Abstract
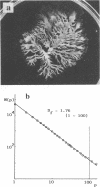
The ability to form a fractal colony was shown to be common among several species of the family Enterobacteriaceae. Bacterial spreading growth in a two-dimensional field of nutrient concentration was indicated to be important for this experimental self-similar morphogenesis. As a basic analogy, the diffusion-limited aggregation model was suggested. Fractal dimensions of colonies were mostly in the range of values from 1.7 to 1.8, similar to those of the two-dimensional diffusion-limited aggregation model. Bacterial characteristics and culture conditions inducing changes in fractal patterns and growth rates were identified. The contribution of the bacterial multicellular nature to fractal morphogenesis is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belas R., Erskine D., Flaherty D. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J Bacteriol. 1991 Oct;173(19):6279–6288. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta F, Stanley HE, Eldred WD, Daccord G, Hausman RE, Nittmann J. Physical mechanisms underlying neurite outgrowth: A quantitative analysis of neuronal shape. Phys Rev Lett. 1990 Jan 1;64(1):95–98. doi: 10.1103/PhysRevLett.64.95. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Firth W. J. Chaos--predicting the unpredictable. BMJ. 1991 Dec 21;303(6817):1565–1568. doi: 10.1136/bmj.303.6817.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger A. L., Rigney D. R., West B. J. Chaos and fractals in human physiology. Sci Am. 1990 Feb;262(2):42–49. doi: 10.1038/scientificamerican0290-42. [DOI] [PubMed] [Google Scholar]
- Lewis M., Rees D. C. Fractal surfaces of proteins. Science. 1985 Dec 6;230(4730):1163–1165. doi: 10.1126/science.4071040. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Kaneda K., Ishizuka I., Toida T., Yano I. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J Bacteriol. 1990 Jun;172(6):3015–3022. doi: 10.1128/jb.172.6.3015-3022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Sogawa M., Nakagawa Y. Fractal spreading growth of Serratia marcescens which produces surface active exolipids. FEMS Microbiol Lett. 1989 Oct 15;52(3):243–246. doi: 10.1016/0378-1097(89)90204-8. [DOI] [PubMed] [Google Scholar]
- Matsuyama T., Uetake H. Chromosomal locations of Salmonella conversion phages: mapping of prophages g 341, 15, and 34 in Salmonella anatum. Virology. 1972 Aug;49(2):359–367. doi: 10.1016/0042-6822(72)90488-6. [DOI] [PubMed] [Google Scholar]
- Meakin P. A new model for biological pattern formation. J Theor Biol. 1986 Jan 7;118(1):101–113. doi: 10.1016/s0022-5193(86)80011-x. [DOI] [PubMed] [Google Scholar]
- Obert M., Pfeifer P., Sernetz M. Microbial growth patterns described by fractal geometry. J Bacteriol. 1990 Mar;172(3):1180–1185. doi: 10.1128/jb.172.3.1180-1185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumgartner D., Losa G., Weibel E. R. Resolution effect on the stereological estimation of surface and volume and its interpretation in terms of fractal dimensions. J Microsc. 1981 Jan;121(Pt 1):51–63. doi: 10.1111/j.1365-2818.1981.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Serushago B. A., Mitsuyama M., Handa T., Koga T., Nomoto K. Role of antibodies against outer-membrane proteins in murine resistance to infection with encapsulated Klebsiella pneumoniae. J Gen Microbiol. 1989 Aug;135(8):2259–2268. doi: 10.1099/00221287-135-8-2259. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Hsu C. Escherichia coli K-12 cell-cell interactions seen by time-lapse video. J Bacteriol. 1989 Nov;171(11):5963–5974. doi: 10.1128/jb.171.11.5963-5974.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]