Abstract
The photoreversibility of plant phytochromes enables continuous surveillance of the ambient light environment. Through expression of profluorescent, photoinsensitive Tyr-to-His mutant alleles of Arabidopsis thaliana phytochrome B (PHYBY276H) and Arabidopsis phytochrome A (PHYAY242H) in transgenic Arabidopsis plants, we demonstrate that photoconversion is not a prerequisite for phytochrome signaling. PHYBY276H-expressing plants exhibit chromophore-dependent constitutive photomorphogenesis, light-independent phyBY276H nuclear localization, constitutive activation of genes normally repressed in darkness, and light-insensitive seed germination. Fluence rate analyses of transgenic plants expressing PHYBY276H, PHYAY242H, and other YGAF mutant alleles of PHYB demonstrate that a range of altered light-signaling activities are associated with mutation of this residue. We conclude that the universally conserved GAF domain Tyr residue, with which the bilin chromophore is intimately associated, performs a critical role in coupling light perception to signal transduction by plant phytochromes.
INTRODUCTION
Life forms living at or near the earth's surface possess a diverse array of photosensory receptors that trigger cellular signaling cascades to regulate adaptative biological responses to light at the organismal level (Chen et al., 2004; Franklin and Whitelam, 2004; Schäfer and Nagy, 2005). The cohort of photoreceptors is most complex in photosynthetic organisms that must cope with the dual threat of too much or too little light (Falciatore and Bowler, 2005; Lariguet and Dunand, 2005). Land plants possess red/far-red light (R/FR)–absorbing phytochromes (Rockwell et al., 2006), UV-A/blue light (B)–sensing crytochromes and phototropins (Briggs and Christie, 2002; Lin and Todo, 2005), and UV-B photoreceptors (Ballaré, 2003) for perception of the full range of the solar light spectrum (Franklin et al., 2005; Wang, 2005). In flowering plants, phytochromes are encoded by a small family of genes that have arisen by repeated gene duplication of a eukaryote phytochrome progenitor during the course of evolution (Mathews, 2006). The phytochrome family in the model dicot species Arabidopsis thaliana consists of five genes denoted PHYA-E (Sharrock and Quail, 1989; Clack et al., 1994), while monocot species appear only to possess representatives of the PHYA-C families (Sawers et al., 2005). Genetic analyses have facilitated characterization of the functions of individual phytochrome family members in plants, and null mutants of all five Arabidopsis phytochromes have been isolated. Light-labile phyAs appear primarily responsible for sensing very low light fluences and for adaptation to FR-enriched shade environments (reviewed in Casal et al., 1997; Sineshchekov, 2004), while the light-stable phytochromes phyB-E mediate the classic R/FR photoreversible responses and adaptation to elevated fluence rates of R (Reed et al., 1993; Franklin et al., 2003a, 2003b). The overlapping and distinct functional roles of phytochromes are further underscored by the recent observation that phyA can also mediate irradiance-dependent responses to R (Franklin et al., 2007).
Our current understanding of the structure and molecular function of phytochromes has greatly benefited from the isolation of photoreceptor mutants (reviewed in Quail, 1997). Although the majority of phytochrome mutants so far identified have been loss-of-function alleles (for a recent list of phy mutant alleles, see Supplemental Table 2 in Rockwell et al. [2006]), alleles of phytochromes with increased sensitivity to light and weak constitutive photomorphogenesis (COP) phenotypes have also been reported (Kretsch et al., 2000; Weller et al., 2004; Dieterle et al., 2005; Mateos et al., 2006). The latter are still dependent on light for function, so their functions cannot be analyzed without activating other photobiological processes. Despite robust screens for their identification, constitutively active mutants of phytochromes have not yet been isolated. Constitutively active alleles would thus be invaluable tools for characterizing the unique molecular functions of individual phytochromes.
This investigation reports the unexpected discovery of a new class of plant phytochrome mutants that exhibit light-independent signaling activity. Here, we document that dominant gain-of-function activity is caused by mutation of a conserved GAF domain Tyr residue (YGAF) previously shown to be critical for photoactivation of the cyanobacterial phytochrome Cph1 (Fischer and Lagarias, 2004; Fischer et al., 2005). Mutation of this Tyr also strongly inhibits photoactivation of plant phytochromes (Fischer et al., 2005), so we expected that YGAF mutants of Arabidopsis phyA and phyB would exhibit reduced light-signaling activity. To the contrary, the poorly photoactive, profluorescent Tyr276His allele of PHYB (PHYBY276H) not only complements phyB mutants as effectively as the wild-type PHYB allele but leads to COP development, light-independent activation of gene expression, and general R/FR insensitivity. Transgenic plants expressing the Tyr242His allele of PHYA (PHYAY242H) also display weak COP development and dominant-negative, phyA-deficient phenotypes under continuous FR. Comparative analyses of transgenic plants expressing other YGAF mutant alleles of PHYB demonstrate a critical role of this Tyr in coupling light perception with downstream signaling.
RESULTS
PHYBY276H Is a Dominant, Biologically Active Phytochrome B Allele That Can Complement PhyB-Deficient Arabidopsis Mutants
To investigate the biological activity of YGAF mutants of Arabidopsis phytochromes, we prepared transgenic lines expressing cDNA and genomic constructs of wild-type and YGAF mutant alleles of PHYA and PHYB under control of the cauliflower mosaic virus 35S promoter or the native PHY promoters (see Supplemental Figure 1 online for T-DNA maps and Supplemental Table 1 online for transgenic lines constructed). Multiple transformants were selected in various genetic backgrounds (typically more than five), from which multiple homozygous T3 lines were generated. Phenotypic data for representative plant lines for each construct are discussed below, and detailed phenotypic data for all lines are compiled in the supplemental data online.
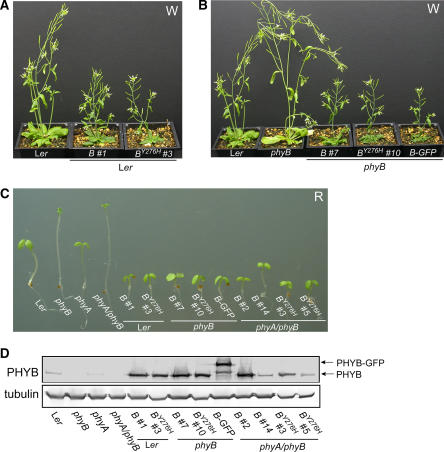
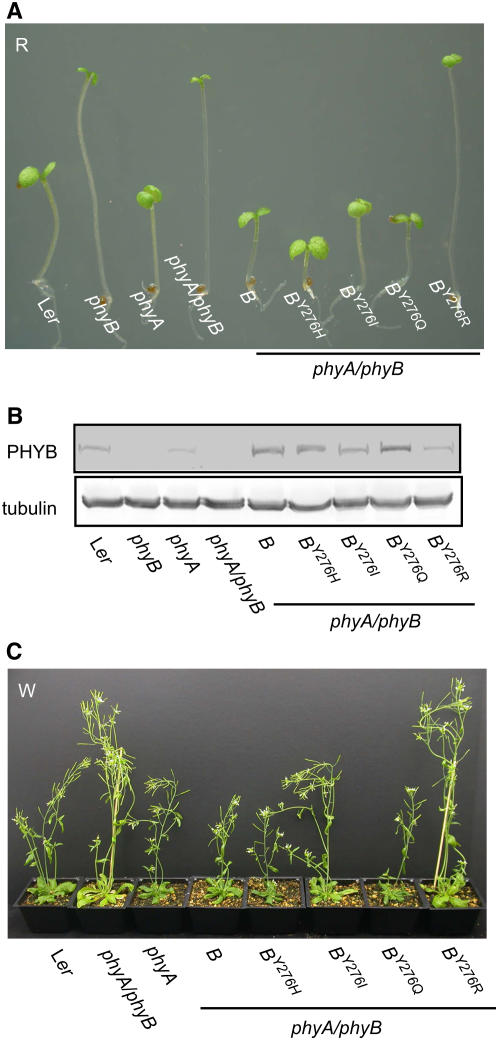
Our initial studies focused on PHYBY276H transgenic plant lines expressing the cauliflower mosaic virus 35S promoter–driven cDNA construct. Since overexpression of wild-type PHYB confers enhanced white (W) and R light sensitivity to transgenic plants (Wagner et al., 1991), we compared light-grown wild-type PHYB- and PHYBY276H-expressing transgenic plants in both Landsberg erecta (Ler) wild-type and phyB-5 null mutant backgrounds (Figures 1A and 1B). Despite the poor photoconvertibility of phyBY276H (Fischer et al., 2005; see Supplemental Figure 2 online), the apparent light-exaggerated phenotypes of transgenic plants overexpressing PHYBY276H were indistinguishable from those of plants overexpressing the wild-type PHYB allele. Hypocotyl and internode lengths of PHYBY276H-expressing transgenic plants were considerably shorter than those of untransformed Ler wild-type plants (see Supplemental Figure 3 online), and PHYBY276H plants possessed smaller rosettes under continuous white light (data not shown). PHYBY276H overexpression also rescued the characteristic elongated phenotype of phyB null mutants as effectively as both the wild-type PHYB allele and a previously described wild-type PHYB-green fluorescent protein (GFP) chimera (Yamaguchi et al., 1999; Figure 1). These results indicate that phyBY276H is biologically active in light-grown plants.
Figure 1.
The Y276H Mutant of Phytochrome B Is Functionally Active in Vivo.
(A) Five-week-old transgenic Ler plants expressing PHYB wild-type (B) and PHYBY276H (BY276H) mutant cDNA constructs grown under continuous W at a fluence rate of 50 μmol m−2 s−1 are hypersensitive to light.
(B) PHYBY276H expression rescues the phenotype of the phyB-5 (phyB) null mutant under the same conditions as (A). Ler wild-type, untransformed phyB-5, and phyB-5 mutant transformed with the wild-type PHYB cDNA construct (B) or the PHYB-GFP chimera cDNA (B-GFP) (Yamaguchi et al., 1999) are shown for comparative purposes.
(C) Six-day-old seedlings expressing cDNA and genomic constructs of wild-type PHYB (B) or the PHYBY276H mutant were grown under 20 μmol m−2 s−1 continuous R. One representative line from transformation of Ler wild-type (i.e., B#1 and BY276H#3 cDNAs), phyB-5 (i.e., B#7 and BY276H#10 cDNAs), and phyA-201/phyB-5 (i.e., B#2 and BY276H#3 cDNAs; and B#14 and BY276H#5 genes) is shown, along with R-grown untransformed parent and PHYB-GFP/phyB plant lines.
(D) Immunoblot analysis of PHYB protein level in wild-type, phyB, phyA, phyA phyB mutants, and PHYBY276H-expressing transgenic plants using monoclonal anti-PHYB antibodies. Total protein extracts (40 μg) from 6-d-old dark-grown seedlings were loaded on each lane. Tubulin was used as loading control.
We next compared the phenotypes of PHYBY276H- and PHYB-expressing transgenic lines grown under continuous R light. R is the optimal light quality for conversion of the red-absorbing Pr form of phytochrome to the FR-absorbing, signaling-active Pfr form (Furuya and Schäfer, 1996). Both cDNA and genomic constructs were introduced into Ler wild-type, phyB single mutant and phyA phyB double mutant backgrounds. As shown in Figure 1C and Supplemental Figure 3A online, overexpression of PHYBY276H, wild-type PHYB, or PHYB-GFP alleles all strongly inhibited hypocotyl growth under R regardless of the genetic background. The elongated hypocotyl phenotypes of R-grown phyB and phyA phyB seedlings were strongly suppressed by expression of the genomic PHYBY276H allele despite the near wild-type level of PHYB protein production (Figure 1D). Indeed, phyBY276H appeared more active than wild-type phyB as evidenced by the greater suppression of hypocotyl growth by PHYBY276H compared with the wild-type PHYB allele in the phyA phyB double mutant background. Taken together, these results indicate that PHYBY276H encodes a functional phytochrome.
Dark-Grown PHYBY276H-Expressing Transgenic Plants Exhibit COP
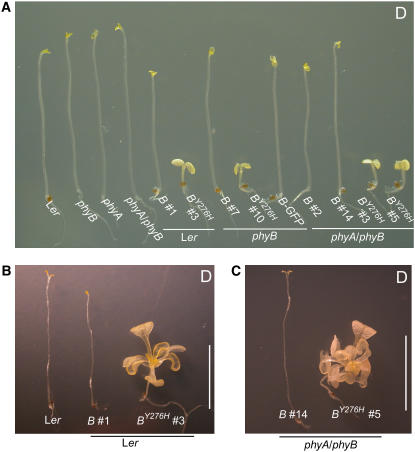
Flowering plants grown in darkness etiolate, undertaking an adaptive developmental program characterized by rapid hypocotyl/mesocotyl/epicotyl elongation growth, repression of hook opening, cotyledon/leaf expansion, and altered plastid development, known as skotomorphogenesis (Von Arnim and Deng, 1996). Etiolation facilitates emergence from soil until sufficient light is available for photoautotrophic growth. Although phytochromes are critical photosensors that regulate deetiolation of developing seedlings following skotomorphogenesis, phyA, phyB, and phyA phyB null mutants etiolate normally, demonstrating that phyA and phyB do not actively repress photomorphogenesis in darkness (Smith et al., 1997), nor does phytochrome overexpression affect etiolation, because dark-grown seedlings overexpressing phytochromes are indistinguishable from wild-type and null mutants (Smith, 1994). It was thus surprising to observe that PHYBY276H-expressing plants were fully deetiolated in complete darkness (Figure 2; see Supplemental Figure 3C online). This COP phenotype included inhibition of hypocotyl elongation, pronounced hood opening, and cotyledon expansion. Because chlorophyll synthesis requires light, neither control lines nor PHYBY276H transgenic plants greened in darkness. The PHYBY276H COP phenotype starkly contrasts with skotomorphogenetic development of untransformed Ler wild-type, phyA, phyB, and phyA phyB null mutants as well as all transgenic lines expressing the wild-type PHYB or PHYB-GFP alleles (Figure 2A).
Figure 2.
COP Phenotypes of PHYBY276H-Expressing Transgenic Plants.
(A) Six-day-old dark-grown seedlings expressing cDNA and genomic Y276H alleles of PHYB exhibit COP on sucrose-free Murashige and Skoog (MS) medium. The lines shown here are the same as in Figure 1.
(B) Four-week-old dark-grown Ler plants expressing PHYBY276H exhibit light-grown development in darkness on MS medium containing 1% (w/v) sucrose. Shown for comparison are untransformed Ler and wild-type PHYB in Ler transgenic plant line.
(C) phyA phyB double mutant plants with or without the PHYBY276H transgene were grown as in (B). Bars = 1 cm.
In T2 generations, dark-grown seedlings of multiple independent transformants of both cDNA and genomic alleles of PHYBY276H displayed COP phenotypes regardless of their genetic backgrounds. For PHYBY276H cDNA transformations, we observed six of nine COP kanamycin-resistant segregants in Ler, 16 of 18 in phyB, and 7 of 8 in phyA phyB backgrounds. For transformation of the phyA phyB background with the PHYBY276H genomic allele, COP phenotypes were observed in 8 of 8 transformants. Segregation analyses of heterozygous lines established that PHYBY276H is a dominant allele; one copy proved sufficient to confer COP phenotypes in all genetic backgrounds analyzed (data not shown). These results indicate that PHYBY276H is a completely penetrant, dominant gain-of-function allele.
Immunoblot analysis indicated that the COP phenotype mediated by the genomic PHYBY276H allele was not due to elevated expression of the PHYBY276H mutant protein (Figure 1D). PHYBY276H-dependent COP phenotypes were also unaltered by FR irradiation immediately following imbibition (data not shown), a treatment that would photorevert a hypothetical stable pool of Pfr present in seeds prior to imbibition if such a pool was present. Moreover, PHYBY276H-expressing seedlings continued to develop true leaves when sucrose was present as a carbon source, while seedlings expressing wild-type PHYB alleles showed arrested development with very long hypocotyls, unexpanded cotyledons, and no true leaves (Figures 2B and 2C). Taken together, these results indicate that PHYBY276H alleles encode constitutively active phytochrome mutants that do not require light for activation.
Bilin Chromophore Is Required for the Gain-of-Function Activity of the PHYBY276H Mutant Protein
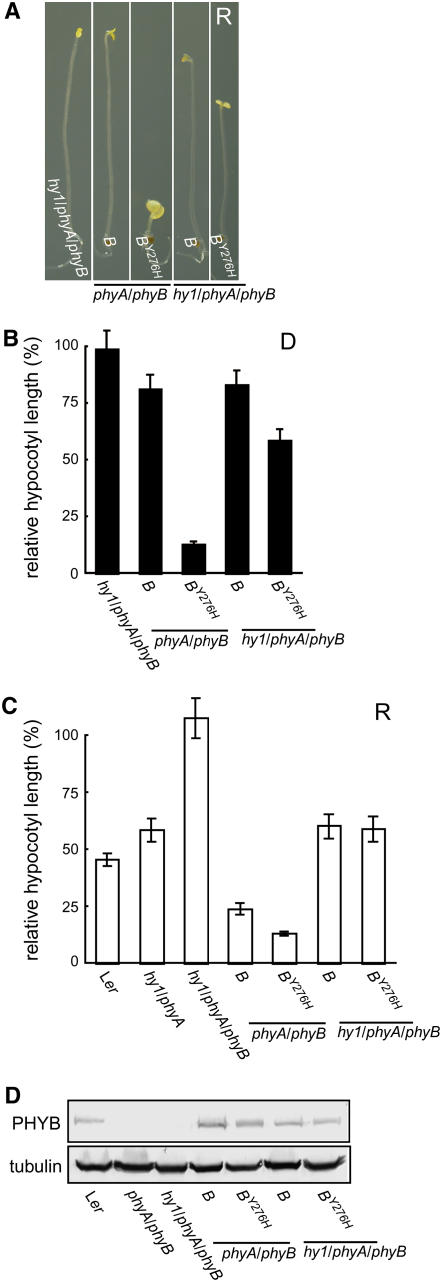
It is well established that the bilin chromophore of phytochromes is essential for light signaling (Koornneef and Kendrick, 1994; Terry, 1997; Montgomery et al., 2001; Sawers et al., 2002). To test the hypothesis that bilin chromophore is required for the gain-of-function activity of the PHYBY276H mutant, we compared the phenotypic consequence of introducing PHYBY276H and wild-type PHYB genomic alleles into both bilin-deficient hy1 phyA phyB triple and bilin-producing phyA phyB double mutant backgrounds. These analyses show that hy1-1, a null allele that is deficient in the bilin chromophore biosynthetic enzyme heme oxygenase (Muramoto et al., 1999), strongly suppressed the gain-of-function activity of the PHYBY276H allele as measured by seedling hypocotyl growth (Figure 3; see Supplemental Figure 4 online). Although dark-grown PHYBY276H transgenics in the hy1 phyA phyB background possessed shorter hypocotyls than the untransformed hy1 phyA phyB parent, PHYBY276H transgenics were significantly more etiolated in bilin-deficient hy1 phyA phyB backgrounds compared with PHYBY276H transgenics in bilin-producing phyA phyB backgrounds that exhibited a strong COP phenotype (Figures 3A and 3B). By contrast, this phenomenon was not observed in hy1 phyA phyB and phyA phyB backgrounds transformed with the wild-type PHYB genomic allele, both of which showed normal skotomorphogenesis.
Figure 3.
Phytochrome Chromophore Biosynthesis Is Required for the Gain-of-Function Activity of PHYBY276H.
(A) Transgenic plants with or without the hy1 mutation were grown in darkness for 6 d on sucrose-free media.
(B) Relative hypocotyl length of dark-grown seedlings in (A) normalized to that of Ler shows the differential activity of PHYBY276H in chromophore-replete phyA phyB and chromophore-deficient hy1 phyA phyB backgrounds. Values are mean ± sd (n = 50).
(C) Relative hypocotyl length of seedlings grown on sucrose-free media under 20 μmol m−2 s−1 continuous R. Mean hypocotyl lengths (± sd; n = 50) are normalized to those of dark-grown Ler seedlings. The shorter hypocotyl lengths of hy1/phyA, PHYB/hy1/phyA/phyB, and PHYBY276H/hy1/phyA/phyB plants compared with the hy1/phyA/phyB parent line indicate that sufficient bilin chromophore is present in the hy1 mutant background to maintain reduced but significant signaling activity of phyB and phyBY276H.
(D) Immunoblot analysis of PHYB protein level was performed as in Figure 1.
To test whether the incomplete suppression of the PHYBY276H-dependent COP phenotype by hy1 reflected partial synthesis of bilin chromophore via one of the three other heme oxygenases in Arabidopsis (Davis et al., 2001; Muramoto et al., 2002; Emborg et al., 2006), we performed an additional set of phenotypic comparisons under R (Figure 3C). These experiments showed that the hy1 mutation does not fully eliminate the light response as long as some PHYB protein is present. This indicates that residual chromophorylation of PHYB protein occurs in the hy1 mutant. This conclusion is supported by the R-dependent hypocotyl growth inhibition of hy1 phyA seedlings, whose hypocotyl lengths were ∼66% of dark-grown wild-type Ler (Figure 3C). Strikingly, the hypocotyl lengths of hy1 phyA plants under R are indistinguishable from those of hy1 phyA phyB mutants that had been transformed with either PHYB or PHYBY276H genomic alleles (Figure 3C). The latter suggests that the residual activity of wild-type phyB in the hy1 phyA phyB background is nearly identical with the light-independent activity of the phyBY276H mutant in the same genetic background under R. Taken together, these results indicate that bilin chromophore is required for the light-independent signaling activity of the PHYBY276H protein.
PhyBY276H Holoprotein Constitutively Localizes to Nuclear Bodies and Activates the Expression of Light-Regulated Genes in Both Light and Darkness
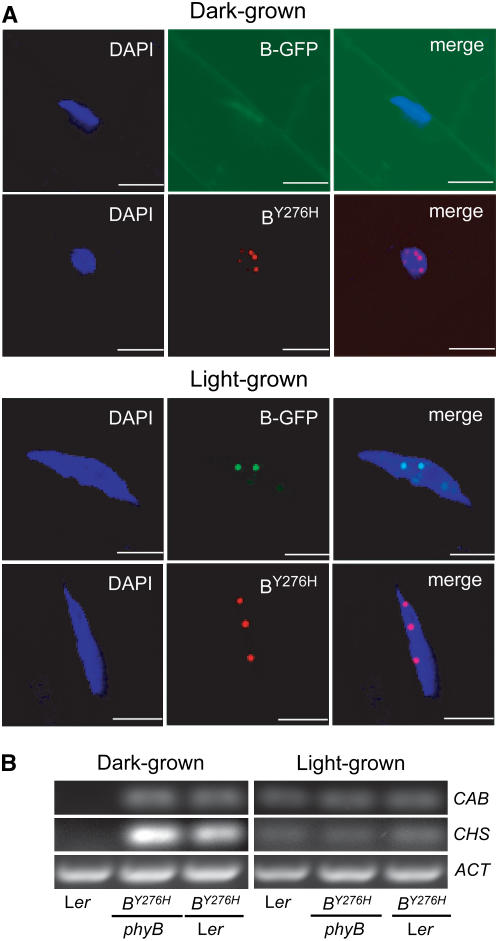
The subcellular localization of phytochrome is tightly correlated with its photoregulatory activity (Yamaguchi et al., 1999; Kircher et al., 2002). Cytoplasmically tethered phytochrome is functionally inactive even in the presence of light (Huq et al., 2003), while nuclear-localized phytochrome requires light activation for regulatory function (Matsushita et al., 2003). Therefore, nuclear-localized Pfr is needed for full signaling activity. Once in the nucleus, phytochrome accumulates in nuclear bodies or speckles, and the size of phyB nuclear bodies depends on continuous Pfr generation by light (Chen et al., 2003). Nuclear body formation also correlates with hypocotyl growth inhibition, and phyB mutants deficient in speckle formation are poorly active, supporting the conclusion that nuclear body formation plays a direct role in signal transduction (Chen et al., 2005). Because of the constitutive signaling activity of the phyBY276H mutant, we tested whether light is required for its nuclear localization. The phyBY276H holoprotein is strongly fluorescent (Fischer et al., 2005), so its localization could readily be monitored by fluorescence microscopy. In contrast with the light-dependent nuclear localization of a wild-type phyB-GFP chimera used as a control (Yamaguchi et al., 1999), phyBY276H was found in the nucleus in both dark- and in light-grown plants (Figure 4A). The presence of red fluorescent nuclear bodies in dark-grown PHYBY276H transgenics clearly indicates that both nuclear translocation and nuclear body formation of phyBY276H are light independent, a result fully consistent with the constitutive signaling activity of this mutant. The facile detection of phyBY276H fluorescence in planta also implies that the full-length mutant has similar photochemical properties to those previously reported for recombinant truncations in vitro (Fischer et al., 2005; see Supplemental Figure 2 online).
Figure 4.
PhyBY276H Localizes to the Nucleus, Forms Nuclear Bodies (Speckles), and Activates the Expression of Light-Regulated Genes in Darkness.
(A) Comparative subcellular localization of phyB-GFP and PHYBY276H-derived phyB proteins in 5-d-old dark- and continuous W-grown (80 μmol m−2 s−1) seedlings was performed by fluorescence confocal microscopy. PhyB-GFP (B-GFP) was visualized using GFP optics (green), phyBY276H (BY276H) was visualized using Texas Red optics (red), and nuclei were identified by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue). Merged images represent overlapping DAPI and GFP images (for phyB-GFP) or overlap of the DAPI with Texas Red images (for phyBY276H). Bars = 10 μm.
(B) Light-independent expression of CAB and CHS transcripts was observed in dark-grown PHYBY276H plants by RT-PCR. Ethidium bromide–stained agarose gels contain 10 μL of PCR product per lane. ACT transcript levels are shown as a control.
To ascertain whether the COP phenotype of dark-grown PHYBY276H transgenics reflects misexpression of light-regulated genes, RT-PCR analysis was performed for chalcone synthase (CHS) and chlorophyll a/b binding protein (CAB)—two well-studied light-responsive genes (Quail, 1991). While the untransformed Ler wild type requires light for measurable expression of either gene, phyBY276H strongly activated expression of both genes in darkness regardless of the presence of wild-type PHYB alleles (Figure 4B). For light-grown plants, little difference was detected in the amount of CAB or CHS gene expression between Ler wild-type and PHYBY276H transformants. Taken together, the observed light-independent nuclear localization of phyBY276H and misexpression of light-regulated genes indicate that the Tyr276His mutation bypasses both light-dependent signaling processes.
Other YGAF Alleles of PHYB Complement the PhyB-Deficient Phenotype of the phyA phyB Double Mutant
Saturation mutagenesis of the YGAF residue in the cyanobacterial phytochrome Cph1 showed that, although this Tyr is not essential for bilin attachment, it is critical for normal photochemistry (Fischer et al., 2005). Besides the intensely fluorescent YGAFH mutant of Cph1, three other amino acid substitutions (Glu, Gln, and Trp) similarly enhanced Cph1 fluorescence. By contrast, most other amino acid substitutions yielded poorly photoactive and nonfluorescent Cph1 holoproteins with more cyclic, deprotonated bilin chromophores, while the Cph1Y176R mutant bound a porphyrin (Fischer et al., 2005). To compare the phenotypic consequences of the different classes of YGAF mutant of phyB in planta, we transformed the phyA phyB null background with genomic PHYBY276Q, PHYBY276I, and PHYBY276R alleles to produce photoreceptors predicted to have fluorescent/extended, nonfluorescent/cyclic, and porphyrin chromophores, respectively. We also performed comparative spectroscopic analysis of recombinant versions of these PHYB mutant alleles expressed in Escherichia coli (see Supplemental Figures 2C and 2D online). With the exception of PHYBY276R, which failed to form a tetrapyrrole adduct, the spectroscopic properties of the YGAF mutants of phyB were in good agreement with those of the equivalent YGAF mutants of Cph1 (Fischer et al., 2005). Like phyBY276H, all of the YGAF mutants of phyB proved to be essentially photoinactive, with <10% photoactivity of wild-type phyB.
Homozygous PHYBY276Q, PHYBY276I, and PHYBY276R lines were selected and grown under R for phenotypic comparison with untransformed Ler wild-type, phyA, phyB, and phyA phyB controls as well as with phyA phyB transformants harboring PHYB wild-type and PHYBY276H alleles (Figure 5A; see Supplemental Figure 5 online). Both PHYBY276Q and PHYBY276I alleles restored R-mediated hypocotyl growth inhibition and cotyledon expansion seen in the Ler wild-type. By contrast, the PHYBY276R allele failed to complement the phyA phyB mutant under the same experimental conditions. Since YGAF mutant protein was present in all transgenic lines, albeit at slightly different levels (Figure 5B), the observed phenotypic complementation under R demonstrates signaling activity for all YGAF mutants except phyBY276R. Phenotypic comparisons of 5-week-old adult plants grown under continuous W were fully consistent with the R-grown seedling experiments (Figure 5C). The PHYBY276H and PHYBY276Q alleles proved more effective than PHYBY276I in complementing the elongated internode phenotype of the phyA phyB mutant in white light, and the PHYBY276R allele similarly proved inactive. These data demonstrate that, although the YGAF residue is critical for phytochrome's photochemical activity, the YGAF residue of phyB can be replaced with other amino acids that considerably inhibit the efficiency of its photoactivation yet still retain significant signaling activity.
Figure 5.
Phenotypic Analysis of Other Light-Grown YGAF Mutants of Phytochrome B Reveals Both Gain-of-Function and Loss-of-Function Phenotypes.
(A) Transgenic plants expressing PHYBY276I, PHYBY276Q, and PHYBY276R mutant alleles of PHYB in the phyA phyB double mutant were grown on sucrose-free media for 6 d under 20 μmol m−2 s−1 of continuous R irradiation. One representative line from each transformation is shown.
(B) Immunoblot analysis of PHYB protein levels was performed as in Figure 1.
(C) Transgenic plants expressing Y276H, Y276I, Y276Q, and Y276R alleles of PHYB were grown for 5 weeks under continuous white light (W) at the fluence rate of 50 μmol m−2 s−1.
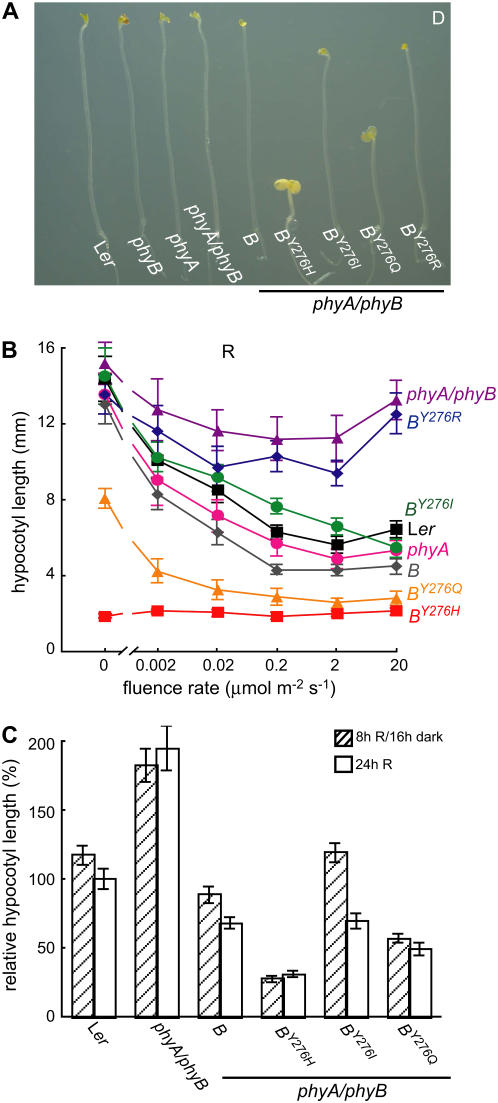
We also examined the dark-grown phenotypes of all transgenic lines. PHYBY276Q-expressing plants exhibited a COP phenotype when grown in darkness, although this phenotype was not as pronounced as in plants expressing the PHYBY276H allele (Figure 6A). While PHYBY276Q-expressing plants had shorter hypocotyls and slightly expanded cotyledons relative to wild-type plants, plants expressing PHYBY276I and PHYBY276R alleles showed normal skotomorphogenesis, demonstrating that these alleles do not encode constitutively active phyBs (Figure 6A; see Supplemental Figure 5D online). Given the comparable levels of PHYB protein accumulation in PHYBY276Q- and PHYBY276H-expressing plants (Figure 5B), these results suggest that phyBY276Q is not as active as phyBY276H.
Figure 6.
PhyBY276Q Is a Constitutively Active Phytochrome, whereas phyBY276I Requires Light for Function and phyBY276R Is Inactive.
(A) Comparative phenotypic analysis of various lines expressing YGAF alleles of PHYB in 6-d-old dark-grown seedlings on sucrose-free MS medium. One representative line from each transformation is shown.
(B) Comparative fluence response curves for hypocotyl growth indicate that phyBY276H-mediated growth suppression is red light independent, while the growth suppression activities of phyBY276Q and phyBY276I are fluence rate dependent and phyBY276R is inactive. Each data point represents the mean of 50 seedlings ± sd.
(C) Mean hypocotyl lengths (± sd; n = 50) of 6-d-old seedlings grown under 20 μmol m−2 s−1 of continuous red light (24h R) or 8-h-R/16-h-dark photoperiods are shown.
To more fully characterize the light responsiveness of the YGAF mutants, fluence rate response measurements of hypocotyl growth were undertaken for seedlings grown under continuous R. Since these experiments employed YGAF transgenic lines in a phyA phyB genetic background, the endogenous phyA and phyB photoreceptors should not interfere with the measurement of YGAF mutant activity. As shown in Figure 6B, phyBY276H proved fully activated under all fluences of R, while no activity of PHYBY276R-expressing plants could be detected at any fluence rate. Hypocotyl lengths of dark-grown PHYBY276H seedlings were indistinguishable from those of seedlings exposed to any fluence rate of R, confirming that the constitutive gain-of-function activity of phyBY276H is independent of red light.
By contrast with PHYBY276H transgenics, the phenotypes of PHYBY276Q- and PHYBY276I-expressing plants were both strongly regulated by the fluence rate of R, similar to those of plants harboring wild-type PHYB alleles (Figure 6B), demonstrating that both PHYBY276Q and PHYBY276I alleles encode functional photoreceptors that require R illumination for full activity. PHYBY276Q-expressing seedlings were shorter than both the Ler wild type and the phyA phyB mutant transformed with a wild-type PHYB allele at all fluence rates of R, indicating that phyBY276Q possesses both light-independent and light-dependent regulatory activity. By contrast, PHYBY276I appears to encode a less active phytochrome that requires continuous irradiation for sustained activation. To test this hypothesis, hypocotyl lengths of YGAF seedlings grown under 8-h-R/16-h-dark cycles were compared with those grown under continuous R. These analyses showed that hypocotyl lengths of the PHYBY276I seedlings were particularly sensitive to the two light regimes (Figure 6C). For PHYBY276I seedlings grown under 8-h-R/16-h-dark cycles, hypocotyls were ∼50% longer than seedlings grown under continuous R, which contrasts with a 20% difference for plants expressing a wild-type PHYB allele (i.e., Ler and PHYB-expressing plants). These results indicate that phyBY276I may require sustained activation for full activity, suggesting enhanced dark reversion of its light-activated Pfr form. PhyB mutants with enhanced dark reversion have been previously described, and many show reduced signaling activity under low fluence rates of light (Elich and Chory, 1997; Sweere et al., 2001).
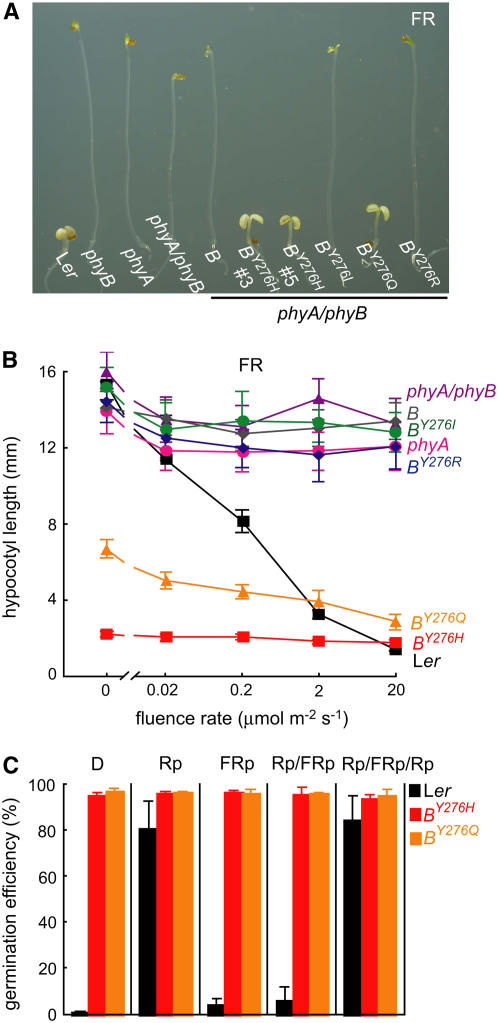
To further characterize the light responsiveness of the YGAF mutants, fluence response measurements of hypocotyl growth were also undertaken for seedlings grown under continuous FR light, conditions that specifically activate phyA signaling (Furuya and Schäfer, 1996). Since the YGAF mutants were introduced into the phyA phyB background, such experiments might reveal whether the YGAF mutations alter the light specificity of phyB signaling. In this regard, both PHYBY276H and PHYBY276Q alleles apparently restore the strong FR-dependent hypocotyl growth inhibition and cotyledon expansion responses that are deficient in the phyA phyB double mutant (Figures 7A and 7B; see Supplemental Figure 5E online). Since both alleles encode constitutively active photoreceptors in darkness (Figure 2), the apparent phyA regulatory activity is likely to be independent of FR irradiation per se. In contrast with the PHYBY276H and PHYBY276Q alleles, the PHYBY276I and PHYBY276R alleles proved inactive under FR (Figures 7A and 7B). Taken together, these experiments show that FR does not reverse the constitutive gain-of-function activities of the phyBY276H and phyBY276Q mutants—a result that strongly contrasts with the striking inhibition of wild-type phyB by FR.
Figure 7.
Signaling Activities of phyBY276H and phyBY276Q Are Not Inhibited by Continuous FR Illumination.
(A) Comparative analysis of seedling development of 6-d-old plants grown under 20 μmol m−2 s−1 continuous FR. One representative line from transformation of phyA/phyB is shown.
(B) Comparative fluence response curves for hypocotyl growth indicate that phyBY276H-mediated growth suppression is FR light independent. Each data point represents the mean of 50 seedlings ± sd.
(C) Comparative analysis of seed germination phenotypes of the wild type and phyA phyB mutant transformed with genomic PHYBY276H or PHYBY276Q alleles.
PHYBY276H- and PHYBY276Q-Expressing Transformants Exhibit Light-Independent Seed Germination
Arabidopsis seed germination is primarily regulated by phyA and phyB (Shinomura, 1997). Since seeds immediately following imbibition possess little phyA owing to its light lability, seed germination of many plant species is primarily promoted by R and inhibited by FR. Indeed, Arabidopsis seed germination is strongly inhibited by a short pulse of far-red light (FRp) that photoconverts the Pfr form of phyB already present in the seed to its inactive Pr form (Shinomura, 1997). The promotive effect of a pulse of red light (Rp) can be strongly reversed by a subsequent FRp via the so-called low fluence response—a response that is mostly mediated by phyB (Shinomura et al., 1996; Shinomura et al., 1998). To test the phenotypic consequence of YGAF alleles on seed germination and to avoid the contribution of endogenous phyA and phyB to this response, we determined the light dependence of seed germination of PHYBY276H- and PHYBY276Q-expressing lines in the phytochrome-deficient phyA phyB background (Figure 7C). Seed germination efficiency of both YGAF mutant lines was ∼100% both in complete darkness and under all light regimes tested (i.e., Rp, FRp, Rp/FRp, and Rp/FRp/Rp). By comparison, the frequencies of seed germination of Ler, phyA, and phyA phyB control lines were strongly stimulated by red light (Rp) and reversed by far-red light (FRp) (Figure 7C; data not shown). These studies not only confirm that the phyBY276H and phyBY276Q mutants are constitutively active but also demonstrate that light-independent promotion of seed germination by both YGAF mutants is not FR inhibited.
The YGAFH Allele of PHYA Exhibits Gain-of-Function Activity in Darkness and Acts as a Dominant-Negative Mutant under Continuous FR Light
To evaluate the biological activity of the YGAFH mutant of PHYA (PHYAY242H), transgenic plants expressing both wild-type PHYA and PHYAY242H mutant alleles under the control of the native PHYA promoter were constructed in Ler wild-type and phyA null mutant backgrounds (see Supplemental Figure 1 and Supplemental Table 1 online). Hypocotyl lengths of dark- and continuous FR–grown seedlings of homozygous lines were then measured (Figure 8; see Supplemental Figure 6 online). As expected (Smith, 1994), expression of the wild-type PHYA allele did not significantly affect skotomorphogenesis of Ler wild-type or PHYA-complemented phyA seedlings (Figure 8A), and the PHYA transformant in the Ler background was hypersensitive to continuous FR compared with Ler (Figures 8B and 8C; see Supplemental Figure 6 online). By contrast, PHYAY242H expression in Ler wild-type and phyA backgrounds yielded dark-grown seedlings with open cotyledons and slightly shorter hypocotyls—a phenotype similar to but not as striking as PHYBY276H-expressing transformants (Figure 8A). However, under FR, PHYAY242H-expressing seedlings in the Ler background developed significantly longer hypocotyls compared with seedlings possessing only wild-type PHYA alleles (Figure 8B). These results indicate that, at this fluence rate of FR, the PHYAY242H allele encodes a dominant-negative protein that attenuates the signaling output activity of wild-type phyA.
Figure 8.
Gain-of-Function Activity of the YGAFH Mutant of Phytochrome A.
(A) Comparative morphogenesis of 6-d-old dark-grown seedlings with or without wild-type PHYA or PHYAY242H transgenes. Plants were grown as in Figure 2.
(B) Comparative morphogenesis of 6-d-old seedlings grown under 20 μmol m−2 s−1 FR.
(C) Immunoblot analysis of PHYA protein levels was performed as in Figure 1 using monoclonal anti-PHYA antibody.
(D) Comparative fluence response curves for hypocotyl growth indicate that PHYAY242H confers gain-of-function FR-independent signaling activity to the phyA-deficient phyA-201 genotype and interferes with the FR high irradiance response of endogenous phyA in the Ler background. Each data point represents the mean of 50 seedlings ± sd.
To more fully examine the photoregulatory activity of the PHYAY242H-derived photoreceptor, fluence rate response measurements for hypocotyl growth inhibition were performed (Figure 8D). These studies showed that the activity of phyAY242H was independent of the fluence rate of FR, in striking contrast with the strong fluence rate dependence of seedlings possessing wild-type PHYA alleles (Figure 8D). Indeed, the wild-type PHYA allele fully complemented the FR-dependent inhibition of hypocotyl elongation of the phyA mutant, restoring the fluence rate response to that of the Ler wild type at fluence rates of FR above 2 μmol m−2 s−1 (Figure 8D). Interestingly, the light-independent gain-of-function activity of phyAY242H in the wild-type Ler background seen in darkness was balanced by a light-dependent dominant-negative activity at higher fluence rates. At low fluence rates of FR, PHYAY242H-expressing seedlings in the Ler background had shorter hypocotyls than the Ler wild type (as observed in darkness), while at high fluence rates, the former had longer hypocotyls (Figure 8D). This result shows that PHYAY242H functions as a constitutive gain-of-function allele in darkness and a dominant-negative allele under elevated fluence rates of FR. Immunoblot analysis further showed that PHYAY242H accumulated to lower levels than wild-type PHYA in all dark-grown genotypes examined (Figure 8C; see Supplemental Figure 6 online). This indicated that the constitutive photomorphogenetic activity of PHYAY242H was not due to elevated accumulation of the PHYAY242H mutant protein. Taken together, these results suggest that phyAY242H adopts a light-independent, signaling active state that is similar to that of phyBY276H but less active than fully activated wild-type phyA.
DISCUSSION
YGAFH Mutants of phyB and phyA Are Constitutively Active
The observation that plants expressing YGAFH mutants of phyA and phyB exhibit constitutive photomorphogenetic development in darkness establishes that the functional consequence of this mutation is the light-independent activation of both photoreceptors. While the extent of the gain-of-function activities is difficult to quantify from our data, the signal output appears greater for phyBY276H than phyAY242H. In this regard, hypocotyl growth inhibition and cotyledon expansion of dark-grown seedlings appeared fully activated in PHYBY276H transformants, while PHYAY242H seedlings only showed partial activation of these photomorphogenetic responses. We interpret the observed gain-of-function activities of both photoreceptors as reflecting the same structural consequence (i.e., that the YGAFH mutation confers a conformation that mimics the photoactivated Pfr form for both phytochromes).
Fluence rate response curves indicate that the regulatory output activity of phyBY276H is fluence rate independent, exceeding that of fully light-activated wild-type phyB (Figure 6). We attribute the light-independent hyperactivity of phyBY276H to two factors: one, the inability to fully photoconvert wild-type phyB to 100% Pfr; and two, the lack of phyBY276H dark reversion. In this regard, red light can only produce a maximum of 85% Pfr for phyA owing to the spectral overlap of Pr and Pfr forms (Lagarias et al., 1987). The percentage of photoconversion is even lower for phyB due to dark reversion, especially under lower fluence rates of light (Hennig and Schäfer, 2001). Since the light-independent activation of the phyBY276H mutant is not constrained by the photoequilibrium limitations of the wild type and its lack of dark reversion would sustain its activation in darkness (or under low fluence rates of light), the hyperactivated phenotype of the PHYBY276H plants is consistent with a photoinsensitive, non-dark-reverting allele of activated phyB.
Our studies also show that the activity of phyAY242H is not affected by illumination with FR light in the phyA genetic background (Figure 8D). We therefore conclude that phyAY242H also adopts a light-independent activated conformation, although one that is apparently less active than fully FR-activated wild-type phyA. The partial activity of phyAY242H can be attributed to the different modes of action of phyA and phyB photoreceptors (Reed et al., 1994; Furuya and Schäfer, 1996). Owing to its light lability and to the negative feedback of its own transcription, phyA accumulation in plants is strongly light regulated (Quail, 1991). This light lability is responsible for the low abundance of phyA in light-grown plants and for its elevated level in dark-grown plants. Indeed, preliminary immunoblot analysis indicates that PHYA levels are extremely low in light-grown PHYAY242H plants, very similar to those in wild-type Ler (data not shown). The COP phenotype of dark-grown PHYAY242H-expressing seedlings is therefore unlikely to represent previously light-activated phyA in seed embryos, as was recently reported for plants constitutively expressing a PHYA-GFP-β-glucuronidase chimera (Mateos et al., 2006).
The dominant-negative phenotype of PHYAY242H-expressing transgenic plants in wild-type Ler backgrounds also is consistent with the reduced specific activity of phyAY242H compared with fully photoactivated wild-type phyA. Since phyA is an obligate homodimer (Jones and Quail, 1986; Hennig and Schäfer, 2001), heterodimers between phyAY242H and wild-type phyA subunits could explain the dominant-negative phenotype. Indeed, heterodimerization could also explain the dominant-negative phenotypes observed by expression of variously truncated (and potentially weakly functional alleles) of both PHYA and PHYB in wild-type genetic backgrounds (Boylan et al., 1994; Wagner et al., 1996). Based on an elegant two-pulse experimental setup, Shinomura et al. (1996) proposed that the active form of phyA that mediates the FR high irradiance response is not Pfr but a Pr species that has been cycled through Pfr. We therefore conclude that the dominant-negative regulatory activity of phyAY242H under high fluence rates of FR reflects a photostable Pfr-like state that is not as active as cycled Pr. Hence, in the Ler genetic background, heterodimers between wild-type phyA and phyAY242H mutant subunits can never be fully activated by FR, leading to the dominant-negative phenotype at elevated fluence rates.
The Biological Activities of Other YGAF Mutants of PhyB Implicate a Critical Role of the GAF Domain Tyr Residue in Coupling Light Perception to Signaling Output
Our studies show that the phenotypic consequence of the mutation of the YGAF residue of phytochrome B is strongly dependent on the particular amino acid substitution chosen. His and Gln substitutions both confer gain-of-function COP activity in darkness, suggesting that the two amino acid substitutions have similar effects on structural and biological properties of phyB. In contrast with the light-independent constitutive activity of phyBY276H, phyBY276Q exhibits some light R sensitivity (Figure 6B). It is interesting that both YGAFH and YGAFQ substitutions have similar effects on the spectroscopic properties of Cph1 (i.e., the two mutants are strongly fluorescent and both possess extended, protonated bilin chromophores) (Fischer et al., 2005). This suggests that the structural perturbations in the chromophore binding pocket that give rise to the fluorescence gain-of-function of these YGAF mutants mimic the light activation of phytochrome.
The correlation of biological activity with the spectral properties of specific YGAF mutations is further underscored by the phenotypic consequences of the expression of PHYBY276I and PHYBY276R alleles—neither of which confers a COP-like phenotype to transgenic plants. The observation that PHYBY276I complements the phyB deficiency of the phyA phyB double mutant under both W and R is of particular interest. Indeed, full complementation by PHYBY276I was unexpected in view of its poor photoconvertibility and the cyclic, deprotonated chromophore of the phyBY276I mutant observed in vitro (see Supplemental Figure 2 online). PHYBY276I-derived mutant plants are considerably taller than those possessing wild-type PHYB alleles, particularly under light/dark cycles. This supports the interpretation that the light-activated Pfr form of phyBY276I is unstable, possibly exhibiting enhanced reversion to the inactive Pr form in darkness. However, phyB mutant alleles with increased dark reversion are typically hypomorphic (Elich and Chory, 1997). These results suggest that either the specific activity of the light-activated Pfr form of phyBY276I exceeds that of wild-type phyB or that continuous prolonged exposure to light enables the phyBY276I chromophore to adopt a more extended, photoactive form more similar to the wild type. Regardless of the structural basis of the unique phenotypes conferred by the various YGAF mutants, their distinct regulatory activities highlight the functional importance of the GAF domain Tyr residue in coupling light perception to downstream signaling.
Mechanistic Implications of YGAF Mutant Studies: An Emerging Model for Phytochrome Signaling
The constitutive signaling activity of YGAFH (and YGAFQ) mutant alleles of plant phytochromes indicates that these mutant receptors bypass light-dependent processes. Light activation not only induces translocation of phytochromes into the nucleus (Nagatani, 2004), but sustained light activation is required for their interaction with, and regulation of, nuclear factors that modulate gene transcription (Khanna et al., 2004). Based on functional analysis of deletion mutants of phyB in transgenic plants, a molecular mechanism for phytochrome signaling has emerged in which the signaling activity of the N-terminal PSD of phytochrome is repressed by interaction with regulatory domains within the C terminus. In support of this hypothesis, Matsushita et al. (2003) report that a severely truncated PSD is sufficient for full phyB signaling. Their studies also imply that this activity requires an NLS and a protein dimerization motif, both of which were removed by the truncation. Based on these results and on the discovery that the C terminus of phyB harbors a cryptic NLS, Chen et al. (2005) suggest that the initial biological process following light activation is the exposure of the NLS triggering phyB translocation into the nucleus. Within the nucleus, phytochrome must be maintained in the Pfr form for full and sustained regulatory activity, a property that is correlated with the amount and size of nuclear bodies (speckles) (Chen et al., 2005). Taken together, these observations support the hypothesis that the PSD is held in a repressed state by its interaction with CTRDs both within the cytosol and within the nucleus.
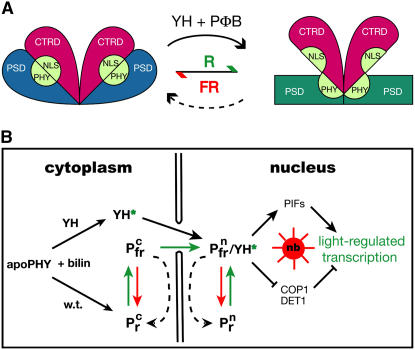
Using fluorescence microscopy, our studies establish that nuclear localization and speckle formation of the YGAFH mutant of phytochrome B are constitutive, a result fully consistent with the light-independent disruption of the intradomain interactions that inhibit both of these signal transfer steps. Our investigation also shows that chromophore binding is required for full derepression because the gain-of-function activity of phyBY276H is absent in the chromophore-deficient hy1 background. We therefore conclude that the gain-of-function activity of phyBY276H reflects a chromophore-induced protein conformational change of the PHYBY276H apoprotein that mimics light activation of the wild-type photoreceptor. As shown in Figure 9A, we envisage a model for phytochrome signaling in which activation involves a chromophore-dependent perturbation of residues within the GAF domain of the PSD that releases its interaction with the CTRD—a domain-uncoupling process that is light dependent for wild-type phytochrome but light independent for both phyBY276H and phyAY242H.
Figure 9.
Proposed Mechanism of Light-Independent Light Signaling by YGAFH Mutant Phytochromes.
(A) A proposed model of phytochrome protein conformational changes is shown. In YGAFH PHYB (or PHYA) apoproteins and the Pr form of the wild type (left), the photosensory domains (PSDs) are tightly associated with the C-terminal regulatory domains (CTRDs). This association masks a cryptic nuclear localization signal (NLS) located in the PAS repeat region within the CTRD that is specific to plant phytochromes (Chen et al., 2005). Activation occurs by Pr-to-Pfr photoconversion for the wild-type (green arrow) or by assembly of the YGAFH mutant apoprotein (YH) with phytochromobilin (PΦB) to produce the activated holoprotein species YH*. This results in release (or uncoupling) of the CTRD domain from the PSD by chromophore-mediated allosteric changes within the GAF domain that potentially are transduced via the PHY subdomain of the PSD. We envisage that this conversion exposes the CTRD-localized NLS, triggering nuclear translocation of phytochrome. For the wild type, this conversion is metastable and can be reversed both by FR irradiation (red arrow) or by dark reversion.
(B) A cellular model for phytochrome signaling. For wild-type phytochromes, nuclear migration, nuclear body (speckle) formation, and transcription of light-regulated genes require both PΦB chromophore binding (black arrows on left) and red light activation (green arrows). While the relationship of nuclear body (nb) formation to PIF-dependent transcription, proteosome-mediated protein turnover of these factors, and COP1- and DET1-dependent repression pathways remains unresolved, all of these phyB-mediated signaling processes are reversed by FR light (red arrows) and/or by dark reversion (black dashed arrows). By contrast, PΦB binding is sufficient for light-independent activation of the YGAFH mutant of phytochrome B, since YH* activates these processes in the absence of light (solid black arrows). The superscript “c” and “n” refer to cytoplasmic and nuclear localization, respectively.
Despite similar signaling outputs, the molecular mechanism underlying signal activation within the GAF domains of wild-type and YGAFH mutants may not be identical. The YGAF Tyr lies within 3.6 Å of the chromophore D-ring in the recent high-resolution crystal structure of the bacteriophytochrome DrBphP (Wagner et al., 2007). YGAF also lies within 3.5 Å of the conserved aromatic residue at position 203 in DrBphP. Residue 203 is part of a conserved, solvent-accessible surface in the available crystal structures of truncated PSDs (Wagner et al., 2005, 2007; Rockwell and Lagarias, 2006) that may constitute a docking site for domains absent in these structures, such as the PHY domain (see Montgomery and Lagarias [2002] for discussion of the various domains of phytochromes). Indeed, recent work has shown that the PHY domain can be cross-linked to a residue lying near this surface in the bacteriophytochrome Agp1 (Noack et al., 2007). The YGAF mutations may therefore disrupt photochemistry due to the proximity of this residue to, and its interaction with, the chromophore that can derepress signaling by distorting a domain–domain interaction surface. The distinct biological activities of the various YGAF mutants examined here strongly imply that this Tyr performs a critical role in coupling light perception to signal transduction.
With regard to transcriptional regulation, these studies show that two representative light-regulated genes, CHS and CAB, are activated in PHYBY276H seedlings grown in darkness. Misregulation of CHS expression is notable since its transcription is mainly B/UV-light dependent in Arabidopsis (Batschauer et al., 1996), while CAB transcription is known to be strongly phytochrome dependent (Chory et al., 1993). The observed light-independent expression of these two light-regulated genes implies that phyBY276H constitutively regulates the nuclear factors that mediate the transcription of these genes. Notable among these are members of the PIF3 family of basic helix-loop-helix transcription factors (Bailey et al., 2003), components of COP1 and DET1-containing complexes that target the degradation of the bZIP transcription factor HY5 (Yi and Deng, 2005), and factors that participate in nuclear body formation (Chen et al., 2003). Recent work has shown that photoactivated phyB can interact with various members of the PIF3 family within the nucleus, presaging their phosphorylation and protein turnover (Oh et al., 2004; Al-Sady et al., 2006). The previously reported interaction between phyB's C terminus and COP1 (Yang et al., 2001) suggests that photoactivated phyB may directly interact with COP1 to regulate HY5 accumulation. In this regard, a direct interaction between the Pfr form of phyA and COP1 has been shown to be responsible for the rapid turnover of phyA (Seo et al., 2004). This type of light-dependent interaction could also influence the E3-ligase activity of COP1 toward other regulators of photomorphogenesis—an interaction that we propose to be light independent for phyBY276H. Figure 9B compares the light-independent signaling processes that appear constitutively activated by the phyBY276H mutant (in black) and the light-dependent processes that are regulated by wild-type phytochromes (in green and red) when bilin chromophore is present.
Agronomic Applications of YGAF Mutants to Regulate Light Responses of Crop Plant Species
Identification of a constitutively active allele of plant phytochromes not only represents a new tool to elucidate the molecular mechanism of phytochrome signaling but also holds great potential for crop improvement. We have shown that seed germination can be dramatically altered by expression of PHYBY276H (and PHYBY276Q); hence, the potential for enhancing germination of certain crop plant species in shade environments is quite real. PHYBYGAFH alleles also should be particularly effective for inhibiting shade avoidance responses. Based on the data presented here, we expect that the constitutive activity of phyBY276H will counteract the enhanced elongation growth response of plants to FR-enriched shade- and reflected-light environments (Schmitt et al., 1995; Smith and Whitelam, 1997; Franklin and Whitelam, 2005; Izaguirre et al., 2006). Phytochrome-mediated shade avoidance responses are often counterproductive in high-density agricultural venues, where they contribute to significant losses in crop yield (Kasperbauer, 1987; Smith et al., 1990). With the appropriate choice of promoter, expression of PHYBYGAFH alleles ultimately may also prove useful for regulation of many aspects of plant development that are affected by light, including seed germination, elongation growth, flowering, seed yield, and senescence.
METHODS
Plant Materials, Growth Conditions, and Phenotypic Analyses
Arabidopsis thaliana ecotype Ler wild-type and phyA-201, phyB-5, phyA-201 phyB-5, hy1-1 phyA-201, and hy1-1 phyA-201 phyB-5 mutants (all in Ler ecotype) were obtained from colleagues or The Arabidopsis Information Resource (http://www.arabidopsis.org/). The Pro35S:PHYB-GFP–expressing line PBG-5 in phyB-5 (Yamaguchi et al., 1999) was used as a control for fluorescence microscopy. Seedlings were grown at 20°C on 0.8% (w/v) agar media (Phytoblend; Caisson Laboratories) containing half-strength MS salt, half-strength vitamin solution, and 1% (w/v) sucrose unless stated otherwise. For hypocotyl length measurement, seedlings were grown on sucrose-free media containing 1× MS salt, 1× vitamin solution, and 0.8% (w/v) agar for 6 d, and mean hypocotyl lengths (± sd) of 50 seedlings were determined using ImageJ software (http://rsb.info.nih.gov/ij/). SNAP-LITE light sources (Quantum Devices) were used for red (662 ± 15 nm) and FR light (730 ± 15 nm). Philips F48T12 cool white VHO 1LP fluorescent lights were used as the continuous white light source with a fluence rate of 50 to 100 μmol m−2 s−1. For germination experiments, seeds were surface sterilized and sowed on top of four layers of moist filter papers, followed by treatment with saturated FR light (18 mmol m−2) and kept in the dark at 4°C for 3 d prior to the induction of germination. Imbibed seeds were kept at 20°C in darkness or treated with <5-min saturating pulses of R (1.5 mmol m−2), FR (5 mmol m−2), R (1.5 mmol m−2) followed by FR (5 mmol m−2), or R (1.5 mmol m−2) followed by FR (5 mmol m−2) and then by R (1.5 mmol m−2), and incubated in darkness at 23°C for 6 d before germination efficiencies were scored. The mean of germination efficiency was calculated from at least three independent experiments (∼100 seeds per experiment).
Plant Transformation Constructs
The Arabidopsis PHYB cDNA plant transformation vector pJM61 was described previously (Maloof et al., 2001). Construction of the PHYBY276H cDNA plant transformation vector encoding the PHYBY276H mutant entailed two cloning steps. The pBS-PHYBY276H-ST plasmid was initially constructed by mutagenizing the plasmid pBS-PHYB-ST (Fischer et al., 2005) using the QuikChange site-directed mutagenesis kit (Stratagene) with forward and reverse primers 5′-GGTTATGATCGTGTTATGGTTCATAAGTTTCATGAAGATGAGC-3′ and 5′-GCTCATCTTCATGAAACTTATGAACCATAACACGATCATAACC-3′. The mutagenized region was excised with BamHI and SpeI restriction enzymes and cloned into the similarly restricted PHYB-containing plant transformation vector pJM61 to generate pJM61YH. To generate genomic PHYBY276H, PHYBY276I, PHYBY276Q, and PHYBY276R plant transformation constructs, the PHYB genomic DNA-containing plasmid pJM78 was used for site-directed mutagenesis using the following forward and reverse primer sets: for PHYBY276H (see above); for PHYBY276I (5′-GGTTATGATCGTGTTATGGTTATTAAGTTTCATGAAGATGAGC-3′ and 5′-GCTCATCTTCATGAAACTTAATAACCATAACACGATCATAACC-3′); for PHYBY276Q (5′-GTTATGATCGTGTTATGGTTCAAAAGTTTCATGAAGATGAGC-3′ and 5′-GCTCATCTTCATGAAACTTTTGAACCATAACACGATCATAAC-3′); and for PHYBY276R (5′-TTATGATCGTGTTATGGTTCGTAAGTTTCATGAAGATGAGC-3′ and 5′-GCTCATCTTCATGAAACTTACGAACCATAACACGATCATAA-3′). The mutagenized regions were excised with SacII and PstI and cloned into the similarly restricted PHYB genomic DNA-containing plant transformation vector pJM63 to generate plasmids pJM63YH, pJM63YI, pJM63YQ, and pJM63YR. For PHYAY242H vector construction (encoding the PHYAY242H mutant), the PHYA coding region was amplified with Pfu polymerase (Stratagene) using forward and reverse primers 5′-AGAGCTCATGTCAGGCTCTAGGCCGACT-3′ and 5′-CTAGTCGACCTACTTGTTTGCTGCAGCGAGTTC-3′ and the PHYA cDNA-containing plasmid pA2a (a kind gift of Joanne Chory, Salk Institute, La Jolla, CA) as the DNA template. The resulting PCR product was blunt-end cloned into pBluescript II KS+ restricted with EcoRV to yield pBS-PHYA. Plasmid pBS-PHYAY242H was generated using the QuikChange site-directed mutagenesis kit with plasmid pBS-PHYA as template and the following primers: 5′-GGTATGACAGGGTGATGGCTCATAAGTTTCATGAAGATGATCAC-3′ and 5′-GTGATCATCTTCATGAAACTTATGAGCCATCACCCTGTCATACC-3′. To express PHYA and PHYAY276H cDNAs under the control of the PHYA promoter (ProPHYA), the PHYA promoter was amplified with Pfu polymerase using Columbia genomic DNA as template and the primers 5′-GGAATTCGAATTGCGCTGTCTAGATAAGA-3′ and 5′-AGAGCTCGGATCCCCTTTTTCCTGACACAGAGAC-3′. The PCR product was blunt-end cloned into pBluescript II KS+ restricted with EcoRV to yield pBS-ProPHYA. The PHYA cDNA was restricted from pBS-PHYA with SacI and SalI, and the PHYA promoter region was restricted from pBS-ProPHYA with EcoRI and SacI. The two fragments were cloned into EcoRI- and SalI-restricted pCHF1 to yield pCHF1-ProPhyA:PHYA. An analogous strategy was used to generate pCHF1- ProPhyA:PHYAY242H.
Plant Transformation and Genetic Selection
Arabidopsis ecotype Ler (wild-type), phyA-201, phyB-5, phyA-201 phyB-5, and hy1-1 phyA-201 phyB-5 were transformed with the floral dip technique using Agrobacterium tumefaciens strain GV3101 as the host (Clough and Bent, 1998). Transgenic plants were selected on solid media containing half-strength MS salt, half-strength vitamin solution, 1% (w/v) sucrose, and 0.8% (w/v) agar (Phytoblend; Caisson Laboratories) containing 35 μg/mL of kanamycin (for pJM61 [PHYB and PHYBY276H] cDNA and pJM63 [PHYB, PHYBY276H, PHYBY276I, PHYBY276Q, and PHYBY276R] PHYB genomic constructs) or 100 μg/mL of gentamycin (for pCHF1-based PHYA [PHYA and PHYAY242H] cDNA constructs). Transgenic lines segregating ∼3:1 for antibiotic resistance in the T2 generation were selected, and the T3 or T4 homozygous generation was used for photographs, protein extraction, and phenotypic analyses.
Total Protein Extraction and Immunoblot Analysis
For total protein extraction, 6-d-old dark-grown seedlings were frozen in liquid nitrogen, ground into powder, and extracted with hot SDS buffer (165 mM Tris-HCl, pH 6.8, 5.1% [w/v] SDS, 5 mM EDTA, 5 mM EGTA, 5% [v/v] β-mercaptoethanol, and 1 mM PMSF) and boiling for 1 min. The soluble fraction was clarified by centrifugation, and proteins were precipitated by methanol-chloroform extraction (Wessel and Flugge, 1984). Protein pellets were dissolved in 50 mM Tris-HCl, pH 6.8, containing 2% (w/v) SDS, and total protein concentration was determined by BCA protein assay reagent using BSA as standard (Pierce). Equal amounts of proteins were separated on SDS-PAGE (Laemmli, 1970) and electroblotted to a polyvinylidene difluoride membrane. Mouse monoclonal anti-PHYA 073D, anti-PHYB B6-B3, and anti-α-tubulin antibodies (Sigma-Aldrich) were used for immunodetection of PHYA, PHYB, and tubulin, respectively. After washing, blots were incubated with alkaline phosphatase-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology). Immunoreactive bands were visualized by incubating blots with NBT/BCIP reagent (Pierce).
RT-PCR Analysis
Total RNA was isolated from 7-d-old dark- and light-grown seedlings using TRIzol reagent (Invitrogen). First-strand cDNA was synthesized using the StrataScript first-strand synthesis system (Stratagene), and 1 μL of first-strand cDNA was used for 25 μL of PCR reaction. CAB, CHS, and actin (ACT) transcripts were amplified using the following primer sets: CAB-F, 5′-TAAGGCCGTCAAGCTTTCCCC-3′, and CAB-R, 5′-TACCATGGGCTGCCTGATGG-3′ (Usami et al., 2004); CHS-F, 5′-CGCATCACCAACAGTGAACAC-3′, and CHS-R, 5′-TTCCTCCGTCAGATGCATGTG-3′ (Mehrtens et al., 2005); Actin-F, 5′-ATGAAGATTAAGGTCGTGGCA-3′, and Actin-R, 5′-TCCGAGTTTGAAGAGGCTAC-3′ (Abe et al., 2004). PCR reactions were performed using the following cycle: 94°C for 2 min and 94°C for 30 s, 56°C for 30 s, and 72°C for 40 s for 35 cycles, followed by 72°C for 10 min. Ten microliters of PCR reactions were separated on 2% TAE gels and visualized with ethidium bromide staining.
Fluorescence Microscopy for Phytochrome Localization
Five-day-old dark- and light-grown (continuous white light, 80 μmol m−2 s−1) seedlings were stained with 50 ng/mL of DAPI in PBS buffer for 30 min followed by destaining in PBS buffer for 10 min. DAPI-stained seedlings were transferred to a microscope slide immersed in PBS buffer under a cover slip and examination by fluorescence microscopy at the MCB Microscopy Imaging Facility. An Olympus FV1000 confocal laser scanning microscope equipped with an LD violet diode laser (405 nm, 25 mW), a multiline Ar laser (457 nm, 488 nm, 515 nm, Total 30 mW), a HeNe-G laser (543 nm, 1 mW), and a HeNe-R laser (633 nm, 10 mW) using DAPI (EX 405, EM 425/75), GFP (EX 488, EM500/55), and CY-5 (EX 633, EM 650LP) filter sets were used to visualize DNA, phyB-GFP, and phyBY276H, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Schematic Diagram of Binary Vector Constructs.
Supplemental Figure 2. Spectra of PCB Adducts of Recombinant Wild-Type and YGAF Mutants of At PHYA and At PHYB.
Supplemental Figure 3. Hypocotyl Lengths of Multiple PHYBY276H Transgenic Lines Grown in Continuous Red Light and Darkness.
Supplemental Figure 4. Relative Hypocotyl Lengths of Multiple PHYBY276H Transgenic Lines in Phytochromes and the Chromophore-Deficient Background Grown in Darkness and Continuous Red Light.
Supplemental Figure 5. Hypocotyl Lengths of Multiple Transgenic Lines Expressing PHYBYGAF Mutant Alleles Grown in Continuous Red Light, Darkness, and Continuous Far-Red Light.
Supplemental Figure 6. Hypocotyl Lengths of Multiple PHYAY242H Transgenic Lines Grown in Continuous Far-Red Light and Darkness.
Supplemental Table 1. Transgenic Plant Lines Expressing Wild-Type and YGAF Mutant Phytochromes.
Supplemental Methods.
Supplementary Material
Acknowledgments
We thank Joanne Chory, Jason Reed, Christian Fankhauser, and Julin Maloof for providing phytochrome mutants and PHYA/B clones, Akira Nagatani for providing PBG-5 seed, Peter Quail for providing PHYA monoclonal antibody 073D and PHYB monoclonal antibody B6-B3, Michael Paddy for fluorescence microscopy assistance, Amanda Fischer for recombinant phyA(N599) expression data, and Nathan Rockwell, Wei Hu, and Donna Lagarias for valuable editorial contributions. This work was supported in part by National Institutes of Health Grant GM068552 to J.C.L. and National Science Foundation Grant PHY-0120999 to the Center for Biophotonics Science and Technology.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: J. Clark Lagarias (jclagarias@ucdavis.edu).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Abe, T., Thitamadee, S., and Hashimoto, T. (2004). Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 45 211–220. [DOI] [PubMed] [Google Scholar]
- Al-Sady, B., Ni, W.M., Kircher, S., Schafer, E., and Quail, P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasorne-mediated degradation. Mol. Cell 23 439–446. [DOI] [PubMed] [Google Scholar]
- Bailey, P.C., Martin, C., Toledo-Ortiz, G., Quail, P.H., Huq, E., Heim, M.A., Jakoby, M., Werber, M., and Weisshaar, B. (2003). Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 15 2497–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré, C.L. (2003). Stress under the sun: Spotlight on ultraviolet-B response. Plant Physiol. 132 1725–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer, A., Rocholl, M., Kaiser, T., Nagatani, A., Furuya, M., and Schäfer, E. (1996). Blue and UVA light-regulated CHS expression in Arabidopsis independent of phytochrome A and phytochrome B. Plant J. 9 63–69. [Google Scholar]
- Boylan, M., Douglas, N., and Quail, P.H. (1994). Dominant negative suppression of Arabidopsis photoresponses by mutant phytochrome A sequences identifies spatially discrete regulatory domains in the photoreceptor. Plant Cell 6 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs, W.R., and Christie, J.M. (2002). Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 7 204–210. [DOI] [PubMed] [Google Scholar]
- Casal, J.J., Sanchez, R.A., and Yanovsky, M.J. (1997). The function of phytochrome A. Plant Cell Environ. 20 813–819. [Google Scholar]
- Chen, M., Chory, J., and Fankhauser, C. (2004). Light signal transduction in higher plants. Annu. Rev. Genet. 38 87–117. [DOI] [PubMed] [Google Scholar]
- Chen, M., Schwabb, R., and Chory, J. (2003). Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc. Natl. Acad. Sci. USA 100 14493–14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M., Tao, Y., Lim, J., Shaw, A., and Chory, J. (2005). Regulation of phytochrome B nuclear localization through light-dependent unmasking of nuclear-localization signals. Curr. Biol. 15 637–642. [DOI] [PubMed] [Google Scholar]
- Chory, J., Altschmied, L., Cabrera, H., Li, H.-M., and Susek, R. (1993). Genetic dissection of signal transduction pathways that regulate CAB gene expression. In Cellular Communication in Plants, R.M. Amasino, ed (New York: Plenum Press), pp. 57–62.
- Clack, T., Mathews, S., and Sharrock, R.A. (1994). The phytochrome apoprotein family in Arabidopsis is encoded by five genes - The sequences and expression of PHYD and PHYE. Plant Mol. Biol. 25 413–427. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16 735–743. [DOI] [PubMed] [Google Scholar]
- Davis, S.J., Bhoo, S.H., Durski, A.M., Walker, J.M., and Vierstra, R.D. (2001). The heme-oxygenase family required for phytochrome chromophore biosynthesis is necessary for proper photomorphogenesis in higher plants. Plant Physiol. 126 656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle, M., Bauer, D., Buche, C., Krenz, M., Schäfer, E., and Kretsch, T. (2005). A new type of mutation in phytochrome A causes enhanced light sensitivity and alters the degradation and subcellular partitioning of the photoreceptor. Plant J. 41 146–161. [DOI] [PubMed] [Google Scholar]
- Elich, T.D., and Chory, J. (1997). Biochemical characterization of Arabidopsis wild-type and mutant phytochrome B holoproteins. Plant Cell 9 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg, T.J., Walker, J.M., Noh, B., and Vierstra, R.D. (2006). Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol. 140 856–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falciatore, A., and Bowler, C. (2005). The evolution and function of blue and red light photoreceptors. Curr. Top. Dev. Biol. 68 317–350. [DOI] [PubMed] [Google Scholar]
- Fischer, A.J., and Lagarias, J.C. (2004). Harnessing phytochrome's glowing potential. Proc. Natl. Acad. Sci. USA 101 17334–17339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A.J., Rockwell, N.C., Jang, A.Y., Ernst, L.A., Waggoner, A.S., Duan, Y., Lei, H., and Lagarias, J.C. (2005). Multiple roles of a conserved GAF domain tyrosine residue in cyanobacterial and plant phytochromes. Biochemistry 44 15203–15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, K.A., Allen, T., and Whitelam, G.C. (2007). Phytochrome A is an irradiance-dependent red light sensor. Plant J. 50 108–117. [DOI] [PubMed] [Google Scholar]
- Franklin, K.A., Davis, S.J., Stoddart, W.M., Vierstra, R.D., and Whitelam, G.C. (2003. a). Mutant analyses define multiple roles for phytochrome C in Arabidopsis photomorphogenesis. Plant Cell 15 1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, K.A., Larner, V.S., and Whitelam, G.C. (2005). The signal transducing photoreceptors of plants. Int. J. Dev. Biol. 49 653–664. [DOI] [PubMed] [Google Scholar]
- Franklin, K.A., Praekelt, U., Stoddart, W.M., Billingham, O.E., Halliday, K.J., and Whitelam, G.C. (2003. b). Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 131 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, K.A., and Whitelam, G.C. (2004). Light signals, phytochromes and cross-talk with other environmental cues. J. Exp. Bot. 55 271–276. [DOI] [PubMed] [Google Scholar]
- Franklin, K.A., and Whitelam, G.C. (2005). Phytochromes and shade-avoidance responses in plants. Ann. Bot. (Lond.) 96 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya, M., and Schäfer, E. (1996). Photoperception and signalling of induction reactions by different phytochromes. Trends Plant Sci. 1 301–307. [Google Scholar]
- Hennig, L., and Schäfer, E. (2001). Both subunits of the dimeric plant photoreceptor phytochrome require chromophore for stability of the far-red light-absorbing form. J. Biol. Chem. 276 7913–7918. [DOI] [PubMed] [Google Scholar]
- Huq, E., Al-Sady, B., and Quail, P.H. (2003). Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J. 35 660–664. [DOI] [PubMed] [Google Scholar]
- Izaguirre, M.M., Mazza, C.A., Biondini, M., Baldwin, I.T., and Ballare, C.L. (2006). Remote sensing of future competitors: Impacts on plant defenses. Proc. Natl. Acad. Sci. USA 103 7170–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, A.M., and Quail, P.H. (1986). Quaternary structure of 124-kilodalton phytochrome from Avena sativa L. Biochemistry 25 2987–2995. [Google Scholar]
- Kasperbauer, M.J. (1987). Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 85 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, R., Huq, E., Kikis, E.A., Al-Sady, B., Lanzatella, C., and Quail, P.H. (2004). A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16 3033–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher, S., Gil, P., Kozma-Bognar, L., Fejes, E., Speth, V., Husselstein-Muller, T., Bauer, D., Adam, E., Schäfer, E., and Nagy, F. (2002). Nucleocytoplasmic partitioning of the plant photoreceptors phytochrome A, B, C, D, and E is regulated differentially by light and exhibits a diurnal rhythm. Plant Cell 14 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef, M., and Kendrick, R.E. (1994). Photomorphogenic mutants of higher plants. In Photomorphogenesis in Plants, R.E. Kendrick and G.H.M. Kronenberg, eds (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 601–628.
- Kretsch, T., Poppe, C., and Schäfer, E. (2000). A new type of mutation in the plant photoreceptor phytochrome B causes loss of photoreversibility and an extremely enhanced light sensitivity. Plant J. 22 177–186. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Lagarias, J.C., Kelly, J.M., Cyr, K.L., and Smith, W.O., Jr. (1987). Comparative photochemical analysis of highly purified 124 kilodalton oat and rye phytochromes in vitro. Photochem. Photobiol. 46 5–13. [Google Scholar]
- Lariguet, P., and Dunand, C. (2005). Plant photoreceptors: Phylogenetic overview. J. Mol. Evol. 61 559–569. [DOI] [PubMed] [Google Scholar]
- Lin, C., and Todo, T. (2005). The cryptochromes. Genome Biol. 6 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloof, J.N., Borevitz, J.O., Dabi, T., Lutes, J., Nehring, R.B., Redfern, J.L., Trainer, G.T., Wilson, J.M., Asami, T., Berry, C.C., Weigel, D., and Chory, J. (2001). Natural variation in light sensitivity of Arabidopsis. Nat. Genet. 29 441–446. [DOI] [PubMed] [Google Scholar]
- Mateos, J.L., Luppi, J.P., Ogorodnikova, O.B., Sineshchekov, V.A., Yanovsky, M.J., Braslavsky, S.E., Gartner, W., and Casal, J.J. (2006). Functional and biochemical analysis of the N-terminal domain of phytochrome A. J. Biol. Chem. 281 34421–34429. [DOI] [PubMed] [Google Scholar]
- Mathews, S. (2006). Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol. Ecol. 15 3483–3503. [DOI] [PubMed] [Google Scholar]
- Matsushita, T., Mochizuki, N., and Nagatani, A. (2003). Dimers of the N-terminal domain of phytochrome B are functional in the nucleus. Nature 424 571–574. [DOI] [PubMed] [Google Scholar]
- Mehrtens, F., Kranz, H., Bednarek, P., and Weisshaar, B. (2005). The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 138 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery, B.L., Franklin, K.A., Terry, M.J., Thomas, B., Jackson, S.D., Crepeau, M.W., and Lagarias, J.C. (2001). Biliverdin reductase-induced phytochrome chromophore deficiency in transgenic Nicotiana tabacum cv. Maryland Mammoth. Plant Physiol. 125 266–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery, B.L., and Lagarias, J.C. (2002). Phytochrome ancestry. Sensors of bilins and light. Trends Plant Sci. 7 357–366. [DOI] [PubMed] [Google Scholar]
- Muramoto, T., Kohchi, T., Yokota, A., Hwang, I.H., and Goodman, H.M. (1999). The Arabidopsis photomorphogenic mutant hy1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid heme oxygenase. Plant Cell 11 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramoto, T., Tsurui, N., Terry, M.J., Yokota, A., and Kohchi, T. (2002). Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol. 130 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani, A. (2004). Light-regulated nuclear localization of phytochromes. Curr. Opin. Plant Biol. 7 708–711. [DOI] [PubMed] [Google Scholar]
- Noack, S., Michael, N., Rosen, R., and Lamparter, T. (2007). Protein conformational changes of Agrobacterium phytochrome Agp1 during chromophore assembly and photoconversion. Biochemistry 46 4164–4176. [DOI] [PubMed] [Google Scholar]
- Oh, E., Kim, J., Park, E., Kim, J.I., Kang, C., and Choi, G. (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16 3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail, P.H. (1991). Phytochrome - A light-activated molecular switch that regulates plant gene expression. Annu. Rev. Genet. 25 389–409. [DOI] [PubMed] [Google Scholar]
- Quail, P.H. (1997). An emerging molecular map of the phytochromes. Plant Cell Environ. 20 657–665. [Google Scholar]
- Reed, J.W., Nagatani, A., Elich, T.D., Fagan, M., and Chory, J. (1994). Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 104 1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, J.W., Nagpal, P., Poole, D.S., Furuya, M., and Chory, J. (1993). Mutations in the gene for the red far-red light receptor phytochrome-B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell, N.C., and Lagarias, J.C. (2006). The structure of phytochrome. A picture is worth a thousand spectra. Plant Cell 18 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell, N.C., Su, Y.S., and Lagarias, J.C. (2006). Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57 837–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers, R.J., Sheehan, M.J., and Brutnell, T.P. (2005). Cereal phytochromes: Targets of selection, targets for manipulation? Trends Plant Sci. 10 138–143. [DOI] [PubMed] [Google Scholar]
- Sawers, R.J.H., Linley, P.J., Farmer, P.R., Hanley, N.P., Costich, D.E., Terry, M.J., and Brutnell, T.P. (2002). elongated mesocotyl1, a phytochrome-deficient mutant of maize. Plant Physiol. 130 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, E., and Nagy, F. (2005). Photomorphogenesis in Plants and Bacteria: Function and Signal Transduction Mechanisms, 3rd edition. (Dordrecht, The Netherlands: Springer).
- Schmitt, J., McCormac, A.C., and Smith, H. (1995). A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. Am. Nat. 146 937–953. [Google Scholar]
- Seo, H.S., Watanabe, E., Tokutomi, S., Nagatani, A., and Chua, N.H. (2004). Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 18 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock, R.A., and Quail, P.H. (1989). Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 3 1745–1757. [DOI] [PubMed] [Google Scholar]
- Shinomura, T. (1997). Phytochrome regulation of seed germination. J. Plant Res. 110 151–161. [DOI] [PubMed] [Google Scholar]
- Shinomura, T., Hanzawa, H., Schäfer, E., and Furuya, M. (1998). Mode of phytochrome B action in the photoregulation of seed germination in Arabidopsis thaliana. Plant J. 13 583–590. [DOI] [PubMed] [Google Scholar]
- Shinomura, T., Nagatani, A., Hanzawa, H., Kubota, M., Watanabe, M., and Furuya, M. (1996). Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93 8129–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sineshchekov, V.A. (2004). Phytochrome A: Functional diversity and polymorphism. Photochem. Photobiol. Sci. 3 596–607. [DOI] [PubMed] [Google Scholar]
- Smith, H. (1994). Phytochrome transgenics: Functional, ecological and biotechnological applications. Semin. Cell Biol. 5 315–325. [DOI] [PubMed] [Google Scholar]
- Smith, H., Casal, J.J., and Jackson, G.M. (1990). Reflection signals and the perception by phytochrome of the proximity of neighbouring vegetation. Plant Cell Environ. 13 73–78. [Google Scholar]
- Smith, H., and Whitelam, G.C. (1997). The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 20 840–844. [Google Scholar]
- Smith, H., Xu, Y., and Quail, P.H. (1997). Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol. 114 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweere, U., Eichenberg, K., Lohrmann, J., Mira-Rodado, V., Baurle, I., Kudla, J., Nagy, F., Schäfer, E., and Harter, K. (2001). Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science 294 1108–1111. [DOI] [PubMed] [Google Scholar]
- Terry, M.J. (1997). Phytochrome chromophore-deficient mutants. Plant Cell Environ. 20 740–745. [Google Scholar]
- Usami, T., Mochizuki, N., Kondo, M., Nishimura, M., and Nagatani, A. (2004). Cryptochromes and phytochromes synergistically regulate Arabidopsis root greening under blue light. Plant Cell Physiol. 45 1798–1808. [DOI] [PubMed] [Google Scholar]
- Von Arnim, A., and Deng, X.W. (1996). Light control of seedling development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 215–243. [DOI] [PubMed] [Google Scholar]
- Wagner, D., Koloszvari, M., and Quail, P.H. (1996). Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell 8 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, D., Tepperman, J.M., and Quail, P.H. (1991). Overexpression of phytochrome-B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell 3 1275–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, J.R., Brunzelle, J.S., Forest, K.T., and Vierstra, R.D. (2005). A light-sensing knot revealed by the structure of the chromophore binding domain of phytochrome. Nature 438 325–331. [DOI] [PubMed] [Google Scholar]
- Wagner, J.R., Zhang, J., Brunzelle, J.S., Vierstra, R.D., and Forest, K.T. (2007). High resolution structure of Deinococcus bacteriophytochrome yields new insights into phytochrome architecture and evolution. J. Biol. Chem. 282 12298–12309. [DOI] [PubMed] [Google Scholar]
- Wang, H. (2005). Signaling mechanisms of higher plant photoreceptors: A structure-function perspective. Curr. Top. Dev. Biol. 68 227–261. [DOI] [PubMed] [Google Scholar]
- Weller, J.L., Batge, S.L., Smith, J.J., Kerckhoffs, L.H., Sineshchekov, V.A., Murfet, I.C., and Reid, J.B. (2004). A dominant mutation in the pea PHYA gene confers enhanced responses to light and impairs the light-dependent degradation of phytochrome A. Plant Physiol. 135 2186–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel, D., and Flugge, U.I. (1984). A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138 141–143. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.Q., Tang, R.H., and Cashmore, A.R. (2001). The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13 2573–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, C., and Deng, X.W. (2005). COP1 - From plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 15 618–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.