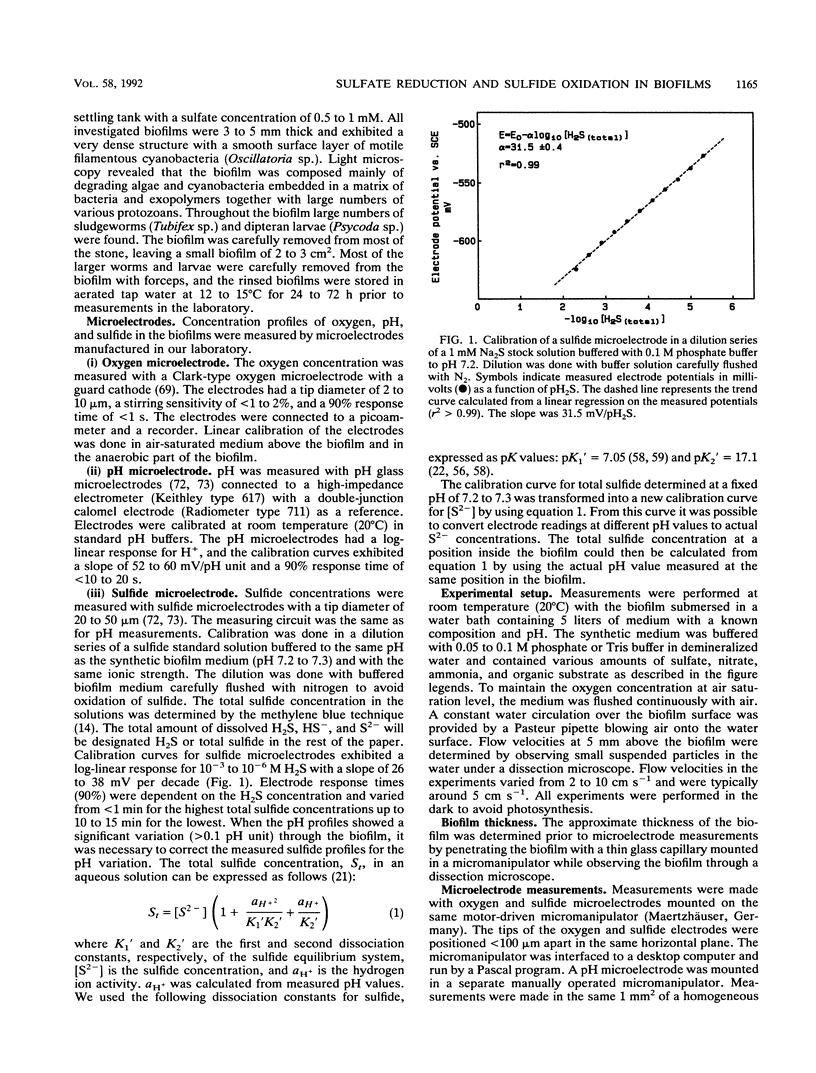
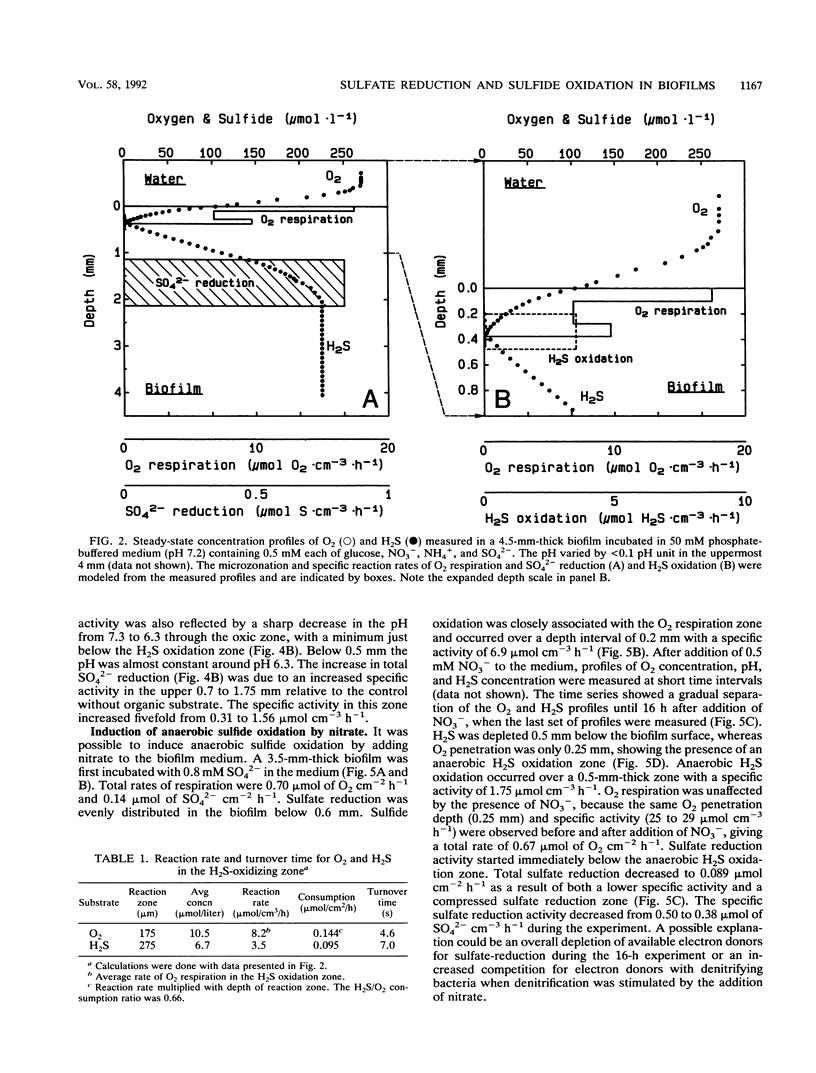
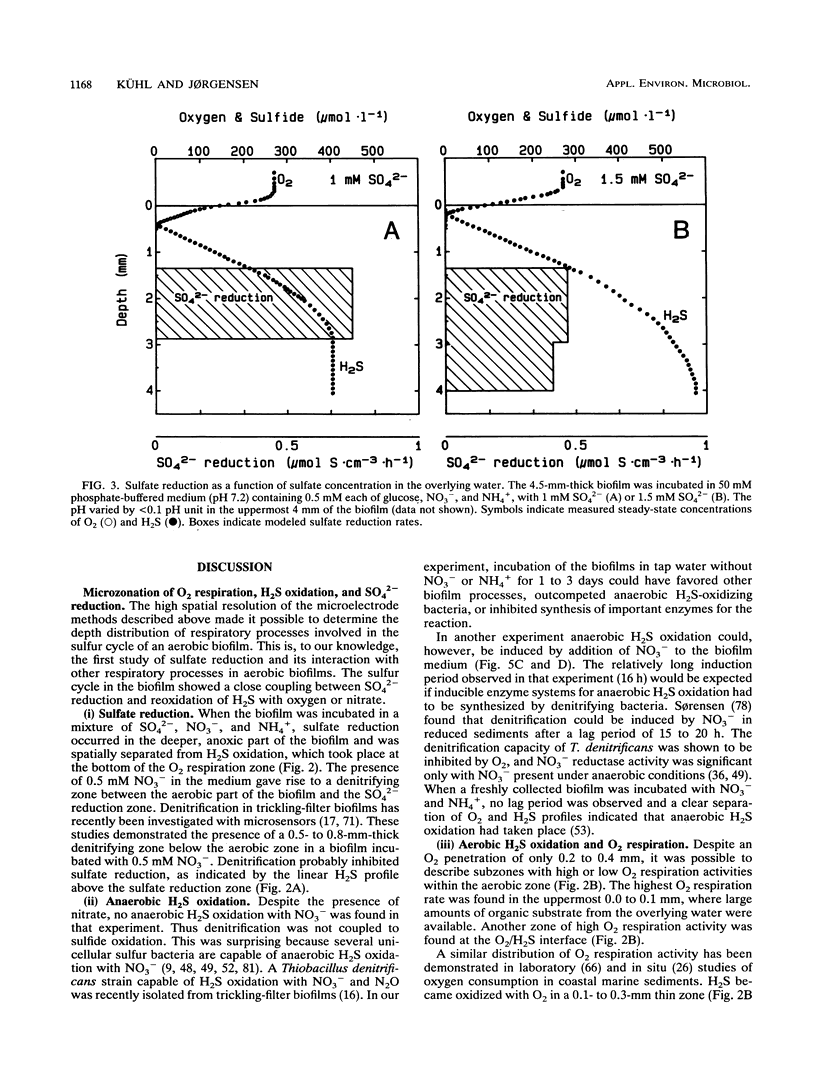
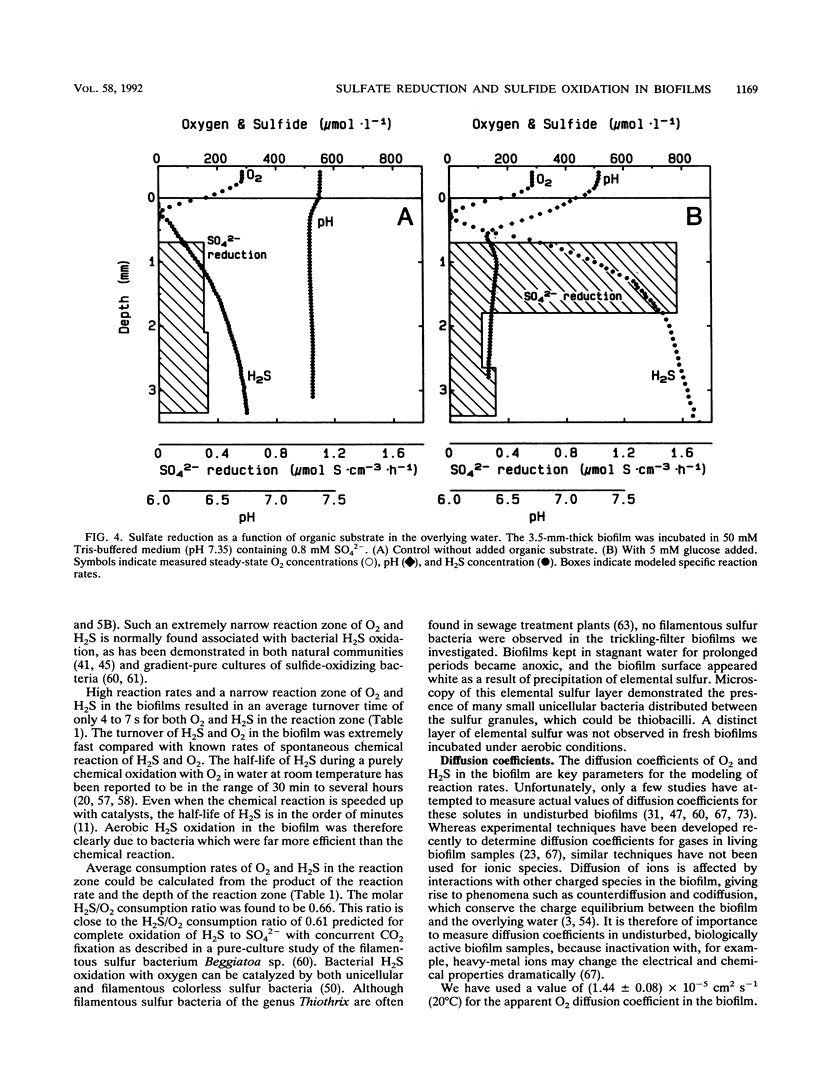
Abstract
The microzonation of O2 respiration, H2S oxidation, and SO42- reduction in aerobic trickling-filter biofilms was studied by measuring concentration profiles at high spatial resolution (25 to 100 μm) with microsensors for O2, S2-, and pH. Specific reaction rates were calculated from measured concentration profiles by using a simple one-dimensional diffusion reaction model. The importance of electron acceptor and electron donor availability for the microzonation of respiratory processes and their reaction rates was investigated. Oxygen respiration was found in the upper 0.2 to 0.4 mm of the biofilm, whereas sulfate reduction occurred in deeper, anoxic parts of the biofilm. Sulfate reduction accounted for up to 50% of the total mineralization of organic carbon in the biofilms. All H2S produced from sulfate reduction was reoxidized by O2 in a narrow reaction zone, and no H2S escaped to the overlying water. Turnover times of H2S and O2 in the reaction zone were only a few seconds owing to rapid bacterial H2S oxidation. Anaerobic H2S oxidation with NO3- could be induced by addition of nitrate to the medium. Total sulfate reduction rates increased when the availability of SO42- or organic substrate increased as a result of deepening of the sulfate reduction zone or an increase in the sulfate reduction intensity, respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canfield D. E., Des Marais D. J. Aerobic sulfate reduction in microbial mats. Science. 1991 Mar 22;251:1471–1473. doi: 10.1126/science.11538266. [DOI] [PubMed] [Google Scholar]
- Christensen P. B., Nielsen L. P., Revsbech N. P., Sørensen J. Microzonation of denitrification activity in stream sediments as studied with a combined oxygen and nitrous oxide microsensor. Appl Environ Microbiol. 1989 May;55(5):1234–1241. doi: 10.1128/aem.55.5.1234-1241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. A. Sulphate-reducing bacteria and anaerobic corrosion. Annu Rev Microbiol. 1985;39:195–217. doi: 10.1146/annurev.mi.39.100185.001211. [DOI] [PubMed] [Google Scholar]
- Jorgensen B. B., Des Marais D. J. The diffusive boundary layer of sediments: oxygen microgradients over a microbial mat. Limnol Oceanogr. 1990;35(6):1343–1355. doi: 10.4319/lo.1990.35.6.1343. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B. Ecology of the bacteria of the sulphur cycle with special reference to anoxic-oxic interface environments. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):543–561. doi: 10.1098/rstb.1982.0096. [DOI] [PubMed] [Google Scholar]
- Jørgensen B. B., Revsbech N. P. Colorless Sulfur Bacteria, Beggiatoa spp. and Thiovulum spp., in O(2) and H(2)S Microgradients. Appl Environ Microbiol. 1983 Apr;45(4):1261–1270. doi: 10.1128/aem.45.4.1261-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenen J. G., Beudeker R. F. Microbiology of thiobacilli and other sulphur-oxidizing autotrophs, mixotrophs and heterotrophs. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):473–497. doi: 10.1098/rstb.1982.0093. [DOI] [PubMed] [Google Scholar]
- Nelson D. C., Jørgensen B. B., Revsbech N. P. Growth Pattern and Yield of a Chemoautotrophic Beggiatoa sp. in Oxygen-Sulfide Microgradients. Appl Environ Microbiol. 1986 Aug;52(2):225–233. doi: 10.1128/aem.52.2.225-233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. C., Revsbech N. P., Jørgensen B. B. Microoxic-Anoxic Niche of Beggiatoa spp.: Microelectrode Survey of Marine and Freshwater Strains. Appl Environ Microbiol. 1986 Jul;52(1):161–168. doi: 10.1128/aem.52.1.161-168.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. H. Biofilm Dynamics and Kinetics during High-Rate Sulfate Reduction under Anaerobic Conditions. Appl Environ Microbiol. 1987 Jan;53(1):27–32. doi: 10.1128/aem.53.1.27-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Nielsen L. P., Christensen P. B., Sørensen J. Combined oxygen and nitrous oxide microsensor for denitrification studies. Appl Environ Microbiol. 1988 Sep;54(9):2245–2249. doi: 10.1128/aem.54.9.2245-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


