Abstract
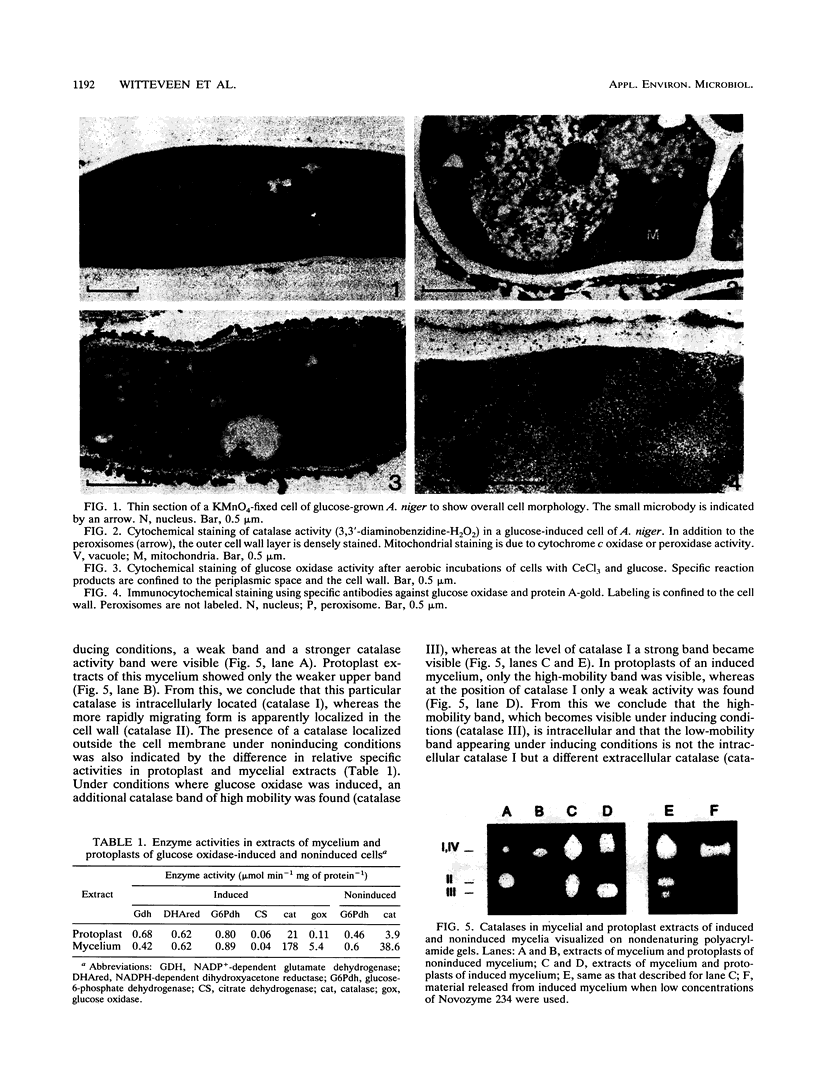
The subcellular localization of glucose oxidase (EC 1.1.3.4) in Aspergillus niger N400 (CBS 120.49) was investigated by (immuno)cytochemical methods. By these methods, the bulk of the enzyme was found to be localized in the cell wall. In addition, four different catalases (EC 1.11.1.6) were demonstrated by nondenaturing polyacrylamide gel electrophoresis of crude extracts of induced and noninduced cells. Comparison of both protoplast and mycelial extracts indicated that, of two constitutive catalases, one is located outside the cell membrane whereas the other is intracellular. Parallel with the induction of glucose oxidase, two other catalases are also induced, one located intracellularly and one located extracellularly. Furthermore, lactonase (EC 3.1.1.17) activity, catalyzing the hydrolysis of glucono-δ-lactone to gluconic acid, was found to be exclusively located outside the cell membrane, indicating that gluconate formation in A. niger occurs extracellularly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bruinenberg P. M., van Dijken J. P., Scheffers W. A. An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol. 1983 Apr;129(4):965–971. doi: 10.1099/00221287-129-4-965. [DOI] [PubMed] [Google Scholar]
- Chary P., Natvig D. O. Evidence for three differentially regulated catalase genes in Neurospora crassa: effects of oxidative stress, heat shock, and development. J Bacteriol. 1989 May;171(5):2646–2652. doi: 10.1128/jb.171.5.2646-2652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick K. R., Tung J., Emerick R. S., Masiarz F. R., Chamberlain S. H., Vasavada A., Rosenberg S., Chakraborty S., Schopfer L. M., Schopter L. M. Glucose oxidase from Aspergillus niger. Cloning, gene sequence, secretion from Saccharomyces cerevisiae and kinetic analysis of a yeast-derived enzyme. J Biol Chem. 1990 Mar 5;265(7):3793–3802. [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Visualization of catalase on acrylamide gels. Anal Biochem. 1974 Mar;58(1):57–62. doi: 10.1016/0003-2697(74)90440-0. [DOI] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- KUSAI K., SEKUZU I., HAGIHARA B., OKUNUKI K., YAMAUCHI S., NAKAI M. Crystallization of glucose oxidase from Penicillium amagasakiense. Biochim Biophys Acta. 1960 Jun 3;40:555–557. doi: 10.1016/0006-3002(60)91406-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi-Torii K., Hayashi S., Nakamoto H., Nakamura S. Properties of Aspergillus niger catalase. J Biochem. 1982 Nov;92(5):1449–1456. doi: 10.1093/oxfordjournals.jbchem.a134069. [DOI] [PubMed] [Google Scholar]
- Kleppe K. The effect of hydrogen peroxide on glucose oxidase from Aspergillus niger. Biochemistry. 1966 Jan;5(1):139–143. doi: 10.1021/bi00865a018. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pazur J. H., Kleppe K., Cepure A. A glycoprotein structure for glucose oxidase from Aspergillus niger. Arch Biochem Biophys. 1965 Aug;111(2):351–357. doi: 10.1016/0003-9861(65)90196-7. [DOI] [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., van Dijken J. P., Harder W. Cytochemical studies on the localization of methanol oxidase and other oxidases in peroxisomes of methanol-grown Hansenula polymorpha. Arch Microbiol. 1976 Dec 1;111(1-2):123–135. doi: 10.1007/BF00446559. [DOI] [PubMed] [Google Scholar]
- Verduyn C., Giuseppin M. L., Scheffers W. A., van Dijken J. P. Hydrogen peroxide metabolism in yeasts. Appl Environ Microbiol. 1988 Aug;54(8):2086–2090. doi: 10.1128/aem.54.8.2086-2090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman B. P., Hultin H. O. Effect of deglycosylation on the stability of Aspergillus niger catalase. Arch Biochem Biophys. 1981 Dec;212(2):385–392. doi: 10.1016/0003-9861(81)90379-9. [DOI] [PubMed] [Google Scholar]
- Witteveen C. F., van de Vondervoort P., Dijkema C., Swart K., Visser J. Characterization of a glycerol kinase mutant of Aspergillus niger. J Gen Microbiol. 1990 Jul;136(7):1299–1305. doi: 10.1099/00221287-136-7-1299. [DOI] [PubMed] [Google Scholar]