Abstract
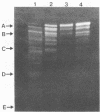
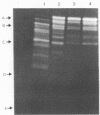
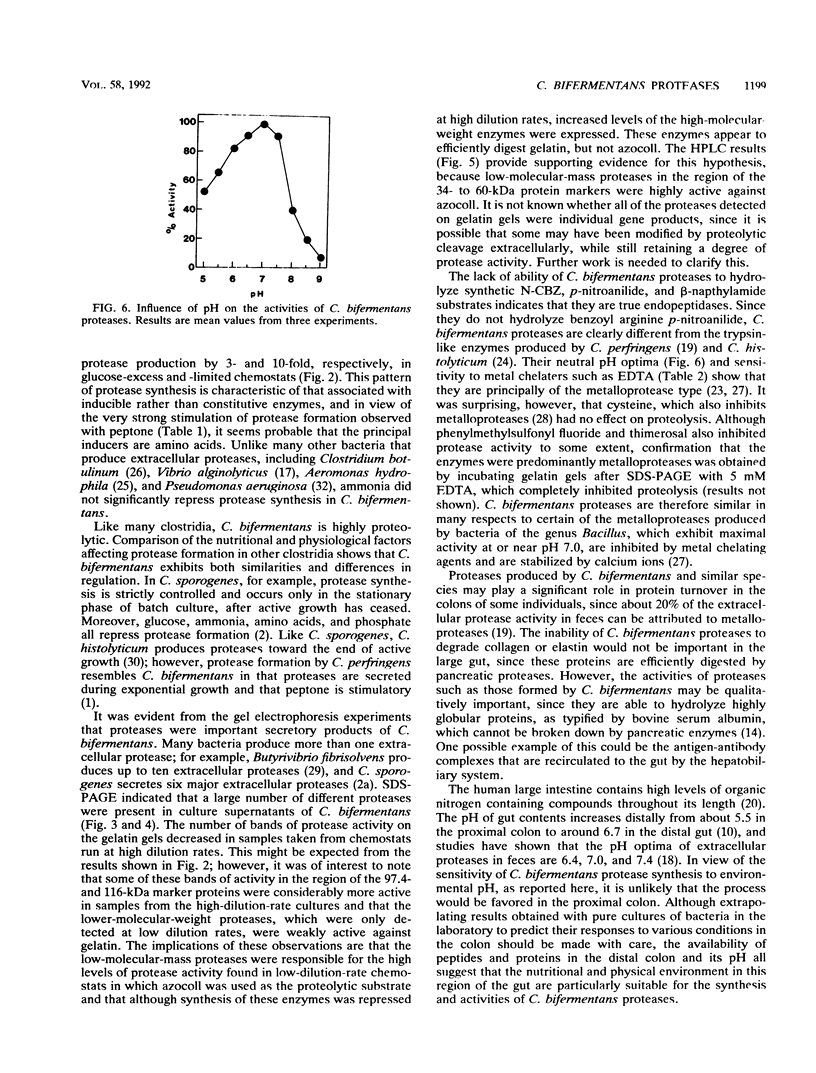
Extracellular protease production by Clostridium bifermentans NCTC 2914 occurred throughout the growth phase in batch culture. In both glucose-excess and -limited chemostats, protease formation was inversely related to the dilution rate, over the range D = 0.03 to 0.70 h-1. At high dilution rates (D greater than 0.25 h-1), protease activities were greatest under excess glucose conditions. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of chemostat culture effluents showed the presence of up to 18 bands of protease activity at low dilution rates, with apparent molecular masses ranging from about 36 to 125 kDa. High-performance liquid chromatography gel filtration of culture supernatants gave four peaks of activity at 34, 42, 60, and 102 kDa. Glucose, peptone, and phosphate stimulated protease formation, but ammonia concentrations up to 10 g liter-1 had little effect on the process. Culture pH in glucose-excess chemostats strongly influenced protease synthesis, which was maximal during growth at pH 6.4. The optimal pH of protease activity was 7.0. Although a wide variety of proteins were hydrolyzed by C. bifermentans proteases, none of the enzymes were collagenolytic. Of 21 different p-nitroanilide, beta-naphthylamide, and N-carbobenzoyl substrates tested, none were hydrolyzed. With the exception of Ca2+, divalent metal ions inhibited proteolysis. Experiments with protease inhibitors demonstrated that 1 mM EDTA inhibited protease activities in culture supernatants by over 90%, indicating that the enzymes were principally of the metalloprotease type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison C., Macfarlane G. T. Regulation of protease production in Clostridium sporogenes. Appl Environ Microbiol. 1990 Nov;56(11):3485–3490. doi: 10.1128/aem.56.11.3485-3490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe M., Borgström A., Genell S., Ohlsson K. Determination of immunoreactive trypsin, pancreatic elastase and chymotrypsin in extracts of human feces and ileostomy drainage. Digestion. 1983;27(1):8–15. doi: 10.1159/000198913. [DOI] [PubMed] [Google Scholar]
- Chacko A., Cummings J. H. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut. 1988 Jun;29(6):809–815. doi: 10.1136/gut.29.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Macfarlane G. T. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991 Jun;70(6):443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Macfarlane G. T. Characterization of proteases formed by Bacteroides fragilis. J Gen Microbiol. 1988 Aug;134(8):2231–2240. doi: 10.1099/00221287-134-8-2231. [DOI] [PubMed] [Google Scholar]
- Gibson S. A., Macfarlane G. T. Studies on the proteolytic activity of Bacteroides fragilis. J Gen Microbiol. 1988 Jan;134(1):19–27. doi: 10.1099/00221287-134-1-19. [DOI] [PubMed] [Google Scholar]
- Gibson S. A., McFarlan C., Hay S., MacFarlane G. T. Significance of microflora in proteolysis in the colon. Appl Environ Microbiol. 1989 Mar;55(3):679–683. doi: 10.1128/aem.55.3.679-683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood G. P., Edwards R. Proteolytic activities of a rumen bacterium, Bacteroides ruminicola R8/4. J Gen Microbiol. 1981 Jul;125(1):11–15. doi: 10.1099/00221287-125-1-11. [DOI] [PubMed] [Google Scholar]
- Long S., Mothibeli M. A., Robb F. T., Woods D. R. Regulation of extracellular alkaline protease activity by histidine in a collagenolytic Vibrio alginolyticus strain. J Gen Microbiol. 1981 Nov;127(1):193–199. doi: 10.1099/00221287-127-1-193. [DOI] [PubMed] [Google Scholar]
- MACLENNAN J. D. The histotoxic clostridial infections of man. Bacteriol Rev. 1962 Jun;26:177–276. [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G. T., Allison C., Gibson S. A., Cummings J. H. Contribution of the microflora to proteolysis in the human large intestine. J Appl Bacteriol. 1988 Jan;64(1):37–46. doi: 10.1111/j.1365-2672.1988.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Macfarlane S., Gibson G. R. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J Appl Bacteriol. 1989 Nov;67(5):520–527. [PubMed] [Google Scholar]
- Patterson-Curtis S. I., Johnson E. A. Regulation of neurotoxin and protease formation in Clostridium botulinum Okra B and Hall A by arginine. Appl Environ Microbiol. 1989 Jun;55(6):1544–1548. doi: 10.1128/aem.55.6.1544-1548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Seifter S. New culture conditions for Clostirdium histolyticum leading to production of collagenase of high specific activity. J Appl Bacteriol. 1972 Dec;35(4):647–657. doi: 10.1111/j.1365-2672.1972.tb03746.x. [DOI] [PubMed] [Google Scholar]
- Whooley M. A., O'Callaghan J. A., McLoughlin A. J. Effect of substrate on the regulation of exoprotease production by Pseudomonas aeruginosa ATCC 10145. J Gen Microbiol. 1983 Apr;129(4):981–988. doi: 10.1099/00221287-129-4-981. [DOI] [PubMed] [Google Scholar]