Abstract
The immunological consequences of apoptosis have been hotly debated. Apoptosis was originally described as a set of cellular morphological changes that occur in the absence of inflammation but the term has been redefined on the basis of a set of conserved molecular events that include the activation of caspases. Though the apoptosis occurring during normal development is immunologically bland or even tolerizing, the apoptotic death after viral infection or after the ligation of Fas can trigger powerful innate and adaptive immune responses. The molecular machinery at the nexus of apoptosis and inflammation includes caspase-1 – an activator of IL-1β and IL-18 – as well as the double-stranded-RNA-dependent protein kinase pathway and RNaseL pathway, which are key effectors of antiviral immunity. New proapoptotic vaccines induce immune responses that may be able to prevent or treat infectious disease and cancer.
Introduction
There is a great deal of death in the normal life of an organism and most of this death is apoptotic. During development, apoptotic death of cells results in the separation of the digits, the creation of the gyri in the brain and the formation of a variety of tubular structures, such as those in the digestive and respiratory systems. The death occurring during development is not inflammatory and hence from an immunological standpoint could be considered bland. Indeed, developmental apoptosis may even be immunologically tolerizing.
We will address three major questions in this review. First, is apoptotic death always bland? Second, what is the molecular basis of both the inflammation that accompanies the apoptotic death that occurs during a viral infection and of the apoptotic death that is induced by the interaction of Fas and its ligand? And finally, perhaps most importantly, can inflammatory signals produced during apoptotic death be used to enhance vaccine function?
Apoptosis is generally referred to in the literature without any modifiers, as if it were of a single type. Further, it is generally thought of as being an immunologically innocuous event that does not activate immune cells, such as dendritic cells (DCs) [1•-3•]. However, rather than being a single entity, apoptosis is complex, adaptable and flexible and the immunological effects of apoptosis vary depending on the circumstances in which it occurs. We argue here that apoptosis can be either bland or inflammatory depending on how it is initiated, in what cell type it occurs and whether or not particular co-factors, for example type I interferons, are present. In this short review, we hope to describe the molecular pathways that trigger inflammation and the activation of both innate and adaptive immunity. We will also explore how these immune responses might be useful in the develpoment of vaccines for infectious diseases and cancer.
Redefining apoptosis
Though programmed cell death was initially described by Carl Vogt in 1842, the term apoptosis was coined in 1972 by Kerr et al. [4] and is derived from ancient Greek (‘apo’ means ‘off’, ‘ptosis’ means ‘a falling’ e.g. the way that leaves fall from a tree). The original descriptions of apoptosis focus on the characteristic morphological appearance of the dying cells: organelles appear shrunken and nuclear chromatin appears condensed and fragmented. Dense blebs develop and become apoptotic bodies that are phagocytosed by other cells. The lack of inflammation was another hallmark property of death by apoptosis [4,5].
Apoptosis was originally defined in contrast to necrosis, a process resulting from sudden and catastrophic cellular destruction, such as that caused by hypoxia, hyperthermia or physical injury. Necrotic cells have a have a loss of membrane integrity and the entire cell, as well as nucleus and mitochondria, swells. The chromatin initially clumps then disperses in a process known as karyolysis. Thus, cells dying by apoptosis have a very different appearance from those dying by necrosis. Furthermore, in sharp contrast to apoptosis, necrosis was described as “usually” being associated with inflammation [5].
A process of redefinition was initiated by Andrew Wyllie — one of the original authors of the paper [4] in which the term apoptosis was coined — when he observed [6] that the morphological changes that were originally described were “…closely associated with excision of nucleosome chains from nuclear chromatin, apparently through activation of an intracellular, but nonlysosomal, endonuclease…”. Since these early studies, the molecular components of the apoptotic death machinery have been further defined. The premier genetic system for studying the molecular machinery of apoptotic death is Caenorhabditis elegans, in which death genes and their protein products have been defined. In mammals, orthologs of these molecules have been identified and further characterized in vitro and in vivo.
The concept that has now become well accepted is that apoptosis involves a shared biochemical suicide program that exists in most cells and is turned on by a variety of normal developmental and pathogenic triggers, as well as by experimental manipulations. Though the original definition of apoptosis excluded inflammation, it has now become apparent that, in some cell types under certain circumstances, the generation of inflammatory signals can be an intrinsic component of the apoptotic machinery.
How members of the TNF-receptor family can induce death and inflammation
Our own interest in inflammatory apoptosis came from experiments in which we studied the expression of Fas ligand (FasL; also known as CD95L or APO-1L) by tumor cells [7,8,9•,10,11]. It was initially believed that Fas and its ligand mediated immune privilege and, furthermore, that FasL transfection could make transplantation easier and enable tumors to escape [12]. However, it was found that expression of FasL on β cells in the pancreas resulted in rapid rejection of islet cells accompanied by a massive inflammatory response. Tumors expressing FasL also are rapidly rejected (summarized in [9•]) and the sites of these regressing tumors coalesce into abscesses, thus linking the activity of a death receptor and an inflammatory immune response.
The way in which these events are linked with cellular death are becoming clearer. Initiation of apoptosis occurs when FasL — a member of the TNF family of proteins — interacts with its receptor, Fas. Not surprisingly, Fas is a member of the TNF receptor (TNFR) family of proteins that also includes a handful of other death receptors [13•]. The intracytoplasmic death-domains of these receptors activate a cascade of cysteine proteases — called caspases — via a set of adaptor molecules [13•,14,15].
There is ample evidence that, in some cell types, inflammatory signals can be an intrinsic part of this caspase cascade and this can activate innate and adaptive immune responses. One caspase at the nexus of death and inflammation is caspase-1, also known as IL-1β-converting enzyme (ICE) (Figure 1). Other ICE-like caspases that may be important for inflammation are caspase-4, -5, -11, -12 and -13 but their full activities remain incompletely elucidated [16-18]. ICE is rapidly activated by some apoptosis-inducing stimuli, including ligation of Fas by its ligand.
Figure 1.
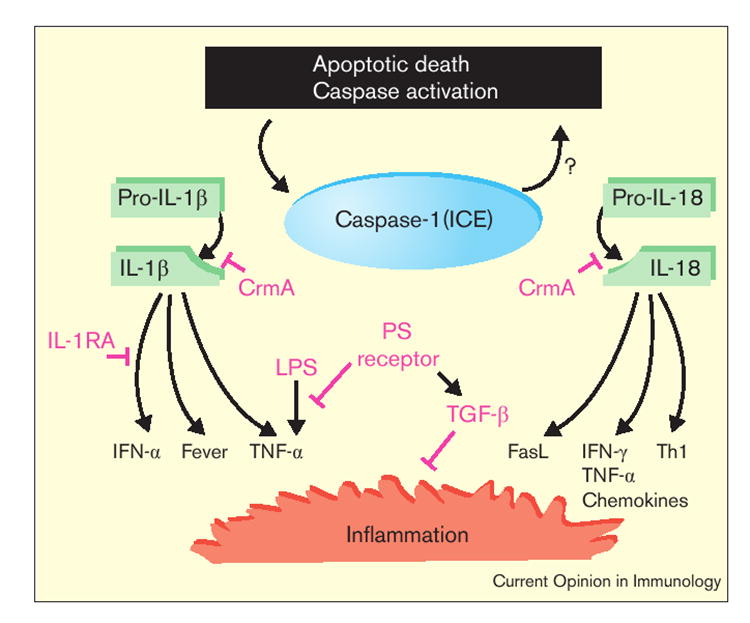
Death and inflammation: how caspase-1 (ICE) activates IL-1β and IL-18 to induce innate and adaptive immunity and how this inflammation may be modulated. Apoptotic death induced by a variety of factors (such as ligation of Fas by its ligand) triggers a caspase cascade that can include ICE. Caspase-11 may be involved in the activation of ICE or may form a complex with it [16]. Activated ICE is then capable of cleaving the pro-forms of IL-1β and IL-18, which in turn trigger other proinflammatory cytokines. There is also evidence that activated ICE plays a role in the induction of apoptosis under some conditions (hence the question mark) [19••]. During poxviral infection, CrmA can block the activity of ICE. A number of other factors may regulate or even abrogate the activation of innate and adaptive immune responses: ICE, or its substrates IL-1β and IL-18, may not be expressed by the dying cell; IL-1-receptor antagonist (IL-1RA) may modulate or block the effects of IL-1β; and macrophages that take up apoptotic cells through the phosphatidylserine (PS) receptor do not produce TNF-α in response to lipopolysaccharide (LPS) but may produce the anti-inflammatory cytokine, TGF-β [34••].
In addition to being activated during death, there is some evidence that ICE itself can be involved in the induction of apoptotic death. Indeed, ICE is a requisite component of the apoptotic death induced by Shigella flexneri in macrophages [19••]. However, ICE is not an essential component of developmental apoptosis: ICE-knockout mice develop normally. Its true function is as a mediator of the inflammatory events that can accompany apoptosis and ICE mediates this inflammation by activating IL-1β and IL-18.
In experiments using a variety of mouse tumors transfected with FasL, signaling through Fas has been directly linked to production of IL-1β [20]. Ligation of Fas is also known to induce IL-18 and — as a consequence — the inflammatory chemokine, IL-8 [21]. ICE-activated IL-1β augments DC maturation [22] and migration [23], and can activate NF-κB, triggering a variety of immune-activating cytokines and chemokines. IL-1β receptors can be found in one form or another on most cell types [24,25,26•]. In addition, IL-1β is the most potent known endogenous pyrogen [27•,28].
The cleavage of pro-IL-18 into active IL-18 by activated ICE is structurally similar to the cleavage of pro-IL-1β. Like IL-1β, only the mature form of IL-18 is bioactive and pro-IL-18 lacks a signal sequence. The external receptors for IL-1β and IL-18 are different but the intracellular signaling pathways are identical [29]. Previously known as IFN-γ-inducing factor (IGIF), IL-18 induces NF-κB and has a number of other activities including the induction of FasL, TNF-α and chemokines, the augmentation of IFN-γ release after IL-12 stimulation and the stimulation of a Th1-type response [30-32].
It is known that Fas can induce apoptotic death through two different pathways but it is not known how these pathways differentially promote inflammation [33]. Inflammatory components of apoptosis may be regulated, in part, by controlling the expression of the ‘pro’ forms of IL-1 and IL-18, which are primarily produced in monocytes and macrophages. A receptor antagonist has been described for IL-1 that may counteract the inflammatory activities of IL-1β. Finally, potentially anti-inflammatory cytokines, including transforming growth factor β (TGF-β), are produced by macrophages upon the uptake of apoptotic cells via the phosphatidylserine receptor that may regulate or abrogate inflammatory signals [34••]. Regulatory events at the nexus of death and inflammation are likely to determine whether apoptosis is bland or inflammatory.
How double-stranded RNA induces both death and inflammation: the PKR and RnaseL pathways
Another example of inflammatory apoptosis is cell death associated with the recognition of double-stranded RNA (dsRNA). Many viruses, especially some RNA-based viruses, mediate the expression of dsRNA during transcription of overlapping RNA species or during viral replication. Higher organisms have evolved a number of redundant and complementary pathways for the recognition of dsRNA, including the dsRNA-dependent protein kinase (PKR) pathway and the 2′-5′-oligoadenylate-synthetase/RNaseL pathway. Activation of either of these pathways can induce apoptotic death. Though less is known about RnaseL pathway, the PKR and RNaseL systems can also induce robust innate and adaptive immunity (Figure 2).
Figure 2.
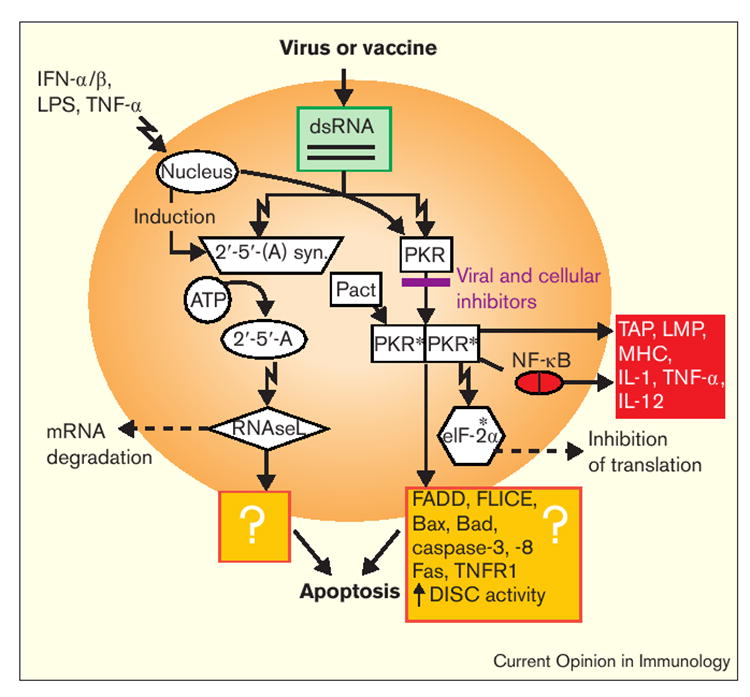
How both apoptotic death and immunity may be triggered by dsRNA. The cellular ‘death-inducing’ events after exposure to dsRNA – following virus infection or vaccination – include the RNaseL and PKR pathways, which destroy mRNA and stop translation, respectively. Though the mechanisms are not fully known, apoptosis is induced in part by enhancing efficiency in the transmission of death signals through the adaptor, FADD, and enhanced activity of the death-induced signaling complex (DISC) [77,78]. Two proapoptotic members of the Bcl-2 family, Bad and Bax, are also induced. The PKR pathway can be activated (phosphorylated; this is indicated by the asterisks) by an endogenous gene product called Pact or inhibited by a variety of viral and cellular inhibitors that can act at a number of points in the pathway; these are summarized in [79]. Activated PKR also induces NF-κB and a variety of molecules that facilitate T cell recognition of infected targets (red box). LPS, lipopolysaccharide; syn., sythetase; TAP, transporter associated with antigen processing.
The RNaseL pathway starts with the IFN-α/β-induced 2′,5′-oligoadenylate synthetases, a family of enzymes capable of linking ATP into oligomers of adenosine [35,36]. These 2′-5′-linked oligomers are capable of activating RNaseL, an enzyme named for its ability to degrade mRNA. The consequent inhibition of protein synthesis is reinforced by the activity of PKR, a serine/threonine kinase capable of dimerization and subsequent autophosphoryla-tion in the presence of dsRNA [37,38].
The recent solution of the structure of human PKR provides insight into the mechanism of its activation by dsRNA [39]. Once PKR is phosphorylated (activated), it can phosphorylate the eukaryotic translation initiation factor 2α (eIF-2α). The phosphorylated eIF-2α can then be chemically linked with guanosine by an enzyme called guanosine exchange factor and thus be inactivated. When functional eIF-2α is phosphorylated, protein translation cannot efficiently occur [40].
Thus, the PKR and RNaseL pathways shut-down translation and cause mRNA degradation. But the death observed after activation of these pathways is not merely due to the inhibition of host protein synthesis. The rapid death is mediated by a caspase cascade and thus is apoptotic. Our own experiments have demonstrated that replicon-containing nucleic-acid constructs (described in more detail below) that are known to mediate the production of dsRNA can induce caspase-dependent apoptotic death [41•]. Furthermore, phosphorylation of PKR has been linked to the elaboration of the death receptors, Fas and TNFR1 and to the activation of caspase-3 and -8 [42] (Figure 2).
In addition to its death-enhancing functions, phosphoryla-tion of PKR also has immune-modulating effects mediated in part through its induction of NF-κB [43]. These include the induction of the expression of a number of molecules—including MHC molecules, the LMP-2 and -7 components of the proteasome, and the ATP-dependent transporters associated with antigen processing (TAPs)—that facilitate T cell recognition of targets. Thus, immune activation and apoptotic death are both intrinsic components of the PKR and RNaseL pathways and form important links between cell death and immunity.
Viral blockade of apoptosis and inflammation
Viral infection of host cells triggers mechanisms that, if effective, can limit viral replication. As described above, cells respond to viral infection with apoptosis and the activation of innate and adaptive immunity. It has long been known that viruses encode proteins that counter these cellular defenses [44•,45-50] but the recent characterization of anti-apoptotic viral genes that double as anti-inflammatories sheds new light on how death and immunity are linked.
One example involves a viral protein called M11L. Myxoma viruses lacking M11L are highly attenuated. Despite their lack of virulence, lesions are characterized by vigorous inflammatory activity that far exceeds the inflammation associated with the wild-type virus [51,52]. In addition to its anti-inflammatory activity, M11L is also antiapoptotic at the mitochondrial checkpoint—downstream caspase-3 is not activated [53]. Interestingly, only the M11L-knockout virus induced apoptosis in monocytes, which are known to be capable of releasing activated IL-1β and IL-18 upon their death by apoptosis. Like another antiapoptotic viral protein, the poxviral serpin cytokine-response modifier A (CrmA) which inhibits ICE [54], observations with M11L provide a link between apoptosis and virally induced inflammation. These viral proteins may mimic the activity of natural inhibitors of ICE activity such as the serpin PI-9, whose downregulation in human vascular smooth muscle cells has recently been implicated in the inflammatory pathogenesis of atherosclerosis [53].
Inflammatory apoptosis compared with bland apoptosis in vaccine design
It may be possible to induce inflammatory apoptosis as a way of enhancing vaccine function. Others and we have focused on developing recombinant and synthetic cancer vaccines [55-60], especially those targeting normal ‘self’ antigens [61••,62-71]. We have previously reported on a new type of ‘naked’ nucleic-acid vaccine in which the gene encoding an antigen is inserted with an RNA replicase from an alphavirus (called a replicon) to form an RNA- or DNA-based immunogen [41•,72•,73,74]. The replicon mediates the copying of positive-stranded RNA to negative-stranded RNA and back again in the cytosol. Replicon-containing plasmids are 100–1000 times more efficient at eliciting immune responses than conventional DNA vaccines. Though initially designed as a way of enhancing antigen production in vivo, replicon-containing plasmids do not produce measurably more antigen that do conventional DNA vaccines. Instead, replicon-based nucleic-acid vaccines may be more effective at inducing immune responses because they employ qualitatively different mechanisms for immune activation: specifically, they induce caspase-dependent death in host cells [41•,42,43,44•,72•].
The apoptotic death mediated by these vaccines is a likely consequence of the requisite production of dsRNA intermediates that results from the activity of the replicon. dsRNA then can activate the PKR and 2′-5′-oligoadenylate synthetase pathways described above. Though these pathways may limit antigen production by mimicking viral infection [50], they also may activate the immune system. There are a variety of gene products known to modulate these pathways that may be useful in vaccine design (see Figure 2). It is not yet known how viral and cellular modulators of the PKR and 2′-5′-oligoadenylate-synthetase pathways will affect vaccine function.
The critical question from a vaccinologist’s perspective is whether a particular dying cell contains signals that stimulate DC maturation. This activity may not be identical to factors known to induce ‘inflammation’ as described above. DC maturation signals can be obtained from supernatants of freeze-thawed ‘necrotic’ cells but these factors have not yet been fully characterized [1•,2•]. Apoptotic death of some cell types but not other types may induce the migration and maturation of DCs. Candidate signals that mediate these activities include chemokines such as CCR6 [75] and cytokines, for example IL-1β and type I interferons [22,23,76].
Conclusions
Apoptotic cell death can induce inflammation and promote the activation of an immune response. Apoptosis-associated immune effects are mediated by caspase-1 and other potentially inflammatory caspases, and by innate components of the antiviral machinery. The design of more effective vaccines for infectious diseases and cancer may be one of the first applications of a better understanding of the molecules at the nexus of apoptotic death, and innate and adaptive immunity.
Acknowledgments
We thank Wolfgang Leitner and Leroy Hwang for help with experiments and diagrams, Martha Blalock for expert graphics, Pat Hwu, Jim Yang and Steve Rosenberg for daily, wide-ranging discussions and Pierre Henkart for multiple readings the manuscript and for helping to create the ideas presented here.
Abbreviations
- CrmA
cytokine-response modifier A
- DC
dendritic cell
- dsRNA
double-stranded RNA
- eIF-2α
eukaryotic translation initiation factor 2α
- FasL
Fas ligand
- ICE
IL-1β-converting enzyme
- TGF-β
transforming growth factor β
- TNFR
TNF receptor
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1•.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death. Exposure To necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. These investigators report that apoptotic cells are not capable of activating DCs but that necrotic cells can. The authors prepared apoptotic cells using UV light and necrotic cells with 4–5 cycles of rapid freezing and thawing. In light of our new understanding of the complexity and diversity of apoptotic mechanisms it may be interesting to evaluate if the generalizations made in this paper apply to other types of apoptotic death.
- 2•.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. The authors of this paper also (like [1•]) conclude that apoptosis does not activate DCs but again these generalizations are made after examining a very narrow set of conditions. Apoptotic cells were generated by incubation “for 6–8 or 12 hours in 100 M ceramide, with 50 g/ml mitomycin c for 30 min at 37°C followed by overnight incubation in serum-free conditions, then washed several times to eliminate residual apoptotic agents”. As in the paper by Sauter et al. [1•],‘necrotic’ cells are generated by freezing and thawing for 4–5 cycles.
- 3•.Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol. 2000;30:705–708. doi: 10.1002/1521-4141(200002)30:2<705::AID-IMMU705>3.0.CO;2-P. In sharp contrast to the two papers [1•,2•] cited above, the authors of this paper assert that in their experiments neither apoptotic nor necrotic cells induced DC maturation. Instead they found that the maturation of DCs was efficiently effected by mycoplasma, a common infectious agent that is well known to clinicians and laboratory workers alike (it is known to the latter because of its propensity to infect tissue-culture flasks).
- 4.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 6.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 7.Chappell DB, Zaks TZ, Rosenberg SA, Restifo NP. Human melanoma cells do not express Fas (Apo-1/CD95) ligand. Cancer Res. 1999;59:59–62. [PMC free article] [PubMed] [Google Scholar]
- 8.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–3279. [PMC free article] [PubMed] [Google Scholar]
- 9•.Restifo NP. Not so fas: Re-evaluating the role of FasL in autoimmunity and tumor escape. Nat Med. 2000;6:493–495. doi: 10.1038/74955. As a result of early studies, it was hypothesized that expression of FasL by tumor cells enabled them to counterattack the immune system and that transplant rejection could be prevented by expressing FasL on transplanted organs. More recent studies have indicated that the notion of FasL as a mediator of immune privilege needs to be reconsidered and taught a valuable lesson about making broad conclusions based on small amounts of data.
- 10.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–5320. [PMC free article] [PubMed] [Google Scholar]
- 11.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F. Fas counter-attack – the best form of tumor defense? Nat Med. 1999;5:267–268. doi: 10.1038/6477. [DOI] [PubMed] [Google Scholar]
- 13•.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. This is an excellent review of our current understanding of apoptotic mechanisms. A general note concerning nomenclature in the literature: although alternative names for caspases have generally been dropped, a number of names for death receptors (DRs) are still widely used. DR3 is also known as TRAMP or APO-3, DR4 is also designated TRAIL-R1 and DR5 is also called TRAIL-R2. The intracytoplasmic death-domains of these receptors can interact with a set of adaptor molecules (examples of which include FADD, TRADD, Daxx and APAF1), which in turn activate the cascades of cysteine proteases (caspases) that are described in the text.
- 14.Wang J, Lenardo MJ. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J Cell Sci. 2000;113:753–757. doi: 10.1242/jcs.113.5.753. [DOI] [PubMed] [Google Scholar]
- 15.Saikumar P, Dong Z, Mikhailov V, Denton M, Weinberg JM, Venkatachalam MA. Apoptosis: definition, mechanisms, and relevance to disease. Am J Med. 1999;107:489–506. doi: 10.1016/s0002-9343(99)00259-4. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Miura M, Jung Y, Zhu H, Gagliardini V, Shi L, Greenberg AH, Yuan J. Identification and characterization of Ich-3, a member of the interleukin-1-beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J Biol Chem. 1996;271:20580–20587. doi: 10.1074/jbc.271.34.20580. [DOI] [PubMed] [Google Scholar]
- 18.Humke EW, Ni J, Dixit VM. ERICE, a novel FLICE-activatable caspase. J Biol Chem. 1998;273:15702–15707. doi: 10.1074/jbc.273.25.15702. [DOI] [PubMed] [Google Scholar]
- 19••.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. The causative agen of bacillary dysentery, Shigella flexneri, induces both apoptosis and inflammation. These effects are mediated by ICE. Both the IL-1β and IL-18 components of the ICE-mediated inflammatory pathway contribute to the characteristic inflammatory changes that accompany shigellosis. The authors show that macrophage apoptosis is diminished in ICE-knockout mice with Shigella infection and, further, that ICE-knockouts do not develop inflammation and cannot clear the pathogen.
- 20.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1 beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 21.Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFN-gamma-inducing factor) induces IL-8 and IL-1beta via TNF alpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada N, Katz SI. Generation of mature dendritic cells from a CD14+ cell line (XS52) by IL-4, TNF-alpha, IL-1 beta, and agonistic anti-CD40 monoclonal antibody. J Immunol. 1999;163:5331–5337. [PubMed] [Google Scholar]
- 23.Stoitzner P, Zanella M, Ortner U, Lukas M, Tagwerker A, Janke K, Lutz MB, Schuler G, Echtenacher B, Ryffel B, et al. Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1 beta. J Leukoc Biol. 1999;66:462–470. [PubMed] [Google Scholar]
- 24.O’Neill LA, Greene C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants. J Leukoc Biol. 1998;63:650–657. [PubMed] [Google Scholar]
- 25.Rosenwasser LJ. Biologic activities of IL-1 and its role in human disease. J Allergy Clin Immunol. 1998;102:344–350. doi: 10.1016/s0091-6749(98)70118-6. [DOI] [PubMed] [Google Scholar]
- 26•.O’Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000;21:206–209. doi: 10.1016/s0167-5699(00)01611-x. See annotation to [27•].
- 27•.Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179(suppl 2):S294–S304. doi: 10.1086/513856. Once activated by ICE, IL-1β can activate NF-κB, triggering the production of TNF-α, GM-CSF and type I interferons. In addition, IL-1β is the most potent known endogenous pyrogen. IL-1β activates and triggers the production of TNF-α, interleukins including IL-2, IL-6 and IL-8, and ELR sequence (glu–leu–arg)-containing (inflammatory) CXC chemokines. Receptors for IL-1β can be found in one form or another on most cell types including T cells, B cells, monocytes, neutrophils, fibroblasts, keratinocytes, endothelial cells and hepatocytes (also see reviews [25,26•]).
- 28.Alheim K, Bartfai T. The interleukin-1 system: receptors, ligands, and ICE in the brain and their involvement in the fever response. Ann NY Acad Sci. 1998;840:51–58. doi: 10.1111/j.1749-6632.1998.tb09548.x. [DOI] [PubMed] [Google Scholar]
- 29.Akira S. The role of IL-18 in innate immunity. Curr Opin Immuno. 2000;12:59–63. doi: 10.1016/s0952-7915(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 30.Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantuzzi G, Puren AJ, Harding MW, Livingston DJ, Dinarello CA. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1-beta-converting enzyme (caspase-1)-deficient mice. Blood. 1998;91:2118–2125. [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin-18. Methods. 1999;19:121–132. doi: 10.1006/meth.1999.0837. [DOI] [PubMed] [Google Scholar]
- 33.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. During apoptotic death, the asymmetry of the cell membrane is lost but its integrity is largely maintained until late in the process. The lipid phosphatidylserine (PS), normally confined to the inner leaflet, can be found on the outer leaflet of the bilayer and may be important in the uptake of apoptotic cells by macrophages. Thecloning of the PS receptor is described in the present paper. Interestingly, the authors demonstrate that ligation of the PS receptor increases the production of TGF-β and inhibits LPS-induced TNF-α production.
- 35.Rebouillat D, Hovanessian AG. The human 2′,5′-oligoadenylate synthetase family: interferon-induced proteins with unique enzymatic properties. J Interferon Cytokine Res. 1999;19:295–308. doi: 10.1089/107999099313992. [DOI] [PubMed] [Google Scholar]
- 36.Castelli JC, Hassel BA, Maran A, Paranjape J, Hewitt JA, Li XL, Hsu YT, Silverman RH, Youle RJ. The role of 2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell Death Differ. 1998;5:313–320. doi: 10.1038/sj.cdd.4400352. [DOI] [PubMed] [Google Scholar]
- 37.Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. doi: 10.1038/sj.onc.1203127. [DOI] [PubMed] [Google Scholar]
- 38.Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nanduri S, Carpick BW, Yang Y, Williams BR, Qin J. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 1998;17:5458–5465. doi: 10.1093/emboj/17.18.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Haro C, Mendez R, Santoyo J. The eIF-2 alpha kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 41•.Ying H, Zaks TZ, Wang RF, Irvine KR, Kammula US, Marincola FM, Leitner WW, Restifo NP. Cancer therapy using a self-replicating RNA vaccine. Nat Med. 1999;5:823–827. doi: 10.1038/10548. ‘Naked’ nucleic acid vaccines are attractive candidates for the treatment of patients with cancer but their clinical efficacy has yet to be demonstrated. To enhance the immunogenicity of a nucleic-acid vaccine by making it ‘self-replicating’, a gene encoding an RNA replicase polyprotein derived from the Semliki Forest virus (SFV) was used in combination with a model antigen. A single intramuscular injection of a self-replicating RNA immunogen elicited antigen-specific antibody and CD8+ T cell responses at doses as low as 0.1 μg. Therapeutic immunization prolonged the survival of mice bearing established tumors. Interestingly, the self-replicating RNA vectors did not mediate the production of significantly greater quantities of the model antigen when compared with a conventional DNA vaccine in vitro. Instead, the enhanced efficacy in vivo correlated with a caspase-dependent apoptotic death in transfected cells. This death facilitated the uptake of apoptotic cells by dendritic cells, providing a potential mechanism for enhanced immunogenicity.
- 42.Lee SB, Rodriguez D, Rodriguez JR, Esteban M. The apoptosis pathway triggered by the interferon-induced protein kinase PKR requires the third basic domain, initiates upstream of Bcl-2, and involves ICE-like proteases. Virology. 1997;231:81–88. doi: 10.1006/viro.1997.8494. [DOI] [PubMed] [Google Scholar]
- 43.Zamanian-Daryoush M, Mogensen TH, DiDonato JA, Williams BR. NF-kappa-B activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-kappa-B-inducing kinase and I-kappa-B kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem. 1999;274:1541–1548. doi: 10.1074/jbc.274.3.1541. Many of the viral genes that counter apoptosis are similar to host genes involved in the modulation of apoptosis, such as the apoptosis inhibitor (IAP) family of proteins. Antiapoptotic genes, such as those encoding viral Fas-associated death-domain-like IL-1β-converting enzyme (FLICE)-inhibitory proteins (vFLIPs), are encoded by a number of different viruses. Like their cellular cousins (cFLIPS, discussed in the present paper), vFLIPs can interfere with the assembly of the death-induced signaling complex (DISC). Antiapoptotic viral proteins can also interfere with the Bcl-2 checkpoint (see [42]). The adenovirus 14.7 kDa protein interacts directly with FLICE to inhibit Fas-ligand-induced apoptosis (see [43]).
- 45.Meinl E, Fickenscher H, Thome M, Tschopp J, Fleckenstein B. Antiapoptotic strategies of lymphotropic viruses. Immunol Today. 1998;19:474–479. doi: 10.1016/s0167-5699(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 46.Tschopp J, Thome M, Hofmann K, Meinl E. The fight of viruses against apoptosis. Curr Opin Genet Dev. 1998;8:82–87. doi: 10.1016/s0959-437x(98)80066-x. [DOI] [PubMed] [Google Scholar]
- 47.Hardwick JM. Viral interference with apoptosis. Semin Cell Dev Biol. 1998;9:339–349. doi: 10.1006/scdb.1998.0243. [DOI] [PubMed] [Google Scholar]
- 48.Chen P, Tian J, Kovesdi I, Bruder JT. Interaction of the adenovirus 14.7-kDa protein with FLICE inhibits Fas ligand-induced apoptosis. J Biol Chem. 1998;273:5815–5820. doi: 10.1074/jbc.273.10.5815. [DOI] [PubMed] [Google Scholar]
- 49.Cai R, Carpick B, Chun RF, Jeang KT, Williams BR. HIV-I TAT inhibits PKR activity by both RNA-dependent and RNA-independent mechanisms. Arch Biochem Biophys. 2000;373:361–367. doi: 10.1006/abbi.1999.1583. [DOI] [PubMed] [Google Scholar]
- 50.Terenzi F, deVeer MJ, Ying H, Restifo NP, Williams BR, Silverman RH. The antiviral enzymes PKR and RNase L suppress gene expression from viral and non-viral based vectors. Nucleic Acids Res. 1999;27:4369–4375. doi: 10.1093/nar/27.22.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macen JL, Graham KA, Lee SF, Schreiber M, Boshkov LK, McFadden G. Expression of the myxoma virus tumor necrosis factor receptor homologue and M11L genes is required to prevent virus-induced apoptosis in infected rabbit T lymphocytes. Virology. 1996;218:232–237. doi: 10.1006/viro.1996.0183. [DOI] [PubMed] [Google Scholar]
- 52.Graham KA, Opgenorth A, Upton C, McFadden G. Myxoma virus M11L ORF encodes a protein for which cell surface localization is critical in manifestation of viral virulence. Virology. 1992;191:112–124. doi: 10.1016/0042-6822(92)90172-l. [DOI] [PubMed] [Google Scholar]
- 53.Everett H, Barry M, Lee SF, Sun X, Graham K, Stone J, Bleackley RC, McFadden G. M11L. A novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J Exp Med. 2000;191:1487–1498. doi: 10.1084/jem.191.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 55.Restifo NP, Rosenberg SA. Developing recombinant and synthetic vaccines for the treatment of melanoma. Curr Opin Oncol. 1999;11:50–57. doi: 10.1097/00001622-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, Rosenberg SA, Moss B, Restifo NP. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc Natl Acad Sci USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Irvine KR, Chamberlain RS, Shulman EP, Rosenberg SA, Restifo NP. Route of immunization and the therapeutic impact of recombinant anticancer vaccines. J Natl Cancer Inst. 1997;89:390–392. doi: 10.1093/jnci/89.5.390. [DOI] [PubMed] [Google Scholar]
- 59.Surman DR, Dudley ME, Overwijk WW, Restifo NP. Cutting edge: CD4+ T cell control of CD8+ T cell reactivity to a model tumor antigen. J Immunol. 2000;164:562–565. doi: 10.4049/jimmunol.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am. 2000;6(suppl 1):S76–S80. [PMC free article] [PubMed] [Google Scholar]
- 61••.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. The authors show here that antibodies to CTLA-4, an inhibitory receptor on T cells, were able to treat primary prostate tumors in transgenic (TRAMP) mice when administered in combination with an irradiated tumor cell vaccine. This work significantly extends the authors’ previous efforts exploring the uses of CTLA-4 blockade in combination with a GM-CSF-based vaccine (see [58]) in a transplantable mouse B16 melanoma model.
- 62.Kwon ED, Foster BA, Hurwitz AA, Madias C, Allison JP, Greenberg NM, Burg MB. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bronte V, Apolloni E, Ronca R, Zamboni P, Overwijk WW, Surman DR, Restifo NP, Zanovello P. Genetic vaccination with ‘self’ tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res. 2000;60:253–258. [PMC free article] [PubMed] [Google Scholar]
- 65.Touloukian CE, Leitner WW, Topalian SL, Li YF, Robbins PF, Rosenberg SA, Restifo NP. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J Immunol. 2000;164:3535–3542. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of ‘self’-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irvine KR, Parkhurst MR, Shulman EP, Tupesis JP, Custer M, Touloukian CE, Robbins PF, Yafal AG, Greenhalgh P, Sutmuller RP, et al. Recombinant virus vaccination against ‘self’ antigens using anchor-fixed immunogens. Cancer Res. 1999;59:2536–2540. [PMC free article] [PubMed] [Google Scholar]
- 68.Surman DR, Irvine KR, Shulman EP, Allweis TM, Rosenberg SA, Restifo NP. Generation of polyclonal rabbit antisera to mouse melanoma associated antigens using gene gun immunization. J Immunol Methods. 1998;214:51–62. doi: 10.1016/s0022-1759(98)00036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, Pierce RA, Restifo NP, Engelhard VH. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen. Implications for tumor immunotherapy. J Exp Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiang R, Lode HN, Chao TH, Ruehlmann JM, Dolman CS, Rodriguez F, Whitton JL, Overwijk WW, Restifo NP, Reisfeld RA. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci. 2000;97:5492–5497. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Overwijk WW, Lee DS, Surman DR, Irvine KR, Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA, Restifo NP. Vaccination with a recombinant vaccinia virus encoding a ‘self’ antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4(+) T lymphocytes. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72•.Leitner WW, Ying H, Driver DA, Dubensky TW, Restifo NP. Enhancement of tumor-specific immune response with plasmid DNA replicon vectors. Cancer Res. 2000;60:51–55. Replicons derived from the prototype alphavirus, Sindbis, and another alphavirus, Semliki Forest virus, were used to enhance the immunogenicity of nucleic-acid vaccines. These plasmid DNAs launch a self-replicating RNAvector (replicon) that in turn directs the expression of a model tumor antigen. Immunization with plasmid DNA replicons elicited immune responses at doses 100–1000-fold lower than conventional DNA plasmids and effectively treated mice bearing an experimental tumor expressing the model antigen. The replicon-based DNA plasmids did not produce a greater quantity of antigen but were associated with the apoptotic death of the host cells. Plasmid DNA replicons may prove useful to study the relationships between apoptosis and vaccine function.
- 73.Leitner WW, Ying H, Restifo NP. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine. 1999;18:765–777. doi: 10.1016/s0264-410x(99)00271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Restifo NP, Ying H, Hwang L, Leitner WW. The promise of nucleic acid vaccines. Gene Ther. 2000;7:89–92. doi: 10.1038/sj.gt.3301117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 76.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR, Barber GN. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol. 2000;74:1513–1523. doi: 10.1128/jvi.74.3.1513-1523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan SL, Katze MG. The emerging role of the interferon-induced PKR protein kinase as an apoptotic effector: a new face of death? J Interferon Cytokine Res. 1999;19:543–554. doi: 10.1089/107999099313677. [DOI] [PubMed] [Google Scholar]
- 79.Gale MJ, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]


