Abstract
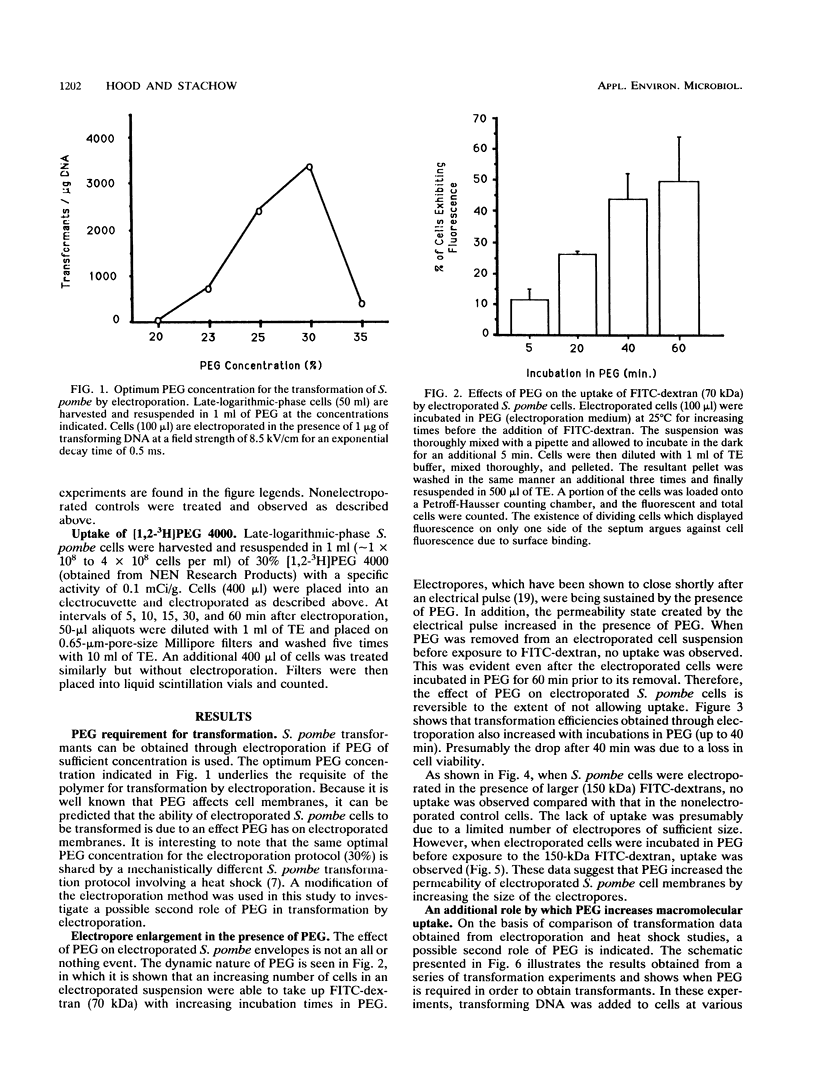
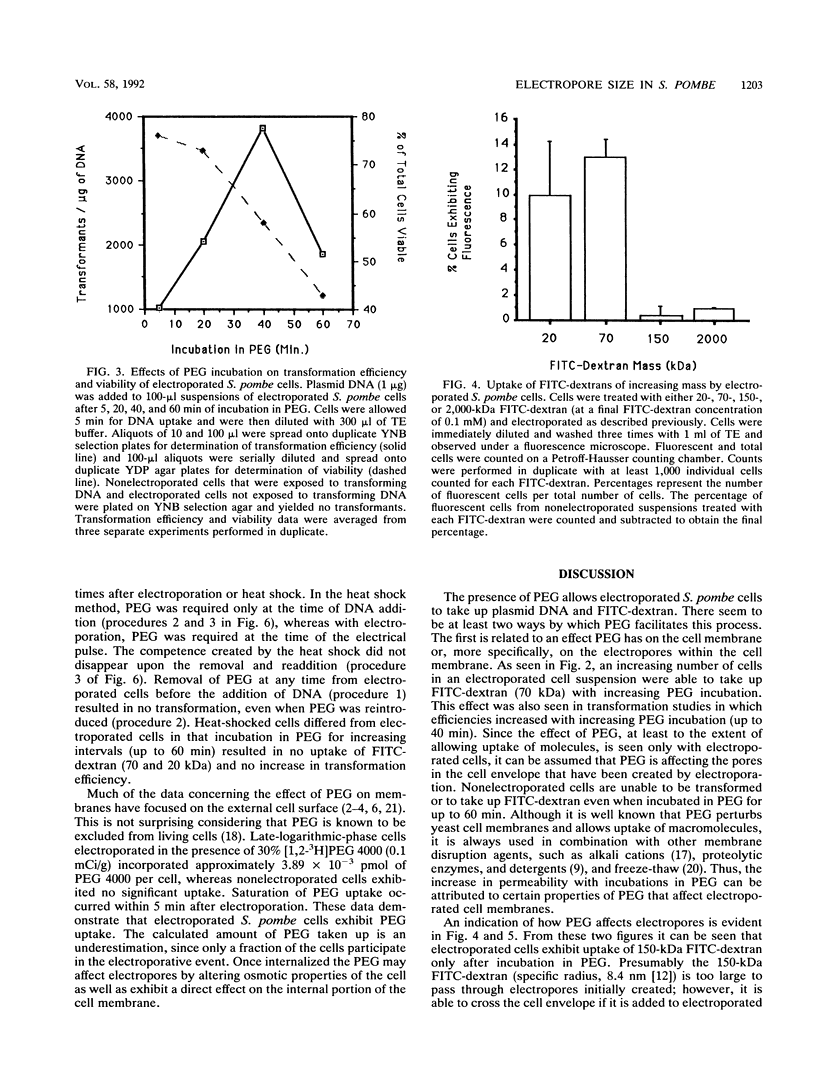
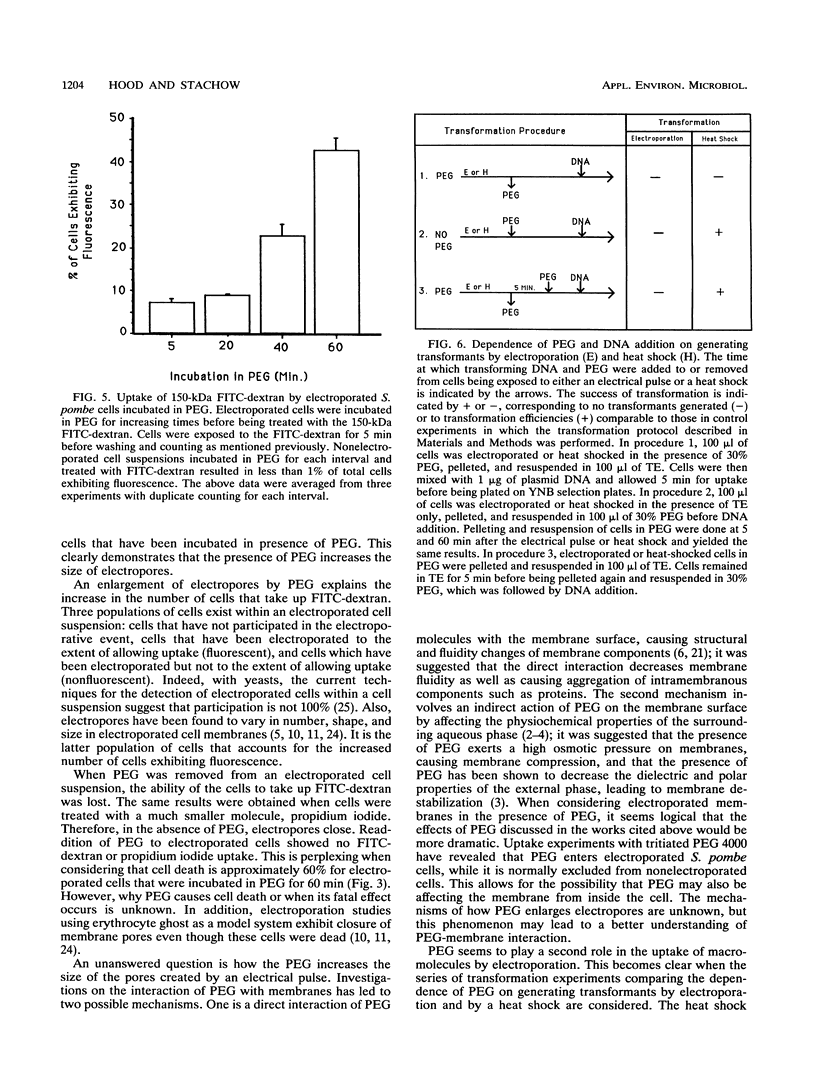
The role of polyethylene glycol (PEG) in the transformation of Schizosaccharomyces pombe by electroporation is investigated by fluorescein isothiocyanate-dextran uptake and transformation studies. It is shown that when S. pombe cells are electroporated in the presence of PEG, the permeability state created is sustained until removal of PEG. In addition, the permeability of electroporated S. pombe envelopes is further increased with longer incubation times in PEG. The increased permeability is apparently a result of enlarged pores (electropores) due to the presence of PEG. Comparison of a heat pulse transformation protocol with electroporation suggests a second role for PEG in the uptake of macromolecules. Since pores are not thought to be created during a heat pulse, the PEG may be facilitating the uptake of plasmid DNA. This facilitation of uptake would also be expected to affect DNA uptake by electroporated cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreason G. L., Evans G. A. Introduction and expression of DNA molecules in eukaryotic cells by electroporation. Biotechniques. 1988 Jul-Aug;6(7):650–660. [PubMed] [Google Scholar]
- Arnold K., Herrmann A., Pratsch L., Gawrisch K. The dielectric properties of aqueous solutions of poly(ethylene glycol) and their influence on membrane structure. Biochim Biophys Acta. 1985 May 28;815(3):515–518. doi: 10.1016/0005-2736(85)90381-5. [DOI] [PubMed] [Google Scholar]
- Arnold K., Pratsch L., Gawrisch K. Effect of poly(ethylene glycol) on phospholipid hydration and polarity of the external phase. Biochim Biophys Acta. 1983 Feb 9;728(1):121–128. doi: 10.1016/0005-2736(83)90444-3. [DOI] [PubMed] [Google Scholar]
- Arnold K., Zschoernig O., Barthel D., Herold W. Exclusion of poly(ethylene glycol) from liposome surfaces. Biochim Biophys Acta. 1990 Mar;1022(3):303–310. doi: 10.1016/0005-2736(90)90278-v. [DOI] [PubMed] [Google Scholar]
- Bartoletti D. C., Harrison G. I., Weaver J. C. The number of molecules taken up by electroporated cells: quantitative determination. FEBS Lett. 1989 Oct 9;256(1-2):4–10. doi: 10.1016/0014-5793(89)81707-7. [DOI] [PubMed] [Google Scholar]
- Boni L. T., Hah J. S., Hui S. W., Mukherjee P., Ho J. T., Jung C. Y. Aggregation and fusion of unilamellar vesicles by poly(ethylene glycol). Biochim Biophys Acta. 1984 Sep 5;775(3):409–418. doi: 10.1016/0005-2736(84)90198-6. [DOI] [PubMed] [Google Scholar]
- Bruschi C. V., Comer A. R., Howe G. A. Specificity of DNA uptake during whole cell transformation of S. cerevisiae. Yeast. 1987 Jun;3(2):131–137. doi: 10.1002/yea.320030209. [DOI] [PubMed] [Google Scholar]
- Brzobohatý B., Kovác L. Factors enhancing genetic transformation of intact yeast cells modify cell wall porosity. J Gen Microbiol. 1986 Nov;132(11):3089–3093. doi: 10.1099/00221287-132-11-3089. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Sowers A. E. Membrane electroporation--fast molecular exchange by electroosmosis. Biochim Biophys Acta. 1990 Mar;1022(3):381–392. doi: 10.1016/0005-2736(90)90289-z. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Herrmann A., Pratsch L., Arnold K., Lassmann G. Effect of poly(ethylene glycol) on the polarity of aqueous solutions and on the structure of vesicle membranes. Biochim Biophys Acta. 1983 Aug 24;733(1):87–94. doi: 10.1016/0005-2736(83)90093-7. [DOI] [PubMed] [Google Scholar]
- Heyer W. D., Sipiczki M., Kohli J. Replicating plasmids in Schizosaccharomyces pombe: improvement of symmetric segregation by a new genetic element. Mol Cell Biol. 1986 Jan;6(1):80–89. doi: 10.1128/mcb.6.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. T., Stachow C. Transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1990 Feb 11;18(3):688–688. doi: 10.1093/nar/18.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I. T., Gee J. M., Price K., Curl C., Fenwick G. R. Influence of saponins on gut permeability and active nutrient transport in vitro. J Nutr. 1986 Nov;116(11):2270–2277. doi: 10.1093/jn/116.11.2270. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Ashikawa I., Saita N., Yoshimura H., Itoh H., Nagayama K., Ikegami A. Electroporation of cell membrane visualized under a pulsed-laser fluorescence microscope. Biophys J. 1988 Jun;53(6):1015–1019. doi: 10.1016/S0006-3495(88)83181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebe R. J., Harriss J. V., Sharp Z. D., Douglas M. G. A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene. 1983 Nov;25(2-3):333–341. doi: 10.1016/0378-1119(83)90238-x. [DOI] [PubMed] [Google Scholar]
- Ohno H., Shimidzu N., Tsuchida E., Sasakawa S., Honda K. Fluorescence polarization study on the increase of membrane fluidity of human erythrocyte ghosts induced by synthetic water-soluble polymers. Biochim Biophys Acta. 1981 Dec 7;649(2):221–228. doi: 10.1016/0005-2736(81)90409-0. [DOI] [PubMed] [Google Scholar]
- Potter H. Electroporation in biology: methods, applications, and instrumentation. Anal Biochem. 1988 Nov 1;174(2):361–373. doi: 10.1016/0003-2697(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Sowers A. E., Lieber M. R. Electropore diameters, lifetimes, numbers, and locations in individual erythrocyte ghosts. FEBS Lett. 1986 Sep 15;205(2):179–184. doi: 10.1016/0014-5793(86)80893-6. [DOI] [PubMed] [Google Scholar]
- Weaver J. C., Harrison G. I., Bliss J. G., Mourant J. R., Powell K. T. Electroporation: high frequency of occurrence of a transient high-permeability state in erythrocytes and intact yeast. FEBS Lett. 1988 Feb 29;229(1):30–34. doi: 10.1016/0014-5793(88)80791-9. [DOI] [PubMed] [Google Scholar]


