Abstract
Computer-aided diagnosis (CAD) has become one of the major research subjects in medical imaging and diagnostic radiology. In this article, the motivation and philosophy for early development of CAD schemes are presented together with the current status and future potential of CAD in a PACS environment. With CAD, radiologists use the computer output as a “second opinion” and make the final decisions. CAD is a concept established by taking into account equally the roles of physicians and computers, whereas automated computer diagnosis is a concept based on computer algorithms only. With CAD, the performance by computers does not have to be comparable to or better than that by physicians, but needs to be complementary to that by physicians. In fact, a large number of CAD systems have been employed for assisting physicians in the early detection of breast cancers on mammograms.
A CAD scheme that makes use of lateral chest images has the potential to improve the overall performance in the detection of lung nodules when combined with another CAD scheme for PA chest images. Because vertebral fractures can be detected reliably by computer on lateral chest radiographs, radiologists’ accuracy in the detection of vertebral fractures would be improved by the use of CAD, and thus early diagnosis of osteoporosis would become possible. In MRA, a CAD system has been developed for assisting radiologists in the detection of intracranial aneurysms. On successive bone scan images, a CAD scheme for detection of interval changes has been developed by use of temporal subtraction images. In the future, many CAD schemes could be assembled as packages and implemented as a part of PACS. For example, the package for chest CAD may include the computerized detection of lung nodules, interstitial opacities, cardiomegaly, vertebral fractures, and interval changes in chest radiographs as well as the computerized classification of benign and malignant nodules and the differential diagnosis of interstitial lung diseases. In order to assist in the differential diagnosis, it would be possible to search for and retrieve images (or lesions) with known pathology, which would be very similar to a new unknown case, from PACS when a reliable and useful method has been developed for quantifying the similarity of a pair of images for visual comparison by radiologists.
1. Introduction
Recently, computer-aided diagnosis (CAD) has become a part of the routine clinical work for detection of breast cancer on mammograms at many screening sites and hospitals (1-25) in the United States. This seems to indicate that CAD is beginning to be applied widely in the detection and differential diagnosis of many different types of abnormalities in medical images obtained in various examinations by use of different imaging modalities. In fact, CAD has become one of the major research subjects in medical imaging and diagnostic radiology (12-25). Although early attempts at computerized analysis of medical images (26-32) were made in the 1960s, serious and systematic investigation on CAD began in the 1980s with a fundamental change in the concept for utilization of the computer output, from automated computer diagnosis to computer-aided diagnosis. In this article, the motivation and philosophy for early development of CAD schemes are presented together with the current status and future potential of CAD in the environment of picture archiving and communication systems (PACS).
2. Historical review of the development of CAD
Large-scale and systematic research and development of various CAD schemes were begun in the early 1980s at the Kurt Rossmann Laboratories for Radiologic Image Research in the Department of Radiology at the University of Chicago. Prior to and since that time, we have been engaged in some of basic research on the effects of digital images on radiologic diagnosis (33-48), and many investigators in the United States and in Europe have become involved in research and development of some aspects of PACS (49). Although PACS would be useful and advantageous in the management of radiologic images in radiology departments and might be beneficial economically to hospitals, it seemed unlikely at that time that PACS would bring a significant clinical benefit to radiologists. Therefore, we thought that a major benefit of digital images must be realized in radiologists’ daily work. Of course, radiologists’ daily work consists of image reading and radiologic diagnosis. Thus, we came to the question “how can radiologists’ diagnosis be helped by the benefits of digital images?” This led immediately to the concept of computer-aided diagnosis.
In the 1980s, the concept of automated diagnosis or automated computer diagnosis was already known from studies (26-31) in the 1960s, but these early attempts were not successful. (The difference and the common aspects between automated diagnosis and computer-aided diagnosis will be discussed in the next section.) Thus, it appeared to be extremely difficult to carry out a computer analysis on lesions involved in medical images. It was, therefore, not easy to predict whether the development of CAD schemes would be successful or fail. Therefore, we thought that we should select research subjects which had the potential to have a major impact in medicine, if CAD could be developed successfully. Some of the most important subjects in medicine at that time were related to cardiovascular diseases, lung cancer, and breast cancer. Therefore, we decided to select three main research projects: for detection and/or quantitative analysis of lesions involved in vascular imaging by Fujita et al. (50) and Hoffmann et al. (51); detection of lung nodules in chest radiographs by Giger et al. (52,53); and detection of clustered microcalcifications in mammograms by Chan et al. (54).
In addition, our efforts on research and development of CAD have been based on three fundamental ideas from the beginning to the present time, as described below. First, our basic strategy for development of methods and techniques for detection and quantitation of lesions in medical images has been based on the understanding of processes that would be involved in image readings by radiologists (20,25). This strategy appeared quite logical and straightforward because radiologists are the ones who have been carrying out very complex and difficult tasks of image reading and radiologic diagnosis. Therefore, we assumed that computer algorithms should be developed based on the understanding of image readings, such as how radiologists can detect certain lesions, why they may miss some abnormalities, and how they can distinguish between benign and malignant lesions.
The second thought was related to the way in which the success of our efforts could be measured if we were successful in the development of CAD. It appeared that the best proof of our success would be the daily use of CAD in routine clinical work at many hospitals around the world. In order for us to realize this, it would be necessary for many industries to commercialize CAD products for the global medical market. Therefore, we decided to produce and protect intellectual properties related to basic technologies of CAD schemes in the form of patents, and to promote these in communicating with many individuals in medical industries for potential efforts toward commercialization of CAD products. Subsequently, our first patent on CAD (55), filed in 1987, has become the most commonly cited patent in the field of CAD technology.
The third thought was to promote the wide acceptance of the CAD concept and to facilitate the global distribution of CAD research by many investigators at many different institutions. Because the success of CAD would require overwhelmingly large efforts on many aspects of CAD research such as the development of computerized schemes for many different types of lesions in many different modalities, observer performance studies, clinical trials, and commercialization, it appeared that we would not be successful if we were the only research group on CAD. Instead, we could be successful if many researchers from many institutions were involved in many aspects of CAD research. Therefore, all researchers on CAD should be considered as promoters and as our colleagues rather than competitors. Therefore, for more than 20 years from the initial phase of CAD research, we have had large scientific exhibits at the Annual Meetings of the Radiological Society of North America (RSNA) which were held in Chicago. In these exhibits, we have presented a comprehensive demonstration of CAD research in chest, breast, and vascular imaging. In 1993, we set up a film digitizer and a computer for real-time demonstration of CAD for detection of clustered microcalcifications on “unknown” new cases of mammograms. We invited in advance 118 breast radiologists to bring their cases to the RSNA meeting for testing of our CAD scheme. The results of this informal validation test were promising (56). From 1996 to 2001, we carried out observer performance studies with a large number of participants during the RSNA meetings for detection of various lesions on chest images without and with the computer output, so that many radiologists would be able to have their own experience of using CAD. The usefulness of CAD was clearly demonstrated (57,58). These demonstrations appeared successful in promoting the CAD concept widely and quickly.
For CAD research on lung cancer, we attempted to develop a computerized scheme for detection of lung nodules on chest radiographs. At that time and still now, it is well known that the visual detection of lung nodules is a difficult task for radiologists, who may miss up to 30% of the nodules. Radiologists have missed these lesions due to the overlap of normal anatomic structures with nodules, i.e., the normal background in chest images tends to camouflage nodules (59-63). Therefore, it was predicted that the normal background structures in chest images would become a large obstacle in the detection of nodules, even by computer. Thus, the first step in the computerized scheme for detection of lung nodules in chest images would be removal or suppression of background structures in chest radiographs. A method for suppressing the background structures is the difference-image technique (64-68), in which the difference between a nodule-enhanced image and a nodule-suppressed image is obtained. This difference-image technique has been useful in enhancing lesions and suppressing the background not only for nodules in chest images (64,65), but also for microcalcifications (54,66) and masses (67) in mammograms, and for nodules in CT (68).
Thus, at the Rossmann Laboratories in the mid 1980s, we had already developed basic schemes for the detection of lung nodules in chest images (52,53) and the detection of clustered microcalcifications in mammograms (54). Although the sensitivities of these schemes for detection of lesions were relatively high even at that time, the number of false positives was very large. It was therefore quite uncertain and unpredictable whether the output of these computerized schemes could be used by radiologists in routine clinical work. For example, the average number of false positives was four per mammogram in the detection of clustered microcalcifications by computer, although the sensitivity was about 85%. However, in order to examine the possibility of practical uses of CAD in clinical situations, we carried out an observer performance study without and with the computer output. To our surprise, radiologists’ performance in detecting clustered microcalcifications was improved significantly when the computer output was available, even with such a large number of false positives. This result was published in 1990 by H.P. Chan et al. (66), as described in detail later.
3. Computer-aided diagnosis and automated computer diagnosis
Early studies on quantitative analysis of medical images by computer (26-31) were reported in the 1960s. At that time, it was generally assumed that computers could replace radiologists in detecting abnormalities, because computers and machines are better at performing certain tasks than are human beings. Thus, the concept of computer diagnosis or automated diagnosis in radiology was established at that time. Although interesting results were reported, these early attempts were not successful, because computers were not sufficiently powerful, advanced image-processing techniques were not available, and digital images were not easily accessible. However, a serious flaw was an excessively high expectation from computers. In fact, many different approaches to automated computer diagnosis have been attempted as aids in decision-making in many fields of medicine since the 1950s. In the medical subspecialty of hematology, for example, R.L. Engle (32), in his review article on 30 years’ experience, stated in his conclusion, “Thus, we do not see much promise in the development of computer programs to simulate the decision making of a physician. ---- However, after many years we have concluded that we should stop trying to make computers act like diagnosticians.”
In the 1980s, however, another approach emerged which assumed that the computer output could be utilized by radiologists, but not replace them. This concept is currently known as computer-aided diagnosis, which has spread widely and quickly. However, in the early phase of research on and development of CAD schemes, some computer scientists criticized CAD by saying that it simply would not work, which has been proved to be completely wrong. The reason for this strong criticism at that time might have been related to an unsuccessful attempt in previous research efforts toward the development of automated computer diagnosis. This seems to indicate that a failure of a specific research project can lead to a strong “incorrect” bias toward another area of research that may look similar to the failed previous research.
However, these two concepts of automated computer diagnosis and computer-aided diagnosis clearly exist even at present. For example, some investigators have been working seriously on the development of automated computer diagnosis. In addition, there are many researchers who believe that the technologies involved in the current CAD schemes could be applied as a means for primary diagnosis, which would be equivalent to automated computer diagnosis. During the panel discussion sessions at two meetings, in 2002, of CARS in Paris and the AAPM in Montreal, about half of the participants voted for the possibility that CAD would be shifted to automated computer diagnosis within 50 years, whereas the other half voted against this prediction. Therefore, it may be useful to understand some of the common features and also the differences between CAD and automated computer diagnosis. The common approach to both CAD and automated computer diagnosis is that digital medical images are analyzed quantitatively by computers. Therefore, the development of computer algorithms is required for both CAD and computer diagnosis.
A major difference between CAD and computer diagnosis is the way in which the computer output is utilized for the diagnosis. With CAD, radiologists use the computer output as a “second opinion,” and radiologists make the final decisions. Therefore, for some clinical cases in which radiologists are confident about their judgments, radiologists may agree with the computer output, or disagree and then disregard the computer. However, for cases in which radiologists are less confident, it is expected that the final decision can be improved by use of the computer output. This improvement is possible, of course, only when the computer result is correct. The higher the performance of the computer, the better the overall effect on the final diagnosis. However, the performance level of the computer does not have to be equal to or higher than that of radiologists. With CAD, the potential gain is due to the synergistic effect obtained by combining the radiologist’s competence and the computer’s capability. Because of these multiplicative benefits, the current CAD has become widely used in practical clinical situations.
With automated computer diagnosis, however, the performance level of the computer output is required to be very high. For example, if the sensitivity for detection of lesions by computer would be lower than the average sensitivity of physicians, it would be difficult to justify the use of automated computer diagnosis. This is because the patients in most advanced countries would not be able to accept a lower level of diagnostic results by computer than the average level achievable by physicians. In addition, the reimbursement for the cost of an automated computer diagnosis may be refused by insurance companies. It would be difficult also to implement automated computer diagnosis if the number of false-positive detections by computer were large, such as one or more false positives per case. In such instances, because every case analyzed by computer would have a chance of involving an abnormality, the physician would be required to verify all of the cases, and this would then not be considered an automated computer diagnosis. Therefore, high sensitivity and high specificity by computer are required for implementing automated computer diagnosis. This requirement is extremely difficult to meet for researchers who are developing computer algorithms for detection of abnormalities on radiologic images. In fact, although CAD has been used widely for detection of breast cancer on mammograms, it would not be possible for most advanced countries to employ the current computer results for automated computer diagnosis.
Another important and distinctive difference between CAD and automated computer diagnosis is the way that the performance and the usefulness would be evaluated quantitatively. The performance level of automated computer diagnosis is equal to the performance achieved by computer. However, the performance level of CAD is equal to the performance achieved by the physician who makes the final decision by using the computer output as a second opinion. Therefore, it is important and essential for CAD that the computer results be utilized effectively by physicians. It is thus necessary to evaluate quantitatively how much physicians can use the computer results to improve their overall performance. Even if the computer results are poor--for example, if both sensitivity and specificity are low--it is possible for physicians to incorporate the computer results efficiently. For example, if the computer could detect subtle lesions which might be difficult for physicians to detect, and also if physicians could disregard “obvious” computer false positives easily, it would be possible to realize CAD. However, if the computer results were poor, automated computer diagnosis could not be implemented.
Therefore, it is important for CAD to assess not only the computer performance, but also the performance by physicians. It is thus necessary to evaluate quantitatively and accurately by use of receiver operating characteristic (ROC) analysis (69-71) whether the performance by physicians can be improved by use of the computer results. In fact, even if the ROC curve for computer results in detecting clustered microcalcifications on mammograms is substantially lower than that by radiologists, the ROC curve obtained by radiologists using the computer results can be improved. The extent of this improvement due to CAD was confirmed to be statistically significant, as reported by H.P. Chan et al. (66) in 1990, thereby providing the first scientific evidence for the benefits of CAD in the detection of lesions. Thereafter, a number of investigators have reported similar positive findings on the usefulness of CAD in detecting various lesions, including clustered microcalcifications (66) and masses (72) in mammograms, lung nodules (73,74) and interstitial opacities (75) in chest radiographs, lung nodules in CT (76), and intracranial aneurysms in MRA (77). Thus they have accelerated research and development on CAD schemes in many different types of examinations for detection of various lesions.
In summary, although there are some common aspects of CAD and automated computer diagnosis, there are also distinct differences between them. Automated computer diagnosis is a concept based on computer algorithms, whereas CAD is a concept that has been established by taking into account equally the roles of physicians and computers. Because of this fundamental difference, the performance levels required for computer algorithms have resulted in a large difference between the two. With automated computer diagnosis, the performance by computers needs to be comparable to or better than that by physicians. With CAD, however, the performance by computers does not have to be comparable to or better than that by physicians, but needs to be complementary to that by physicians. In fact, a large number of CAD systems in the United States and Europe have been employed for assisting physicians in the early detection of breast cancers on mammograms. It should be noted that the overall performance level of these computers is far below the average performance level achievable by physicians, and yet it can be useful in practical clinical situations.
4. Current status of CAD research
The number of papers related to CAD research presented at the RSNA meetings from 2000 to 2005 is listed in Table 1. The majority of these presentations were concerned with three organs - chest, breast, and colon - but other organs such as brain, liver, and skeletal and vascular systems were also subjected to CAD research. It may be noted that the detection of cancer in the breast (1-6), lung (78-84), and colon (85,86) has been or is being subjected to screening examinations (87,88). A large fraction of these examinations give normal results, and the detection of only a small number of suspicious lesions by radiologists is considered both difficult and time-consuming. Therefore, it appears reasonable that the initial phase of practical CAD in clinical situations has begun in these screening examinations. In fact, commercial CAD systems for detection of these cancers are now available for clinical use. Figure 1 shows a comparison of the previous performance level (87% sensitivity at 1.0 false positive per image) in the detection of clustered microcalcifications by computer when the CAD technology was licensed to a company in 1993, with an estimated current performance level (98% sensitivity at 0.25 false positive per image) of the latest commercial CAD system. It is obvious from this result that a substantial improvement was possible due to efforts by people in industry, and thus a successful clinical CAD system has evolved. This may be a good example showing that a small positive seed that germinated at an academic institution can grow substantially by commercialization efforts in industry.
Table 1.
Number of CAD papers related to seven different organs, presented at the Annual Meetings of the Radiological Society of North America (RSNA) in Chicago from 2000 to 2005
| Year | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 |
|---|---|---|---|---|---|---|
| Chest | 22 | 37 | 53 | 94 | 70 | 48 |
| Breast | 23 | 28 | 32 | 37 | 48 | 49 |
| Colon | 4 | 10 | 21 | 17 | 15 | 30 |
| Brain | - | 4 | 2 | 10 | 9 | 15 |
| Liver | 3 | - | 5 | 9 | 9 | 9 |
| Skeletal | 2 | 7 | 7 | 9 | 8 | 5 |
| Vascular etc | 5 | - | 12 | 15 | 2 | 7 |
| Total | 59 | 86 | 134 | 191 | 161 | 163 |
Fig. 1.
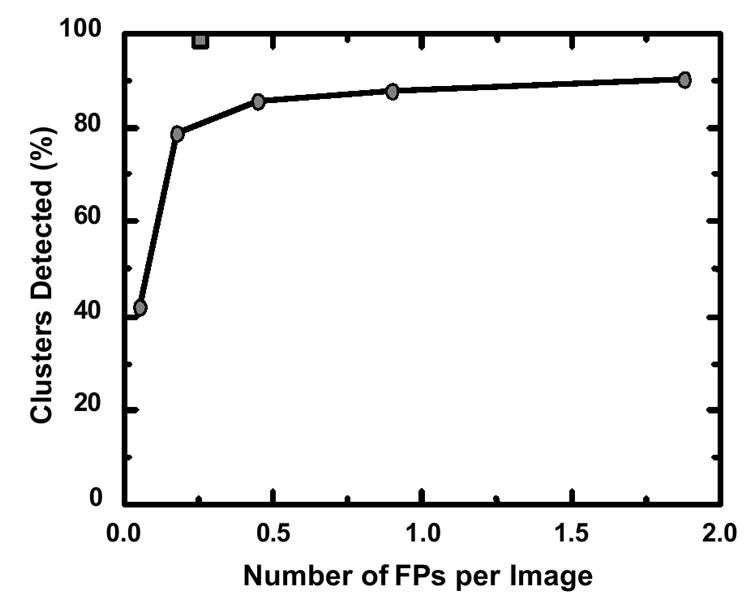
Comparison of the previous performance level marked by circles (87% sensitivity at 1.0 false positive per image) in the detection of clustered microcalcifications by computer in 1993, when the CAD technology was licensed to a company, with the estimated current performance level, marked by a gray square (98% sensitivity at 0.25 false positive per image), of the latest commercial CAD system.
In mammography, investigators (1-6) have reported results from prospective studies on large numbers of screenees, ranging from 8,682 to 115,571, regarding the effect of CAD on the detection rate of breast cancer, as summarized in Table 2. Although there is a large variation in the results, it is important to note that all of these studies indicated an increase in the detection rates of breast cancer with use of CAD. In addition, Cupples et al. (4) reported a 164% increase in the detection of small (less than 1 cm) invasive cancers, and also a reduction of 5.3 years in the mean age of patients at the time of detection, when CAD was used in mammography.
Table 2.
Prospective studies on the usefulness of CAD for detection of breast cancers in screening mammography
Because several review articles on CADs for mammography, chest radiography, thoracic CT, CT colonography, and multi-organ, multi-dimensional CAD are included in this Special Issue of the Journal of Computerized Medical Imaging and Graphics, only selected topics with recent findings are discussed below.
4.1 Detection of lung nodules on PA and lateral chest radiographs
In chest radiography and thoracic CT, a number of CAD schemes have been developed, and some commercial systems are available. CAD schemes in chest radiography include the detection and differential diagnosis of lung nodules (89-91) and of interstitial lung diseases (92-96), and the detection of cardiomegaly (97,98), pneumothorax (99), and interval changes (100-105). However, these CAD methods employed only posterior-anterior (PA) views of chest images, even if both PA and lateral views for the same patient were available. In fact, lateral views of chest images were commonly obtained together with PA views in many medical centers and clinical institutions. Even though different opinions have been expressed in the past about the necessity of obtaining PA and lateral views (106-111), it is reasonable to use a lateral view in supporting the performance of a CAD scheme for the detection of lung nodules, and thus in assisting radiologists’ decision-making, if a lateral view together with a PA view has already been obtained.
Recently, Shiraishi et al. (112) developed a computerized scheme for detection of lung nodules in the lateral views of chest radiographs, in order to improve the overall performance in combination with the CAD scheme for PA views. When the CAD scheme was applied only to PA views, the sensitivity in the detection of lung nodules was 70.5%, with 4.9 false positives per image. Although the performance of the computerized scheme for lateral views was relatively low (60.7% sensitivity with 1.7 false positives per image), the overall sensitivity (86.9%) was improved (6.6 false positives per two views), because 20 (16.4%) of the 122 nodules in 106 pairs of PA and lateral views of chest radiographs used in their study were detected only on lateral views. Figure 2 shows relatively large, but very subtle lung nodules located in the right mediastinum region which were not identified by CAD on the PA view, but were correctly identified by CAD on the lateral view. Thus, the CAD scheme by use of lateral-view images has the potential to improve the overall performance for detection of lung nodules on chest radiographs when combined with a conventional CAD scheme for standard PA views.
Fig. 2.
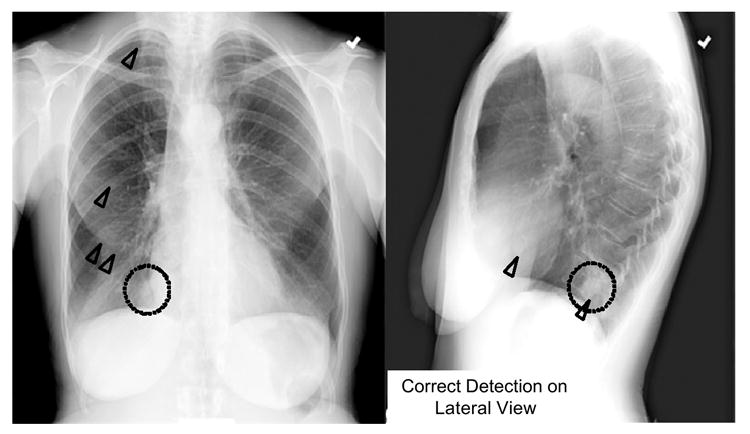
Illustration of a relatively large, but very subtle lung nodule (dotted circles) located in the right mediastinum region which was correctly marked by CAD (triangles) on the lateral view, but was not marked by CAD on the PA view.
4.2 Detection of vertebral fractures on lateral chest radiographs
Vertebral fracture (or vertebral deformity) is a common outcome of osteoporosis, which is one of the major public health concerns in the world. Early detection of vertebral fractures is important because timely pharmacologic intervention can reduce the risk of subsequent additional fractures (113-117). Vertebral fractures can be visible on lateral chest radiographs. However, investigators (118-121) have noted that about 50% of vertebral fractures visible on lateral chest radiographs were underdiagnosed or underreported, even when the fractures were severe. Therefore, Kasai et al. (122,123) developed a computerized method for detection of vertebral fractures on lateral chest radiographs in order to assist radiologists’ image interpretation and thus the early diagnosis of osteoporosis. In the computerized method, a curved search area, which included a number of vertebral end plates, was first extracted automatically, and then was straightened so that vertebral end plates became oriented horizontally. Edge candidates were enhanced by use of a horizontal line-enhancement filter in the straightened image, and a multiple thresholding technique, followed by feature analysis, was used for identification of the vertebral end plates. The height of each vertebra was determined from locations of identified vertebral end plates, and fractured vertebrae were detected by comparison of the measured vertebral height with the expected height.
The cases used in this study were 20 patients with severe vertebral fractures and 118 patients without fractures, as confirmed by the consensus of two radiologists. Preliminary results indicated that the sensitivity of the computerized method for detection of fracture cases was 95% (19/20), with 1.03 (139/135) false-positive fractures per image. For a validation test, the detection accuracy of the computerized method was examined by use of 32 additional fracture cases which were selected independently from training cases. The sensitivity for these cases was 75% (24/32) at 1.03 (33/32) false-positive fractures per image. Figure 3 shows the correct detection of a fractured vertebra on a lateral chest radiograph. Because vertebral fractures can be detected by computer, radiologists can use the detection results as a “second opinion”, and then the detection accuracy of vertebral fractures by radiologists will be improved on lateral chest radiographs, with improvement in the early diagnosis of osteoporosis.
Fig. 3.
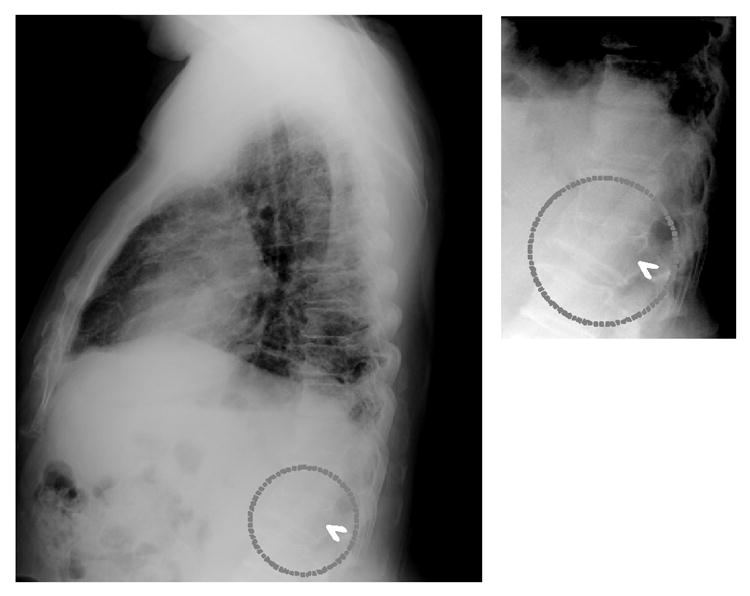
Illustration of the correct detection (arrowhead) by computer of a fractured vertebra (dotted circles) below the diaphragm on a lateral chest radiograph, which can be used as a second opinion. Thus, the accuracy of detection of vertebral fractures by radiologists could be improved on lateral chest radiographs, and the early diagnosis of osteoporosis could be improved.
4.3 Detection of intracranial aneurysms in MRA
During the past decade, there has been considerable interest in the roles of “less invasive” imaging modalities such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) in the detection of intracranial aneurysms (124-131). However, it is still difficult and time-consuming for radiologists to find small aneurysms, and it may not be easy to detect even medium-sized aneurysms on maximum-intensity-projection (MIP) images, because of overlap with adjacent vessels and because of unusual locations. Therefore, a CAD scheme would be useful in assisting radiologists in the detection of intracranial aneurysms, especially those that are small, by use of MRA. Recently, Arimura et al. (132,133) have developed a computerized scheme for automated detection of unruptured intracranial aneurysms in MRA, based on the use of a 3D selective enhancement filter for dots (aneurysms). The isotropic 3D MRA images were processed by use of a selective, multi-scale enhancement filter (134), as illustrated in Fig. 4. The initial candidates were identified by use of a multiple gray-level thresholding technique on the dot-enhanced images and a region-growing technique with monitoring of some image features. All candidates were classified into four types according to the size and local structures based on the effective diameter and the skeleton image of each candidate, i.e., large candidates and three types of small candidates: a short-branch type, a single-vessel type, and a bifurcation type. In each group, a number of false positives were removed by use of different rules on localized image features related to gray levels and morphology. Linear discriminant analysis was employed for further removal of false positives.
Fig. 4.
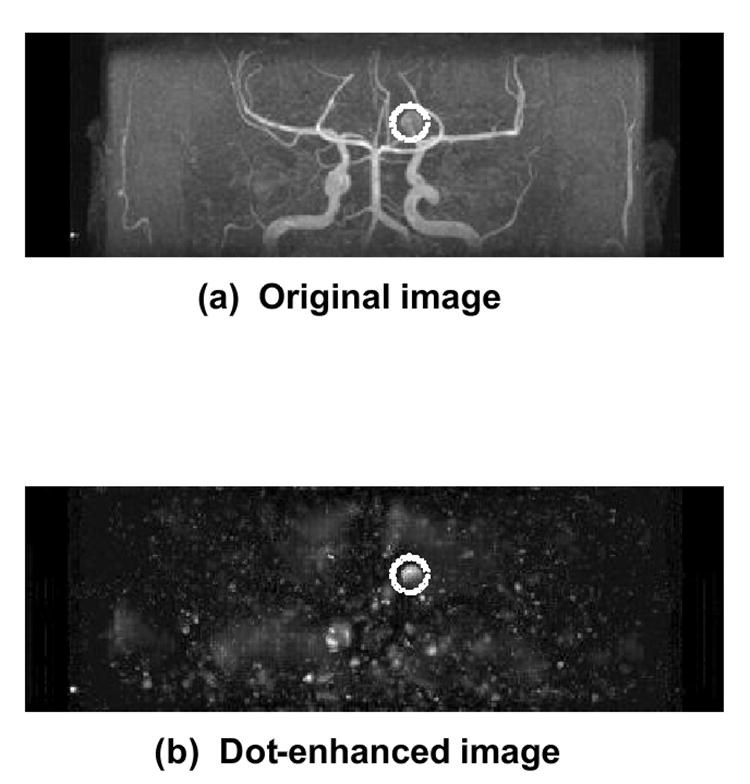
The isotropic 3D MRA image in (a) was processed by use of a selective, multi-scale enhancement filter for detection of an intracranial aneurysm (dotted circles), as illustrated in the dot-enhanced image in (b).
The performance of this CAD scheme was evaluated by use of 207 cases with aneurysms which were obtained from three institutions by use of three different types of MR equipment, and thus cases from each institution were tested independently based on a leave-one-out-by-patient test method. The average sensitivity in detecting intracranial aneurysms with sizes ranging from 1mm to 23 mm was 96%, with 3.2 false positives per patient. An observer study was carried out for examining the effect of the computer output on radiologists’ detection performance by use of 22 cases with aneurysms and 28 cases without. The average area under the ROC curve (Az value) for 15 radiologists in the detection of intracranial aneurysms in MRA was improved from 0.931 to 0.983 (P = 0.001) when the computer output was available (77). Therefore, the CAD system would be useful in assisting radiologists in the detection of intracranial aneurysms in MRA.
4.4 Detection of interval changes in successive whole-body bone scans
Bone scintigraphy is the most frequent examination among various diagnostic nuclear-medicine procedures. It is a well-established imaging modality for the diagnosis of osseous metastases and for monitoring of the osseous-tumor response to chemotherapy and radiation therapy. Although the sensitivity of bone scan examinations for detection of bone abnormalities has been considered to be very high, it is time-consuming to identify multiple lesions such as bone metastases of prostate and breast cancers (135,136). In addition, it is very difficult to detect subtle interval changes between two successive abnormal bone scans, because of variations in patient conditions, the accumulation of radioisotopes during each examination, and the image quality of gamma cameras. Therefore, Shiraishi et al. (137) developed a CAD scheme for the detection of interval changes in successive whole-body bone scans by use of a temporal-subtraction image which was obtained with a nonlinear image-warping technique (103). The computerized scheme consisted of several steps: initial image density normalization on each image, image matching for paired images, temporal subtraction by use of the nonlinear image-warping technique, initial detection of interval changes by use of temporal-subtraction images, image feature extraction of candidates of interval changes, rule-based tests by use of 16 image features for removing some false positives, and display of the computer output for identified interval changes.
Based on the consensus of three radiologists, 107 “gold-standard” interval changes were determined among the 58 pairs of successive bone scans in which each scan included both posterior and anterior views. One hundred seven “gold standard” interval changes included 71 hot lesions (i.e., uptake was increased compared with that in the previous scan, or there was new uptake in the current scan) and 36 cold lesions (i.e., uptake was decreased or disappeared) for anterior and posterior views. The overall sensitivity in the detection of interval changes, including both hot and cold lesions, evaluated by use of the resubstitution and the leave-one-case-out methods were 95.3% with 5.97 false positives per view and 83.2% with 6.02, respectively. Figure 5 shows the temporal subtraction image obtained from previous and current bone scan images, and the correct detection of one cold lesion and two hot lesions by computer. Thus, the temporal-subtraction image for successive whole-body bone scans has the potential to enhance the interval changes between two images. Furthermore, the CAD scheme for the detection of interval changes would be useful in assisting radiologists’ interpretation on successive bone scan images.
Fig. 5.
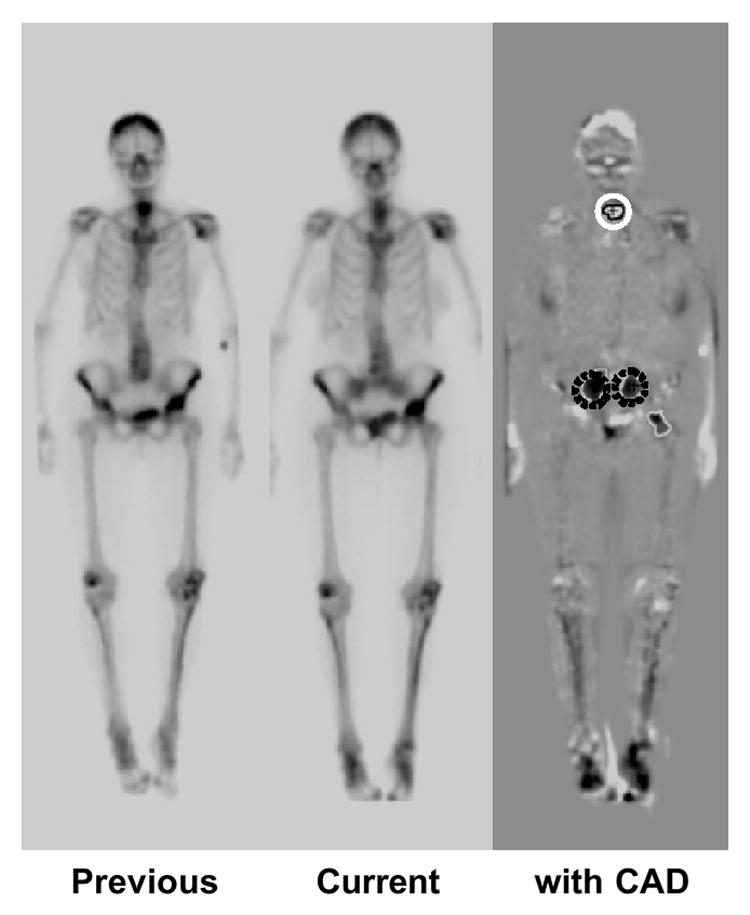
Illustration of temporal subtraction image obtained from previous and current bone scan images. One cold lesion (white solid circle) and two hot lesions (dark dotted circles) on the subtraction image were correctly marked by computer. Thus, the temporal subtraction image for successive whole-body bone scans has the potential to enhance the interval changes between two images.
5. Future potential of CAD in the PACS environment
It is likely that, in the future, some CAD schemes will be included together with other software for image processing in the workstations associated with some specific imaging modalities such as digital mammography, CT, and MRI. However, many other CAD schemes will be assembled as packages and implemented as a part of PACS. For example, the package for chest CAD may include the computerized detection of lung nodules, interstitial opacities, cardiomegaly, vertebral fractures, and interval changes in chest radiographs as well as the computerized classification of benign and malignant nodules and the differential diagnosis of interstitial lung diseases. All of the chest images taken for whatever purpose will be subjected to a computerized search for many different types of abnormalities included in the CAD package, and thus potential sites of lesions together with relevant information such as the likelihood of malignancy and the probability of a certain disease may be displayed on the workstation. For such a package to be used in clinical situations, it is important to reduce the number of false positives as much as possible so that radiologists will not be disturbed by an excessive number of false positives, but will be prompted only or mainly by clinically significant abnormalities.
Radiologists may use this type of CAD package in the workstation in two different ways. One is first to read images without the computer output, and then to request a display of the computer output before making the final decision. If radiologists keep their initial findings in some manner, this mode may prevent a detrimental effect of the computer output on radiologists’ initial diagnosis such as to dismiss incorrectly a subtle lesion because of no computer output, although radiologists were very suspicious of this lesion initially. However, this mode would probably increase the time required for radiologists’ image reading, which is generally undesirable. Another mode is to display the computer output first and then the final decision by the radiologist. With this mode, it is very likely that radiologists can reduce the reading time for image interpretations, but it is uncertain whether they may miss some lesions when no computer output was shown due to computer false negatives. This negative effect can be reduced if the sensitivity in the detection of abnormalities is at a very high level, which may be possible with a package of a number of different, but complementary CAD schemes. For example, although two CAD schemes may miss some lung nodules and other interstitial opacities, it is possible that the temporal subtraction images obtained from the current and previous chest images demonstrate the interval changes clearly, because the temporal-subtraction technique is very sensitive to subtle changes between the two images. This would be one of the potential advantages in packaging of a number of CAD schemes in the PACS environment.
Over the years, investigators have been developing two different types of CAD schemes, one for detection of lesions and another for differential diagnosis of detected lesions based on classification between malignant and benign lesions, and also between different diseases such as different interstitial lung diseases. Although CAD schemes for detection of lesions such as breast lesions on mammograms have been successfully implemented in clinical situations, no serious attempts have been made to apply CAD schemes for differential diagnosis to practical clinical situations, and no commercial systems for CAD for differential diagnosis are available at present. However, the performance levels of CAD schemes for differential diagnosis have been reported to be very high (138-143). For example, a CAD scheme (142,143) for distinction between benign and malignant lung nodules on thoracic CT has been developed based on the determination of the likelihood of malignancy (%) for detected nodules. Feng Li et al. (143) demonstrated the usefulness of this scheme in observer performance studies, in which radiologists’ performance with CAD (Az = 0.853) was greater than that of either radiologists alone (Az = 0.785) or computer output alone (Az = 0.831), with statistically significant differences in Az values. The radiologists generally increased or decreased their confidence level when the likelihood of malignancy was above or below 50%, respectively, and the changes for most nodules tended toward a beneficial effect of the CAD output. Importantly, the correct computer output was able to assist radiologists in improving their decisions on many subtle cases, as illustrated in Fig. 6. For some nodules, however, the radiologists’ initial ratings without CAD were clearly correct; and even when the computer output indicated incorrect results, no serious detrimental effect due to CAD occurred in the radiologists’ ratings, as shown in Fig. 7. Thus, radiologists were able to maintain their own correct judgment when nodules appeared to be obviously benign or malignant despite an incorrect CAD output. Therefore, this study indicated that a synergistic improvement in observers’ interpretation by use of a CAD scheme as a “second opinion” was possible, because the radiologists were able to maintain their own correct opinions on some obvious cases, whereas the computer output assisted in improving their decisions in the majority of subtle cases.
Fig. 6.
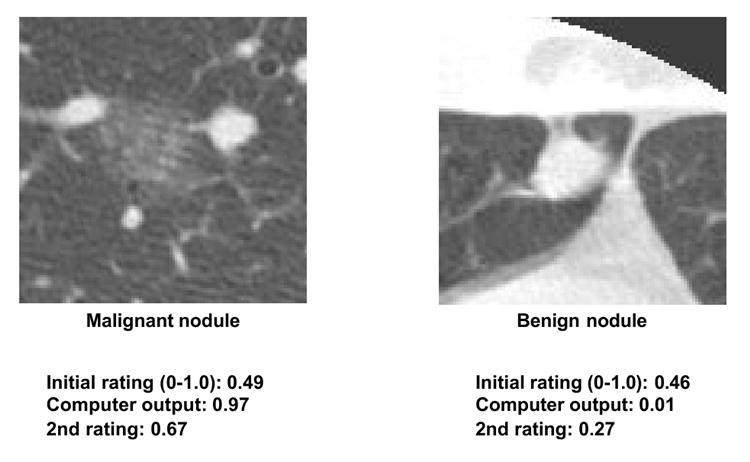
Illustration of subtle, difficult nodules in HRCT. The correct computer output for the likelihood of malignancy was able to assist radiologists in improving their decisions, as indicated by a beneficial change in radiologists’ confidence level toward a correct diagnosis for both malignant and benign nodules. The average confidence ratings by 16 radiologists are shown as initial rating and 2nd rating without and with computer output, respectively, where 0 and 1.0 indicate benign and malignant, respectively.
Fig. 7.
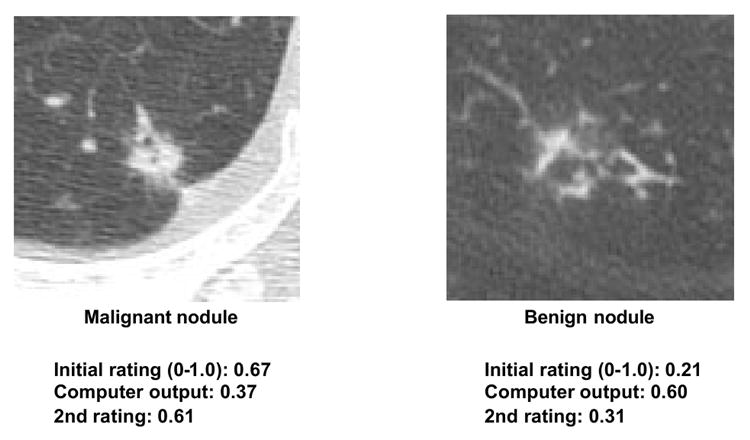
Illustration of “obvious” nodules in HRCT, in which radiologists were able to maintain their correct initial ratings for both malignant and benign nodules, even when the computer output indicated incorrect results. Thus, no serious detrimental effect due to CAD occurred in the radiologists’ ratings.
In order to assist radiologists further in their differential diagnosis in addition to the likelihood of malignancy (%), it would be useful to provide a set of benign and malignant images that are similar to an unknown new case in question. If the new case were considered very similar to one or more benign (or malignant) images by a radiologist, he/she would be more confident in deciding that the new case was benign (or malignant). Therefore, similar images may be employed to supplement the computed likelihood of malignancy for implementing CAD for a differential diagnosis. The usefulness of similar images has been demonstrated in an observer performance study (144) in which the Az value in the distinction between benign and malignant nodules in thoracic CT was improved. Figure 8 illustrates the comparison of an unknown case of a mass in a mammogram in the center with two benign masses on the left and two malignant masses on the right. In this simple example, most observers were able to identify the unknown case correctly as being more similar to malignant masses than to benign ones.
Fig. 8.
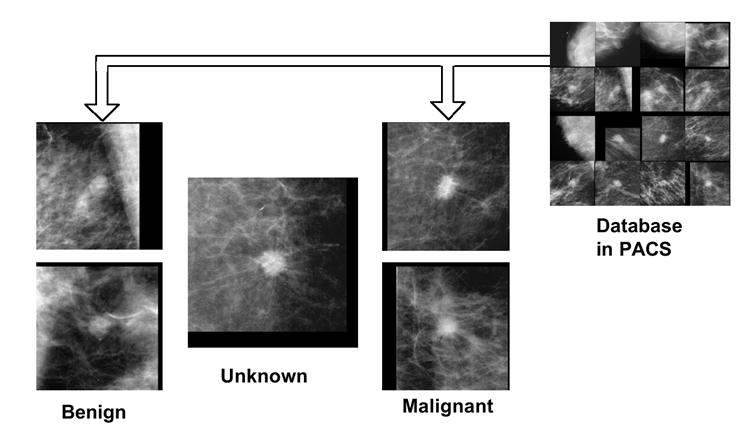
Comparison of an unknown case of a mass in a mammogram in the center with two benign masses on the left and two malignant masses on the right, which may be retrieved from PACS. Most observers were able to identify the unknown case correctly as being more similar to malignant masses than to benign ones.
There are at least two important issues related to the use of similar images in practical clinical situations. One is the need for a unique database that includes a large number of images which can be used as being similar to those of many unknown new cases, and another is a sensitive tool for finding images similar to an unknown case. Although it may require considerable time and effort, a useful database for this purpose can be developed in the future by use of images stored in PACS. At present, the majority of clinical images in PACS have not been used for clinical purposes, except for images of the same patients, such as in comparisons of a current image with previous images. Therefore, it would not be an overstatement to say that the vast majority of images in PACS are currently “sleeping” and need to be awakened in the future for daily use in many clinical situations. It would be possible to search for and retrieve very similar cases with similar images from PACS, if a reliable and useful method were developed for quantifying the similarity on a pair of images (or lesions) for visual comparison by radiologists. Recent studies indicated that the similarity of a pair of lung nodules in CT (144) and of lesions in mammograms (145,146) may be quantified by a psychophysical measure which can be obtained by use of an artificial neural network trained with the corresponding image features and with subjective ratings given by a group of radiologists. However, further investigations are required for examining the usefulness of this type of new tool for searching really similar images in PACS.
6. Conclusion
Computer-aided diagnosis has become a part of clinical work in the detection of breast cancer by use of mammograms, but is still in the infancy of its full potential for applications to many different types of lesions obtained with various modalities. CAD is a concept based on the equal roles of physician and computer, and thus is distinctly different from automated computer diagnosis. In the future, it is likely that CAD schemes will be incorporated into PACS, and that they will be assembled as a package for detection of lesions and also for differential diagnosis. CAD will be employed as a useful tool for diagnostic examinations in daily clinical work.
Acknowledgments
The author is grateful to more than 200 individuals including the faculty, research associates, fellows, residents, research staff, clinical staff, international visitors, and graduate students in the Department of Radiology and the Graduate Programs in Medical Physics, The University of Chicago, who have contributed to research on and development of CAD schemes over the last two decades; to Charles E. Metz, PhD, and Shigehiko Katsuragawa, PhD, for careful reading of this manuscript and providing useful suggestions; and to Mrs. E. Lanzl for improving the manuscript. This study was supported by USPHS Grants CA62625 and CA 98119. K.D. is a shareholder of R2 Technology, Inc., Sunnyvale, CA. CAD technologies developed in the Kurt Rossmann Laboratories for Radiologic Image Research, The University of Chicago, have been licensed to companies including R2 Technology, Deus Technology, Riverain Medical Group, Mitsubishi Space Software Co., Median Technologies, General Electric Corporation, and Toshiba Corporation. It is the policy of the University of Chicago that investigators disclose publicly actual or potential significant financial interests that may appear to be affected by research activities or that may benefit from research activities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freer TW, Ulissey MJ. Screening mammography with computer-aided detection: prospective study of 12,860 patients in a community breast center. Radiology. 2001;220:781–786. doi: 10.1148/radiol.2203001282. [DOI] [PubMed] [Google Scholar]
- 2.Gur D, Sumkin JH, Rockette HE, Ganott M, Hakim C, Hardesty L, Poller WR, Shah R, Wallace L. Changes in breast cancer detection and mammography recall rate after the introduction of a computer-aided detection system. J Natl Cancer Inst. 2004;96:185–190. doi: 10.1093/jnci/djh067. [DOI] [PubMed] [Google Scholar]
- 3.Birdwell RL, Bandodkar P, Ikeda DM. Computer-aided detection with screening mammography in a university hospital setting. Radiology. 2005;236:451–457. doi: 10.1148/radiol.2362040864. [DOI] [PubMed] [Google Scholar]
- 4.Cupples TE, Cunningham JE, Reynolds JC. Impact of computer-aided detection in a regional screening mammography program. AJR. 2005;185:944–950. doi: 10.2214/AJR.04.1300. [DOI] [PubMed] [Google Scholar]
- 5.Morton MJ, Whaley DH, Brandt KR, Amrami KK. Screening mammograms: interpretation with computer-aided detection-prospective evaluation. Radiology. 2006;239:375–383. doi: 10.1148/radiol.2392042121. [DOI] [PubMed] [Google Scholar]
- 6.Dean JC, Ilvento CC. Improved cancer detection using computer-aided detection with diagnostic and screening mammography: prospective study of 104 cancers. AJR. 2006;187:20–28. doi: 10.2214/AJR.05.0111. [DOI] [PubMed] [Google Scholar]
- 7.Destounis SV, DiNitto P, Logan-Young W, Bonaccio E, Zuley ML, Willison KM. Can computer-aided detection with double reading of screening mammograms help decrease the false-negative rate? Initial experience. Radiology. 2004;232:578–584. doi: 10.1148/radiol.2322030034. [DOI] [PubMed] [Google Scholar]
- 8.Butler SA, Gabbay RJ, Kass DA, Siedler DE, O’Shaughnessy KF, Castellino RA. Computer-aided detection in diagnostic mammography: detection of clinically unsuspected cancers. AJR. 2004;183:1511–1515. doi: 10.2214/ajr.183.5.1831511. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa RM, Haldemann RC, Papaioannou J, Giger ML, Lu P, Schmidt RA, Wolverton DE, Bick U, Doi K. Initial experience with a prototype clinical “intelligent” mammography workstation for computer-aided diagnosis. Proc SPIE. 1995;2434:65–71. [Google Scholar]
- 10.Schmidt RA, Nishikawa RM, Osnis RB, Schreibman K, Giger ML, Doi K. Computerized detection of lesions missed by mammography. In: K Doi, Giger ML, Nishikawa RM, Schmidt RA., editors. Digital Mammography. Elsevier Science; Amsterdam: 1996. pp. 105–110. [Google Scholar]
- 11.Warren-Burhenne LJ, Wood SA, D’Orsi CJ, et al. Potential contribution of computer-aided detection to the sensitivity of screening mammography. Radiology. 2000;215:554–562. doi: 10.1148/radiology.215.2.r00ma15554. [DOI] [PubMed] [Google Scholar]
- 12.Giger ML, Huo Z, Kupinski MA, Vyborny CJ. Computer-aided diagnosis in mammography. In: Fitzpatrick JM, Sonka M, editors. The Handbook of Medical Imaging, volume 2 Medical Imaging Processing and Analysis. SPIE; 2000. pp. 915–1004. [Google Scholar]
- 13.Giger ML. Computerized analysis of images in the detection and diagnosis of breast cancer. Seminars in Ultrasound CT and MRI. 2004;25:411–418. doi: 10.1053/j.sult.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Erickson BJ, Bartholmai B. Computer-aided detection and diagnosis at the start of the third millennium. J Dig Imaging. 2002;15:59–68. doi: 10.1007/s10278-002-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Summers RM. Road maps for advancement of radiologic computer-aided detection in the 21st century. Radiology. 2003;229:11–13. doi: 10.1148/radiol.2291030010. [DOI] [PubMed] [Google Scholar]
- 16.Abe H, MacMahon H, Shiraishi J, Li Q, Engelmann R, Doi K. Computer-aided diagnosis in chest radiography. Seminars in Ultrasound, CT and MRI. 2004;25:432–437. doi: 10.1053/j.sult.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Li Q, Li F, Armato SG, III, Suzuki K, Shiraishi J, Abe H, Engelmann R, Nie Y, MacMahon H, Doi K. Computer-aided diagnosis in thoracic CT. Seminars in Ultrasound CT and MRI. 2005;26:357–363. doi: 10.1053/j.sult.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida H, Dachman AH. Computer-aided diagnosis for CT colonography. Seminars in Ultrasound, CT and MRI. 2004;25:404–410. doi: 10.1053/j.sult.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Doi K, Giger ML, MacMahon H, et al. Computer-aided diagnosis: Development of automated schemes for quantitative analysis of radiographic images. Seminars in Ultrasound, CT and MRI. 1992;13:140–152. [PubMed] [Google Scholar]
- 20.Doi K, MacMahon H, Giger ML, Hoffmann KR. Computer Aided Diagnosis in Medical Imaging. Elsevier; Amsterdam: 1999. pp. 3–560. [Google Scholar]
- 21.Doi K, MacMahon H, Katsuragawa S, Nishikawa RM, Jiang Y. Computer-aided diagnosis in radiology: potential and pitfalls. European J Radiology. 1999;31:97 –109. doi: 10.1016/s0720-048x(99)00016-9. [DOI] [PubMed] [Google Scholar]
- 22.Doi K. Advances in Digital Radiography: RSNA Categorical Course in Diagnostic Radiology Physics 2003 Syllabus. 2003. Computer-aided diagnosis in digital chest radiography; pp. 227–236. [Google Scholar]
- 23.Doi K. Overview on research and development of computer-aided diagnostic schemes. Seminars in Ultrasound CT and MRI. 2004;25:404–410. doi: 10.1053/j.sult.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Doi K. Current status and future potential of computer-aided diagnosis in medical imaging. British J Rad. 2005;78(Special Issue):S3–S19. doi: 10.1259/bjr/82933343. [DOI] [PubMed] [Google Scholar]
- 25.Doi K. Diagnostic imaging over the last 50 years: Research and development in medical imaging science and technology. Phys Med Biol. 2006;51:R5–R27. doi: 10.1088/0031-9155/51/13/R02. [DOI] [PubMed] [Google Scholar]
- 26.Lodwick GS, Haun CL, Smith WE, et al. Computer diagnosis of primary bone tumor. Radiology. 1963;80:273–275. [Google Scholar]
- 27.Myers PH, Nice CM, Becker HC, et al. Automated computer analysis of radiographic images. Radiology. 1964;83:1029–1033. doi: 10.1148/83.6.1029. [DOI] [PubMed] [Google Scholar]
- 28.Winsbarg F, Elkin M, May J, et al. Detection of radiographic abnormalities in mammograms by means of optical scanning and computer analysis. Radiology. 1967;89:211–215. [Google Scholar]
- 29.Kruger RP, Towns JR, Hall DL, et al. Automated radiographic diagnosis via feature extraction and classification of cardiac size and shape descriptors. IEEE Transactions on Biomedical Engineering. 1972 May;BME-19(No 3):174–186. doi: 10.1109/TBME.1972.324115. [DOI] [PubMed] [Google Scholar]
- 30.Kruger RP, Thompson WB, Turner AF. Computer diagnosis of pneumoconiosis. IEEE Transactions on Systems, Man, and Cybernetics. 1974 January;SMC-4(No 1):44–47. [Google Scholar]
- 31.Toriwaki J, Suenaga Y, Negoro T, et al. Pattern recognition of chest x-ray images. Computer Graphics and Image Processing. 1973;2:252–271. [Google Scholar]
- 32.Engle RL. Perspectives in Biology and Medicine. Vol. 35. published by The University of Chicago; Winter. 1992. Attempt to use computers as diagnostic aids in medical decision making: A thirty–year experience; pp. 207–218. [DOI] [PubMed] [Google Scholar]
- 33.Ishida M, Kato H, Doi K, Frank PH. Development of a new digital radiographic image processing system. Proc SPIE. 1982;347:42–48. [Google Scholar]
- 34.Ishida M, Frank PH, Doi K, Lehr JL. High-quality digital radiographic images: Improved detection of low-contrast objects and preliminary clinical studies. RadioGraphics. 1983;3:325–338. [Google Scholar]
- 35.Ishida M, Doi K, Loo LN, Metz CE, Lehr JL. Digital image processing: Effect on the detectability of simulated low-contrast radiographic patterns. Radiology. 1984;150:569–575. doi: 10.1148/radiology.150.2.6691118. [DOI] [PubMed] [Google Scholar]
- 36.Giger ML, Doi K. Investigation of basic imaging properties in digital radiography. Part 1: modulation transfer function. Med Phys. 1984;11:287–295. doi: 10.1118/1.595629. [DOI] [PubMed] [Google Scholar]
- 37.Giger ML, Doi K. Investigation of basic imaging properties in digital radiography. Part 2: noise Wiener spectrum. Med Phys. 1984;11:797–805. doi: 10.1118/1.595583. [DOI] [PubMed] [Google Scholar]
- 38.MacMahon H, Vyborny CJ, Powell G, Doi K, Metz CE. The effect of pixel size on the detection rate of early pulmonary sarcoidosis in digital chest radiographic systems. Proc SPIE. 1984;486:14–20. [Google Scholar]
- 39.Giger ML, Doi K. Investigation of basic imaging properties in digital radiography. Effect of pixel size on SNR and threshold contrast. Med Phys. 1985;12:201–208. doi: 10.1118/1.595708. [DOI] [PubMed] [Google Scholar]
- 40.Loo LN, Doi K, Metz CE. Investigation of basic imaging properties in digital radiography. 4. Effect of unsharp masking on the detectability of simple patterns. Med Phys. 1985;12:209–214. doi: 10.1118/1.595775. [DOI] [PubMed] [Google Scholar]
- 41.Fujita H, Doi K, Giger ML. Investigation of basic imaging properties in digital radiography. 6. MTFs of I.I.-TV digital imaging systems. Med Phys. 1985;12:712–729. doi: 10.1118/1.595653. [DOI] [PubMed] [Google Scholar]
- 42.MacMahon H, Vyborny CJ, Sabeti V, Metz CE, Doi K. The effect of digital unsharp masking on the detectability of interstitial infiltrates and pneumothoraces. Proc SPIE. 1985;555:246–252. [Google Scholar]
- 43.Fujita H, Doi K, Giger ML, Chan HP. Investigation of basic imaging properties in digital radiography. 5. Characteristic curves of I.I.-TV digital systems. Med Phys. 1986;13:13–18. doi: 10.1118/1.595932. [DOI] [PubMed] [Google Scholar]
- 44.Giger ML, Doi K, Fujita H. Investigation of basic imaging properties in digital radiography. 7. Noise Wiener spectra of I.I.-TV digital imaging systems. Med Phys. 1986;13:131–138. doi: 10.1118/1.595937. [DOI] [PubMed] [Google Scholar]
- 45.MacMahon H, Vyborny CJ, Metz CE, Doi K, Sabeti V, Solomon SL. Digital radiography of subtle pulmonary abnormalities: An ROC study of the effect of pixel size on observer performance. Radiology. 1986;158:21–26. doi: 10.1148/radiology.158.1.3940383. [DOI] [PubMed] [Google Scholar]
- 46.Ohara K, Chan HP, Doi K, Fujita H, Giger ML. Investigation of basic imaging properties in digital radiography. 8. Detection of simulated low-contrast objects in DSA images. Med Phys. 1986;13:304–311. doi: 10.1118/1.595918. [DOI] [PubMed] [Google Scholar]
- 47.Giger ML, Ohara K, Doi K. Investigation of basic imaging properties in digital radiography. 9. Effect of displayed grey levels on signal detection. Med Phys. 1986;13:312–318. doi: 10.1118/1.595919. [DOI] [PubMed] [Google Scholar]
- 48.Kume Y, Doi K, Ohara K, Giger ML. Investigation of basic imaging properties in digital radiography. 10. Structure mottle of I.I.-TV digital imaging systems. Med Phys. 1986;13:843–849. doi: 10.1118/1.595808. [DOI] [PubMed] [Google Scholar]
- 49.Huang HK. PACS and Imaging Informatics: Basic principles and applications. Published by John Wiley & Sons, Inc; 2004. [Google Scholar]
- 50.Fujita H, Doi K, Fencil LE, Chua KG. Image feature analysis and computer-aided diagnosis in digital radiography. 2. Computerized determination of vessel sizes in digital subtraction angiography. Med Phys. 1987;14:549–556. doi: 10.1118/1.596066. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann KR, Doi K, Chan HP, Fencil L, Fujita H, Muraki A. Automated tracking of the vascular tree in DSA images using a double-square-box region-of-search algorithm. Proc SPIE. 1986;626:326–333. [Google Scholar]
- 52.Giger ML, Doi K, MacMahon H. Computerized detection of lung nodules in digital chest radiographs. Proc SPIE. 1987;767:384–386. [Google Scholar]
- 53.Giger ML, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography. 3. Automated detection of nodules in peripheral lung fields. Med Phys. 1988;15:158–166. doi: 10.1118/1.596247. [DOI] [PubMed] [Google Scholar]
- 54.Chan HP, Doi K, Galhotra S, Vyborny CJ, MacMahon H, Jokich PM. Image feature analysis and computer-aided diagnosis in digital radiography. 1. Automated detection of microcalcifications in mammography. Med Phys. 1987;14:538–548. doi: 10.1118/1.596065. [DOI] [PubMed] [Google Scholar]
- 55.Doi K, Chan H-P, Giger ML. Method and system for enhancement and detection of abnormal anatomic regions in a digital image. United States Patent No 4,907,156. 1990 [Google Scholar]
- 56.Nishikawa RM, Doi K, Giger ML, Schmidt RA, Vyborny CJ, Monnier-Cholley L, Papaioannou J, Lu P. Computerized detection of clustered microcalcifications: Evaluation of performance using mammograms from multiple centers. RadioGraphics. 1995;15:443–452. doi: 10.1148/radiographics.15.2.7761647. [DOI] [PubMed] [Google Scholar]
- 57.Abe H, MacMahon H, Engelmann R, Li Q, Shiraishi J, Katsuragawa S, Aoyama M, Ishida T, Ashizawa K, Metz C, Doi K. Computer-aided diagnosis in chest radiology: Results of large-scale observer tests performed at the 1996-2001 RSNA Scientific Assemblies. RadioGraphics. 2003;23:255–265. doi: 10.1148/rg.231025129. [DOI] [PubMed] [Google Scholar]
- 58.MacMahon H, Engelmann R, Behlen F, Hoffmann KR, Ishida T, Roe C, Metz C, Doi K. Computer-aided diagnosis of pulmonary nodules: Results of a large-scale observer test. Radiology. 1999;213:723–726. doi: 10.1148/radiology.213.3.r99dc27723. [DOI] [PubMed] [Google Scholar]
- 59.Kundel HL, Nodine CF. Interpreting chest radiographs without visual search. Radiology. 1975;116:527–532. doi: 10.1148/116.3.527. [DOI] [PubMed] [Google Scholar]
- 60.Kundel HL, Revesz G. Lesion conspicuity, structured noise, and film reader error. AJR. 1976;126:1233–1238. doi: 10.2214/ajr.126.6.1233. [DOI] [PubMed] [Google Scholar]
- 61.Kundel HL, Nodine CF, Carmody D. Visual scanning, pattern recognition and decision-making in pulmonary nodule detection. Invest Radiol. 1978;13:175–181. doi: 10.1097/00004424-197805000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Kundel HL, Revesz G, Toto L. Contrast gradient and the detection of lung nodules. Invest Radiol. 1979;14:18–22. doi: 10.1097/00004424-197901000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Carmody DP, Nodine CF, Kundel HL. An analysis of perceptual and cognitive factors in radiographic interpretation. Perception. 1980;9:339–344. doi: 10.1068/p090339. [DOI] [PubMed] [Google Scholar]
- 64.Xu XW, Doi K, Kobayashi T, MacMahon H, Giger ML. Development of an improved CAD scheme for automated detection of lung nodules in digital chest images. Med Phys. 1997;24:1395–1403. doi: 10.1118/1.598028. [DOI] [PubMed] [Google Scholar]
- 65.Shiraishi J, Li Q, Suzuki K, Engelmann R, Doi K. Computer-aided diagnostic scheme for the detection of lung nodules on chest radiographs: localized search method based on anatomical classification. Med Phys. 2006;33:2642–2653. doi: 10.1118/1.2208739. [DOI] [PubMed] [Google Scholar]
- 66.Chan HP, Doi K, Vyborny CJ, Schmidt RA, Metz CE, Lam KL, Ogura T, Wu Y, MacMahon H. Improvement in radiologists’ detection of clustered microcalcifications on mammograms: The potential of computer-aided diagnosis. Invest Radiol. 1990;25:1102–1110. doi: 10.1097/00004424-199010000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Yin FF, Giger ML, Vyborny CJ, Doi K, Schmidt RA. Comparison of bilateral-subtraction and single-image processing techniques in the computerized detection of mammographic masses. Invest Radiol. 1993;28:473–481. doi: 10.1097/00004424-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 68.Arimura H, Katsuragawa S, Suzuki K, Li F, Shiraishi J, Doi K. Computerized scheme for automated detection of lung nodules in low-dose CT images for lung cancer screening. Acad Radiol. 2004;11:617–629. doi: 10.1016/j.acra.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Metz CE. ROC methodology in radiologic imaging. Invest Radiol. 1986;21:720–733. doi: 10.1097/00004424-198609000-00009. [DOI] [PubMed] [Google Scholar]
- 70.Metz CE. Some practical issues of experimental design and data analysis in radiological ROC studies. Invest Radiol. 1989;24:234–245. doi: 10.1097/00004424-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Metz CE. Fundamental ROC analysis. In: Beutel J, Kundel HL, Van Metter RL, editors. Handbook of Medical Imaging. Vol. 1. Bellingham, Washington: SPIE Press, The International Society for Optical Engineering ; 2000. pp. 751–770. [Google Scholar]
- 72.Moberg K, Bjurstam N, Wilczek B, Rostgard L, Egge E, Muren C. Computer assisted detection of interval breast cancers. Eur J Radiol. 2001;39:104–110. doi: 10.1016/s0720-048x(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 73.Kobayashi T, Xu X-W, MacMahon H, Metz CE, Doi K. Effect of a computer-aided diagnosis scheme on radiologists’ performance in detection of lung nodules on radiographs. Radiology. 1996;199:843–848. doi: 10.1148/radiology.199.3.8638015. [DOI] [PubMed] [Google Scholar]
- 74.Kakeda S, Moriya J, Sato H, Aoki T, Watanabe H, Nakata H, Oda N, Katsuragawa S, Yamamoto K, Doi K. Improved detection of lung nodules with aid of computerized detection method: Evaluation of a commercial computer-aided diagnosis system. AJR. 2004;182:505–510. doi: 10.2214/ajr.182.2.1820505. [DOI] [PubMed] [Google Scholar]
- 75.Monnier-Cholley L, MacMahon H, Katsuragawa S, Morishita J, Ishida T, Doi K. Computer aided diagnosis for detection of interstitial infiltrates in chest radiographs: Evaluation by means of ROC analysis. AJR. 1998;171:1651–1656. doi: 10.2214/ajr.171.6.9843307. [DOI] [PubMed] [Google Scholar]
- 76.Li F, Arimura H, Suzuki K, Shiraishi J, Li Q, Abe H, Engelmann R, Sone S, MacMahon H, Doi K. Computer-aided diagnosis for detection of missed peripheral lung cancers on CT: ROC and LROC analysis. Radiology. 2005;237:684–690. doi: 10.1148/radiol.2372041555. [DOI] [PubMed] [Google Scholar]
- 77.Hirai T, Korogi Y, Arimura H, Katsuragawa S, Kitajima M, Yamura M, Yamashita Y, Doi K. Intracranial aneurysms at MR angiography: Effect of computer-aided diagnosis on radiologists’ detection performance. Radiology. 2005;237:605–610. doi: 10.1148/radiol.2372041734. [DOI] [PubMed] [Google Scholar]
- 78.Warner EE, Mulshine JL. Lung cancer screening with spiral CT: toward a working strategy. Oncology (Williston Park) 2004;18:564–575. discussion 578, 583-564, 587. [PubMed] [Google Scholar]
- 79.Kaneko M, Eguchi K, Ohmatsu H, Kakinuma R, Naruke T, Suemasu K, Moriyama N. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology. 1996;201:798–802. doi: 10.1148/radiology.201.3.8939234. [DOI] [PubMed] [Google Scholar]
- 80.Sone S, Takashima S, Li F, Yang Z, Honda T, Maruyama Y, Hasegawa M, Yamanda T, Kubo K, Hanamura K, Asakura K. Mass screening for lung cancer with mobile spiral computed tomography scanner. Lancet. 1998;351:1242–1245. doi: 10.1016/S0140-6736(97)08229-9. [DOI] [PubMed] [Google Scholar]
- 81.Henschke CI, MacCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, Smith JP. Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 82.Henschke CI, Yankelevitz DF, Mirtcheva R, McGuinness G, McCauley D, Miettinen OS. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR. 2002;178:1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 83.Li F, Sone S, Abe H, MacMahon H, Armato S, Doi K. Missed lung cancers in low-dose helical CT screening obtained from a general population. Radiology. 2002;225:673–683. doi: 10.1148/radiol.2253011375. [DOI] [PubMed] [Google Scholar]
- 84.Armato SG, III, Li F, Giger ML, MacMahon H, Sone S, Doi K. Performance of automated lung nodule detection applied to cancers missed in a lung screening program. Radiology. 2002;225:685–692. doi: 10.1148/radiol.2253011376. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida H, Masutani Y, MacEneaney P, Rubin D, Dachman AH. Computerized detection of colonic polyps at CT colonography on the basis of volumetric features: pilot study. Radiology. 2002;222:327–336. doi: 10.1148/radiol.2222010506. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida H, Nappi J, MacEneaney P, Rubin D, Dachman AH. Computer-aided diagnosis scheme for the detection of polyps at CT colonography. RadioGraphics. 2002;22:963–979. doi: 10.1148/radiographics.22.4.g02jl16963. [DOI] [PubMed] [Google Scholar]
- 87.Oken MM, Marcus PM, Hu P, et al. Baseline chest radiograph for lung cancer detection in the randomized Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst. 2005;97:1832–1839. doi: 10.1093/jnci/dji430. [DOI] [PubMed] [Google Scholar]
- 88.Byers T, Barrera E, Fontham ET, et al. A midpoint assessment of the American Cancer Society challenge goal to halve the U.S. cancer mortality rates between the years 1990 and 2015. Cancer. 2006;107:396–405. doi: 10.1002/cncr.21990. [DOI] [PubMed] [Google Scholar]
- 89.Nakamura K, Yoshida H, Engelmann R, MacMahon H, Katsuragawa S, Ishida T, Ashizawa K, Doi K. Computerized analysis of the likelihood of malignancy in solitary pulmonary nodules with use of artificial neural networks. Radiology. 2000;214:823–830. doi: 10.1148/radiology.214.3.r00mr22823. [DOI] [PubMed] [Google Scholar]
- 90.Aoyama M, Li Q, Katsuragawa S, MacMahon H, Doi K. Automated computerized scheme for distinction between benign and malignant solitary pulmonary nodules on chest images. Med Phys. 2002;29:701–708. doi: 10.1118/1.1469630. [DOI] [PubMed] [Google Scholar]
- 91.Shiraishi J, Abe H, Engelmann R, Aoyama M, MacMahon H, Doi K. Computer-aided diagnosis to distinguish benign from malignant solitary pulmonary nodules on radiographs: ROC analysis of radiologists’ performance-initial experience. Radiology. 2003;227:469–474. doi: 10.1148/radiol.2272020498. [DOI] [PubMed] [Google Scholar]
- 92.Katsuragawa S, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography: detection and characterization of interstitial lung disease in digital chest radiographs. Med Phys. 1988;15:311–319. doi: 10.1118/1.596224. [DOI] [PubMed] [Google Scholar]
- 93.Katsuragawa S, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography: Classification of normal and abnormal lungs with interstitial disease in chest images. Med Phys. 1989;16:38–44. doi: 10.1118/1.596412. [DOI] [PubMed] [Google Scholar]
- 94.Ishida T, Katsuragawa S, Nakamura K, Ashizawa K, MacMahon H, Doi K. Computerized analysis of interstitial lung diseases on chest radiographs based on lung texture, geometric-pattern features and artificial neural networks. Proc SPIE. 2002;4684:1331–1338. [Google Scholar]
- 95.Ashizawa K, Ishida T, MacMahon H, Vyborny CJ, Katsuragawa S, Doi K. Artificial neural networks in chest radiographs: Application to differential diagnosis of interstitial lung disease. Acad Radiol. 1999;6:2–9. doi: 10.1016/s1076-6332(99)80055-5. [DOI] [PubMed] [Google Scholar]
- 96.Ashizawa K, MacMahon H, Ishida T, Nakamura K, Vyborny CJ, Katsuragawa S, Doi K. Effect of artificial neural network on radiologists’ performance for differential diagnosis of interstitial lung disease on chest radiographs. AJR. 1999;172:1311–1314. doi: 10.2214/ajr.172.5.10227508. [DOI] [PubMed] [Google Scholar]
- 97.Nakamori N, Doi K, Sabeti V, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography: Automated analysis of sizes of heart and lung in digital chest images. Med Phys. 1990;17:342–350. doi: 10.1118/1.596513. [DOI] [PubMed] [Google Scholar]
- 98.Nakamori N, Doi K, MacMahon H, Sasaki Y, Montner S. Effect of heart size parameters computed from digital chest radiographs on detection of cardiomegaly: Potential usefulness for computer-aided diagnosis. Invest Radiol. 1991;26:546–550. doi: 10.1097/00004424-199106000-00008. [DOI] [PubMed] [Google Scholar]
- 99.Sanada S, Doi K, MacMahon H. Image feature analysis and computer-aided diagnosis in digital radiography: Automated detection of pneumothorax in chest images. Med Phys. 1992;19:1153–1160. doi: 10.1118/1.596790. [DOI] [PubMed] [Google Scholar]
- 100.Kano A, Doi K, MacMahon H, Hassell DD, Giger ML. Digital image subtraction of temporally sequential chest images for detection of interval change. Med Phys. 1994;21:453–461. doi: 10.1118/1.597308. [DOI] [PubMed] [Google Scholar]
- 101.Difazio MC, MacMahon H, Xu XW, Tsai P, Shiraishi J, Armato SG, Doi K. Effect of time interval difference images on detection accuracy in digital chest radiography. Radiology. 1997;202:447–452. doi: 10.1148/radiology.202.2.9015072. [DOI] [PubMed] [Google Scholar]
- 102.Ishida T, Katsuragawa S, Nakamura K, MacMahon H, Doi K. Iterative image warping technique for temporal subtraction of sequential chest radiographs to detect interval change. Med Phys. 1999;26:1320–1329. doi: 10.1118/1.598627. [DOI] [PubMed] [Google Scholar]
- 103.Li Q, Katsuragawa S, Doi K. Improved contralateral subtraction images by use of elastic matching technique. Med Phys. 2000;27:1934–1942. doi: 10.1118/1.1287112. [DOI] [PubMed] [Google Scholar]
- 104.Kakeda S, Nakamura K, Kamada K, Watanabe H, Nakata H, Katsuragawa S, Doi K. Observer performance study on the usefulness of temporal subtraction in detection of lung nodules on digital chest images. Radiology. 2002;224:145–151. doi: 10.1148/radiol.2241010719. [DOI] [PubMed] [Google Scholar]
- 105.Kakeda S, Kamada K, Hatakeyama Y, Aoki T, Ohguri T, Korogi Y, Katsuragawa S, Doi K. Effect of temporal subtraction of technique on reading time and diagnostic accuracy in the interpretation of chest radiographs. AJR. 2006 doi: 10.2214/AJR.05.1270. in press. [DOI] [PubMed] [Google Scholar]
- 106.Austin JH, Romney BM, Goldsmith LS. Missed bronchogenic carcinoma: radiographic findings in 27 patients with a potentially resectable lesion evident in retrospect. Radiology. 1992;182:115–122. doi: 10.1148/radiology.182.1.1727272. [DOI] [PubMed] [Google Scholar]
- 107.Forrest JV, Sagel SS. The lateral radiograph for early diagnosis of lung cancer. Radiology. 1979;131:309–310. doi: 10.1148/131.2.309. [DOI] [PubMed] [Google Scholar]
- 108.Muhm JR, Miller WE, Fontana RS, Sanderson DR, Uhlenhopp MA. Lung cancer detected during a screening program using four-month chest radiographs. Radiology. 1983;148:609–615. doi: 10.1148/radiology.148.3.6308709. [DOI] [PubMed] [Google Scholar]
- 109.Quekel LG, Kessels AG, Goei R, van Engelshoven JM. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest. 1999;115:720–724. doi: 10.1378/chest.115.3.720. [DOI] [PubMed] [Google Scholar]
- 110.Stitik FP, Tockman MS. Radiographic screening in the early detection of lung cancer. Radiol Clin North Am. 1978;16:347–366. [PubMed] [Google Scholar]
- 111.Tala E. Carcinoma of the lung. A retrospective study with special reference to pre-diagnosis period and roentgenographic signs. Acta Radiol Diagn (Stockh) 1967;268(Suppl):261+–16. [PubMed] [Google Scholar]
- 112.Shiraishi J, Li F, Doi K. Computer-aided diagnosis for improved detection of lung nodules by use of PA and lateral chest radiographs. Acad Radiol. 2006 doi: 10.1016/j.acra.2006.09.057. in pess. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW, Dequeker J, Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333:1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- 114.Black DR, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 115.Chesnut CH, III, Silverman S, Andriano K, Genant H, Gimona A, Harris S, Kiel D, LeBoff M, Maricic M, Miller P, Moniz C, Peacock M, Richardson P, Watts N, Baylink D. A randomized trial of nasal spray salmon calcitonin in post-menopausal women with established osteoporosis. Am J Med. 2000;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 116.Ettinger E, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 117.Ross DP. Clinical Consequences of Vertebral Fractures. Am J Med. 1997;103(2A):30S–42S. doi: 10.1016/s0002-9343(97)90025-5. [DOI] [PubMed] [Google Scholar]
- 118.Kim N, Rowe BH, Raymond G, Jen H, Colman I, Jackson SA, Siminoski KG, Chahal AM, Folk D, Majumdar SR. Underreporting of vertebral fractures on routine chest radiography. AJR. 2004;182:297–300. doi: 10.2214/ajr.182.2.1820297. [DOI] [PubMed] [Google Scholar]
- 119.Gerlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11:577–82. doi: 10.1007/s001980070078. [DOI] [PubMed] [Google Scholar]
- 120.Lenchik L, Rogers LF, Delmas PD, Genant HK. Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. AJR. 2004;183:949–58. doi: 10.2214/ajr.183.4.1830949. [DOI] [PubMed] [Google Scholar]
- 121.Genant HK, Wu CY, Van KC, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 122.Kasai S, Li F, Shiraishi J, Li Q, Nie Y, Doi K. Development of computerized method for detection of vertebral fractures on lateral chest radiographs. Proc SPIE. 2006;6144:61445D1–D11. [Google Scholar]
- 123.Kasai S, Li F, Shiraishi J, Li Q, Doi K. Computerized detection of vertebral compression fractures on lateral chest radiographs: Preliminary results of a tool for early detection of osteoporosis. Med Phys. 2006 doi: 10.1118/1.2364053. in press. [DOI] [PubMed] [Google Scholar]
- 124.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123:205–221. doi: 10.1093/brain/123.2.205. [DOI] [PubMed] [Google Scholar]
- 125.Ogawa T, Okudera T, Noguchi K, Sasaki N, Inugami A, Uemura K, Yasui N. Cerebral aneurysms : evaluation with three-dimensional CT angiography. Am J Neuroradiol. 1996;17:447–454. [PMC free article] [PubMed] [Google Scholar]
- 126.Korogi Y, Takahashi M, Katada K, Ogura Y, Hasuo K, Ochi M, Utsunomiya H, Abe T, Imakita S. Intracranial aneurysms: Detection with three-dimensional CT angiography with volume rendering – comparison with conventional angiographic and surgical findings. Radiology. 1999;211:497–506. doi: 10.1148/radiology.211.2.r99ma02497. [DOI] [PubMed] [Google Scholar]
- 127.White PM, Teasdale EM, Wardlaw JM, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection – prospective blinded comparison in a large patient cohort. Radiology. 2001;219:739–749. doi: 10.1148/radiology.219.3.r01ma16739. [DOI] [PubMed] [Google Scholar]
- 128.Preda L, Gaetani P, Rodriguez y Baena R, Di Maggio EM, La Fianza A, Dore R, Fulle I, Solcia M, Cecchini A, Infuso L, Campani R. Spiral CT angiography and surgical corrections in the evaluation of intracranial aneurysms. Eur Radiol. 1998;8:739–745. doi: 10.1007/s003300050465. [DOI] [PubMed] [Google Scholar]
- 129.Huston J, III, Nichols DA, Luetmer PH, Goodwin JT, Meyer FB, Wiebers DO, Weaver A. Blinded prospective evaluation of sensitivity of MR angiography to known intracranial aneurysms: importance of aneurysm size. AJNR Am J Neuroradiol. 1994;15:1607–1614. [PMC free article] [PubMed] [Google Scholar]
- 130.Aprile I. Evaluation of cerebral aneurysms with MR-angiography. Riv Neuroradiol. 1996;9:541–550. [Google Scholar]
- 131.Korogi Y, Takahashi M, Mabuchi N, Miki H, Fujiwara S, Horikawa Y, Nakagawa T, O’Uchi T, Watabe T, Shiga H, Furuse M. Intracranial aneurysms. Diagnostic accuracy of three-dimensional, Fourier transform, time-of-flight MR angiography. Radiology. 1994;193:181–186. doi: 10.1148/radiology.193.1.8090889. [DOI] [PubMed] [Google Scholar]
- 132.Arimura H, Li Q, Korogi Y, Hirai T, Abe H, Yamashita Y, Katsuragawa S, Ikeda R, Doi K. Automated computerized scheme for detection of unruptured intracranial aneurysms in three-dimensional MRA. Acad Radiol. 2004;11:1093–1104. doi: 10.1016/j.acra.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 133.Arimura H, Li Q, Korogi Y, Hirai T, Katsuragawa S, Yamashita Y, Tsuchiya K, Doi K. Computerized detection of intracranial aneurysms for 3D MR angiography: Feature extraction of small protrusions based on a shape-based difference image technique. Med Phys. 2006;33:394–401. doi: 10.1118/1.2163389. [DOI] [PubMed] [Google Scholar]
- 134.Li Q, Sone S, Doi K. Selective enhancement filters for nodules, vessels, and airway walls in two- and three-dimensional CT scans. Med Phys. 2003;30:2040–2051. doi: 10.1118/1.1581411. [DOI] [PubMed] [Google Scholar]
- 135.Kagan AR, Bassett LW, Steckel RJ, Gold RH. Radiologic contributions to cancer management. Bone metastases. AJR. 1986;147:305–312. doi: 10.2214/ajr.147.2.305. [DOI] [PubMed] [Google Scholar]
- 136.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 137.Shiraishi J, Li Q, Appelbaum D, Pu Y, Doi K. Development of a computer-aided diagnostic scheme for detection of interval changes in successive whole-body scans. Med Phys. 2006 doi: 10.1118/1.2401044. in press. [DOI] [PubMed] [Google Scholar]
- 138.Jiang Y, Nishikawa RM, Wolverton DE, Metz CE, Giger ML, Schmidt RA, Vyborny CJ, Doi K. Malignant and benign clustered microcalcifications: Automated feature analysis and classification. Radiology. 1996;198:671–678. doi: 10.1148/radiology.198.3.8628853. [DOI] [PubMed] [Google Scholar]
- 139.Jiang Y, Nishikawa RM, Schmidt RA, Metz CE, Giger ML, Doi K. Improving breast cancer diagnosis with computer-aided diagnosis. Acad Radiol. 1999;6:22–33. doi: 10.1016/s1076-6332(99)80058-0. [DOI] [PubMed] [Google Scholar]
- 140.Chan HP, Sahiner B, Helvie MA, Petrick N, Roubidoux MA, Wilson TE, Adler DD, Paramagul C, Newman JS, Sanjay-Gopal S. Improvement of radiologists; characterization of mammographic masses by using computer-aide diagnosis: an ROC study. Radiology. 1999;212:817–827. doi: 10.1148/radiology.212.3.r99au47817. [DOI] [PubMed] [Google Scholar]
- 141.Huo Z, Giger ML, Vyborny CJ, Metz CE. Breast cancer: effectiveness of computer-aided diagnosis-observer study with independent database of mammograms. Radiology. 2002;224:560–568. doi: 10.1148/radiol.2242010703. [DOI] [PubMed] [Google Scholar]
- 142.Aoyama M, Li Q, Katsuragawa S, Li F, Sone S, Doi K. Computerized scheme for determination of the likelihood measure of malignancy for pulmonary nodules on low-dose CT images. Med Phys. 2003;30:387–394. doi: 10.1118/1.1543575. [DOI] [PubMed] [Google Scholar]
- 143.Li F, Aoyama M, Shiraishi J, Abe H, Li Q, Suzuki K, Engelmann R, Sone S, MacMahon H, Doi K. Radiologists’ performance for differentiating benign from malignant lung nodules on high-resolution CT by using computer-estimated likelihood of malignancy. AJR. 2004;183:1209–1215. doi: 10.2214/ajr.183.5.1831209. [DOI] [PubMed] [Google Scholar]
- 144.Li Q, Li F, Shiraishi J, Katsuragawa S, Sone S, Doi K. Investigation of new psychophysical measures for evaluation of similar images on thoracic CT for distinction between benign and malignant nodules. Med Phys. 2003;30:2584–2593. doi: 10.1118/1.1605351. [DOI] [PubMed] [Google Scholar]
- 145.Muramatsu C, Li Q, Suzuki K, Schmidt RA, Shiraishi J, Newstead GM, Doi K. Investigation of psychophysical measure for evaluation of similar images for mammographic masses: Preliminary results. Med Phys. 2005;32:2295–2304. doi: 10.1118/1.1944913. [DOI] [PubMed] [Google Scholar]
- 146.Muramatsu C, Li Q, Schmidt RA, Suzuki K, Shiraishi J, Newstead GM, Doi K. Experimental determination of subjective similarity for pairs of clustered microcalcifications on mammograms: Observer study results. Med Phys. 2006;33:3460–3468. doi: 10.1118/1.2266280. [DOI] [PubMed] [Google Scholar]


