- 40.Hamera E, Schneider J K, Deviney S. J Nerv Ment Dis. 1995;183:559–565. [PubMed] [Google Scholar]
- 41.Goff D S, Henderson D C, Amico E. Am J Psychiatry. 1992;149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- 42.Waldo M C, Cawthra E, Adler L E, Dubester S, Staunton M, Nagamoto H, Baker N, Madison A, Simon J, Scherzinger A, Drebing C, Gerhardt G, Freedman R. Schizophr Res. 1994;12:93–106. doi: 10.1016/0920-9964(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 43.Nagamoto H T, Adler L E, Waldo M C, Freedman R. Biol Psychiatry. 1989;25:549–561. doi: 10.1016/0006-3223(89)90215-1. [DOI] [PubMed] [Google Scholar]
- 44.Siegel C, Waldo M, Mizner G, Adler L E, Freedman R. Arch Gen Psychiatry. 1984;41:607–612. doi: 10.1001/archpsyc.1984.01790170081009. [DOI] [PubMed] [Google Scholar]
- 45.Waldo M C, Carey G, Myles-Worsley M, Cawthra E, Adler L E, Nagamoto H T, Wender P, Byerley W, Plaetke R, Freedman R. Psychiatry Res. 1991;39:257–268. doi: 10.1016/0165-1781(91)90092-4. [DOI] [PubMed] [Google Scholar]
- 46.Coon H, Plaetke R, Holik J, Hoff M, Myles-Worsley M, Freedman R, Byerley W. Biol Psychiatry. 1993;34:277–289. doi: 10.1016/0006-3223(93)90085-r. [DOI] [PubMed] [Google Scholar]
- 47.Chini B, Raimond E, Elgoyhen A, Moralli D, Balzaretti M, Heinemann S. Genomics. 1994;19:379–381. doi: 10.1006/geno.1994.1075. [DOI] [PubMed] [Google Scholar]
- 48.Orr-Urtreger A, Seldin M F, Baldini A, Beaudet A L. Genomics. 1995;26:399–402. doi: 10.1016/0888-7543(95)80228-e. [DOI] [PubMed] [Google Scholar]
- 49.Spitzer R L, Endicott J, Robins E. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 50.Endicott J, Spitzer R L. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 51.Griffith J, Hoffer L D, Adler L E, Zerbe O, Freedman R. Psychophysiology. 1995;32:460–466. doi: 10.1111/j.1469-8986.1995.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 52.Nagamoto H T, Adler L E, Hea R A, Griffith J M, McRae K A, Freedman R. Biol Psychiatry. 1996;40:181–188. doi: 10.1016/0006-3223(95)00371-1. [DOI] [PubMed] [Google Scholar]
- 53.Lathrop G M, Lalouel J-M, Julier C, Ott J. Proc Natl Acad Sci USA. 1984;81:3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ott J. Analysis of Human Genetic Linkage. Baltimore: Johns Hopkins Univ. Press; 1991. [Google Scholar]
- 55.Ott J. Proc Natl Acad Sci USA. 1989;86:4175–4178. doi: 10.1073/pnas.86.11.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elston, R. C. (1995) sibpal, Statistical Analysis for Genetic Epidemiology (Louisiana State Univ. Medical Center, New Orleans), version 2.2.
- 57.Kruglyak L, Daly M J, Reeve-Daly M P, Lander E S. Am J Hum Genet. 1996;58:1347–1363. [PMC free article] [PubMed] [Google Scholar]
- 58.Kruglyak L, Lander E S. Am J Hum Genet. 1995;57:519–527. [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke D J. Br J Psychiatry. 1993;163:680–684. doi: 10.1192/bjp.163.5.680. [DOI] [PubMed] [Google Scholar]
- 60.Sirota P, Frydman M, Sirota L. Br J Psychiatry. 1990;157:433–436. doi: 10.1192/bjp.157.3.433. [DOI] [PubMed] [Google Scholar]
- 61.Melissari M, Giordano G, Crafa P, Martella M, Ricci R. Pathologica. 1995;87:78–81. [PubMed] [Google Scholar]
- 62.Casaubon L K, Melanson M, Lopes-Cendes I, Marineau C, Andersmann E, Andersmann F, Weissenbach J, Prevost C, Bouchard J P, Matheiu J, Rouleau G A. Am J Hum Genet. 1996;58:28–34. [PMC free article] [PubMed] [Google Scholar]
- 63.Calzolari E, Aiello V, Pallazi A, Sensi S, Calzolari D, Orrico L, Calliari L, Hollier H, Marzi C, Belli S, Bernardi F, Patracchini P. Am J Med Genet. 1996;67:154–161. doi: 10.1002/(SICI)1096-8628(19960409)67:2<154::AID-AJMG5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]