Abstract
Epigenetic silencing of genes relocated near telomeres, termed telomeric position effect, has been extensively studied in yeast and more recently in vertebrates. However, protection of a transgene against telomeric position effects by chromatin insulators has not yet been addressed. In this work we investigated the capacity of the chicken β-globin insulator cHS4 to shield a transgene against silencing by telomeric heterochromatin. Using telomeric repeats, we targeted transgene integration into telomeres of the chicken cell line HD3. When the chicken cHS4 insulator is incorporated to the transgene, we observe a sustained gene expression of single-copy integrants that can be maintained for >100 days of continuous culture. However, uninsulated single-copy clones showed an accelerated gene expression extinction profile. Unexpectedly, telomeric silencing was not reversed with trichostatin A or nicotidamine. In contrast, significant reactivation was obtained with 5-aza-2′-deoxycytidine, consistent with the subtelomeric DNA methylation status. Strikingly, insulated transgenes integrated into telomeric regions were enriched in histone methylation, such as H3K4me2 and H3K79me2, but not in histone acetylation. Furthermore, the cHS4 insulator counteracts telomeric position effects in an upstream stimulatory factor-independent manner. Our results suggest that this insulator has the capacity to adapt to different chromatin propagation signals in distinct insertional epigenome environments.
Keywords: chromatin insulator, DNA methylation, heterochromatin, histone modifications, epigenetic silencing
The eukaryotic genome is partitioned in two classes of chromatin: euchromatin and heterochromatin. Centromeric and telomeric sequences represent the main source of constitutive heterochromatin. Telomeres, which are composed of TTAGGG repeats and subtelomeric regions, are gene-poor genomic areas that adopt particular heterochromatin conformation (1, 2). In addition to their contribution to genome stability and their protective role, telomeres can also influence the expression of genes integrated nearby through a phenomenon known as telomeric position effect (TPE) (3, 4).
There are two sorts of position effects, position effect variegation and chromosomal position effect (CPE) (4). Position effect variegation is defined as the variegated pattern of expression from cell to cell when a gene is translocated into the proximity of dominant heterochromatin (3, 4), whereas CPE are alterations of transgene expression associated with a distinct insertion into the epigenome milieu (5). In both cases, transgene expression is affected by changes in chromatin conformation, such as those associated with histone deacetylation, H3K9me3, and DNA methylation, that can extend over considerable genomic distances (3, 4). Many studies have focused on trying to understand such phenomena, but little has been done to study the capacity of vertebrate chromatin insulators to protect against TPE (5–10).
Chromatin domain boundaries or insulators are epigenomic components that contribute to the formation and maintenance of genomic domains (9, 10). Recent evidence supports an active role of insulators in the optimal topology conformation of chromosomal domains in the nucleus (10–12). In particular, the chicken cHS4 β-globin insulator can protect a transgene against CPE via the recruitment of histone acetyltransferases and methyltransferases by USF1 (5, 13). This process further supports the idea that the cHS4 insulator is capable of blocking heterochromatin propagation.
Here we ask whether the cHS4 insulator is able to protect a transgene against a dominant source of constitutive heterochromatin, like telomeres. To this end we targeted the integration of a transgene into telomeric regions by incorporating telomeric (TTAGGG)n repeats on one side of our constructs. Our results show that the cHS4 insulator is able to protect a transgene against TPE. Reactivation experiments demonstrated that DNA methylation is one of the predominant causes of uninsulated transgene silencing. Surprisingly, the insulated transgene is enriched in histone methylation, which contrasts with an absence of histone acetylation marks. Furthermore, we demonstrate that this insulator protects from TPE independent of upstream stimulatory factor (USF). This study provides evidence that a chromatin insulator protects a transgene against TPE and suggests that insulators are able to adapt themselves to protect a transgene against distinct epigenomic environments.
Results
Experimental System to Study CPE.
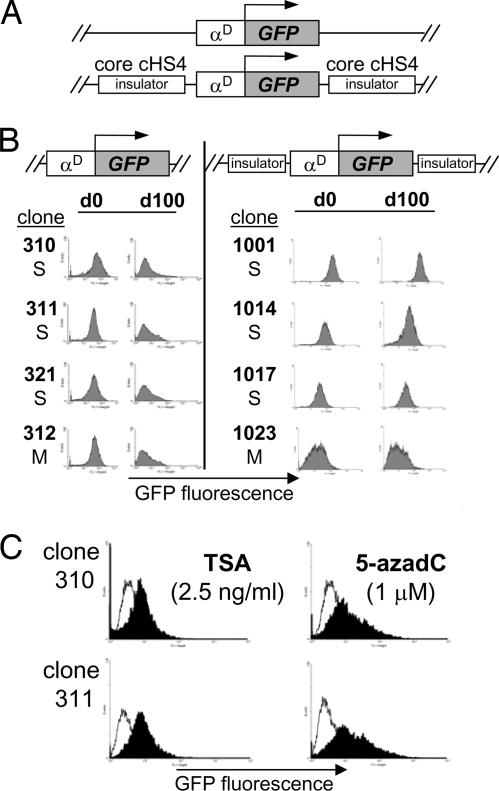
To study CPE and TPE we used the enhanced EGFP as a reporter gene under the control of the chicken adult αD gene promoter, which is susceptible to strong CPE (Fig. 1A and data not shown). We first validated our assay by flanking the transgene on both sides with two copies of the core (2 × 250 bp) chicken β-globin cHS4 insulator element (7). This reporter was randomly integrated into the avian transformed erythroblast HD3 cell line. Southern blot analysis confirmed the integrity and copy number of the transgene in 10 independently isolated lines (data not shown). We performed fluorescence cytometry to measure GFP expression in individual, stable HD3 clones (Fig. 1B). Using this reporter gene, promoter, and cell line, we confirmed the previous observation that the core cHS4 insulator is capable of contributing to sustained expression over >100 days of continuous cell culture in the absence of drug selection (7). As expected, uninsulated transgenes showed a gradual silencing of GFP expression began after ≈40–50 days of continuous cell culture (data not shown). To validate the progressive epigenetic silencing of the uninsulated transgene, we performed reactivation experiments using histone deacetylase [trichostatin A (TSA)] and DNA methylation [5-aza-2′-deoxycytidine (5-azadC)] inhibitors. Our results showed that both DNA methylation and histone deacetylation are responsible for maintaining silencing of uninsulated transgenes (Fig. 1C), and the core cHS4 insulator is able to counteract CPE (6, 7).
Fig. 1.
The cHS4 insulator protects against CPE. (A) Scheme of the vectors used for stable transfections. (B) Stable integrant of HD3 cells transfected with an uninsulated or insulated GFP transgene was maintained in continuous cell culture for 100 days (d100). FACS analysis was performed for each clone every 2 weeks. Representative FACS profiles are shown for several single or multicopy integrants. (C) Reactivation assays were performed with silenced uninsulated clones by incubating with TSA or 5-azadC for 24 and 48 h, respectively.
Transgene Targeting to Telomeric Regions in Chicken HD3 Cells.
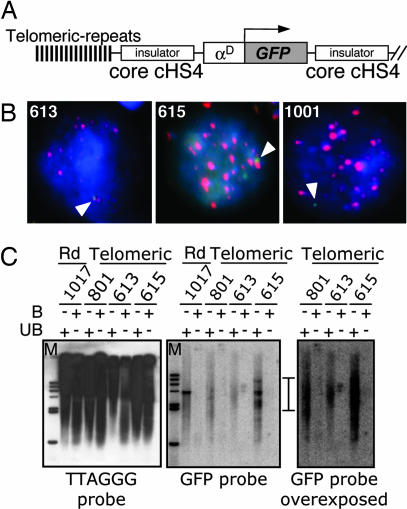
To analyze the effects of an insulated transgene in the context of telomeric heterochromatin, we targeted our constructs to telomeric regions. We flanked both the uninsulated and the core cHS4-insulated transgene with 1.6 kb of telomeric (TTAGGG)n repeats (Fig. 2A) (14, 15). To analyze whether the transgene was integrated in a telomeric region, we evaluated the colocalization of telomeric repeats with the transgene by in situ hybridization (Fig. 2B). The clones 613 and 615 showed a reproducible colocalization with telomeric repeats supporting their integration in these repressive sites. As a control, we generated stable lines with random integration of transgene lacking telomeric repeats, which do not colocalize with telomeric sequences (Fig. 2B Right). The telomeric integration of the transgenes was confirmed by the smearing pattern obtained from genomic DNA digested with DraIII and by Southern blot [Fig. 2C and supporting information (SI) Fig. 7] (14, 16, 17). Through the enrichment of telomeric fractions, we were able to identify the clone 613, in which the transgene was integrated into a telomeric region. The pull-down of telomeric fractions of the clone 615 did not reveal any GFP signal in the bound fraction. However, the unbound fraction for this clone revealed a smear GFP signal, which would be expected in the case of telomeric integration (17). One possibility is that the transgene is integrated near an interstitial (TTAGGG) repeat region (15) (SI Fig. 7). We believe that the avian genome represents an attractive model for TPE because of its high density of telomeric (TTAGGG)n repeats in macrochromosomes and microchromosomes (15).
Fig. 2.
Telomeric insertion of insulated and uninsulated transgenes. (A) Transgene vector with the telomeric (TTGAAA)n repeats. (B) Colocalization of transgene with telomeric repeats. In situ hybridization was performed with the clones 613 and 615 and the random integrated clone 1001. Transgene signal was amplified and detected with a FITC-labeled antibody against anti-digoxigenin (green). Telomeric repeats were hybridized with biotinylated oligonucleotides and identified with streptavidin coupled to Alexa Fluor 568 (red). Cells were counterstained with DAPI. Arrows indicate the location of the transgene. (C) Sequence specificity of the purification of telomeres. DraIII-digested HD3 genomic DNA was annealed to telomeric-specific biotinylated oligonucleotides, and telomere–oligonucleotides complexes were captured with streptavidin-coated magnetic beads. The bound DNA was resolved on an agarose gel and probed with a 32P-labeled GFP probe or with an 32P-labeled (TTAGGG)7 oligonucleotide.
The Core cHS4 Insulator Protects Against TPE.
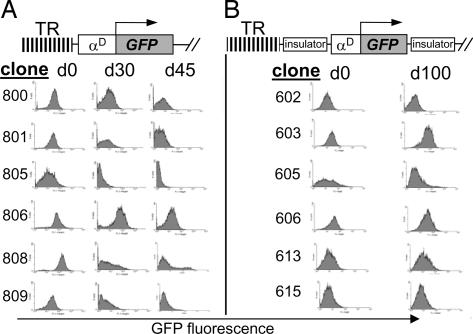
We next analyzed the effects of insulators flanking both sides of the transgene on the expression of the EGFP gene (Fig. 3A). We performed FACS analysis on eight independent, insulated and noninsulated, integrants (Fig. 3A). Uninsulated lines (800 series) showed a rapid decrease in transgene expression, exemplified by almost no fluorescence after 30 days of continuous cell culture. In contrast, insulated transgenes (600 series) demonstrated sustained expression over >100 days of cell culture (Fig. 2B). In the insulated lines, levels of GFP fluorescence varied over time and were not as intense as in random integration (compare Figs. 1B and 3B) (6, 7). This may be the result of the insulator shielding the transgene against a strong source of heterochromatin, such as telomeres (3).
Fig. 3.
The cHS4 insulator protects against TPE. HD3 cells were stably transfected with uninsulated (A) or insulated (B) transgenes. Isolated single-copy and multicopy clones were analyzed by FACS. TR, telomeric (TTGAAA)n repeats.
Uninsulated Telomeric Transgenes Are Preferentially Silenced Through DNA Methylation and Not by Histone Deacetylation.
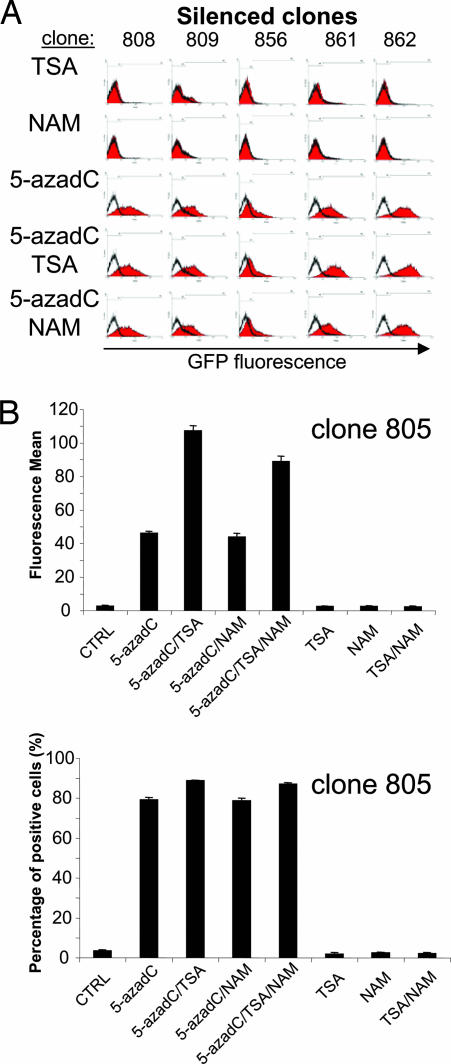
To assess the chromatin landscape of the uninsulated telomeric transgenes, we did reactivation experiments incorporating, in addition to TSA and 5-azadC, the type-III Sir2 NAD(+)-dependent deacetylase inhibitor nicotidamine (Fig. 4) (18–20). Our results showed that neither TSA nor nicotidamine was able to reverse transgene silencing of uninsulated transgenes. In contrast, the DNA methylation inhibitor 5-azadC induced significant transgene reactivation in several silenced clones (Fig. 4A). Nevertheless, the addition of TSA to 5-azadC-treated clones improved, in some cases, the reactivation efficiency by 2-fold, but not the relative number of reactivated cells (Fig. 4 A, clones 861 and 862, and B, clone 805). This effect was not observed when nicotidamine was added in the presence of any of the other inhibitors. This finding indicates that the major source of telomeric transgene silencing is DNA methylation and suggests that the core cHS4 insulator is able to block such epigenetic silencing, allowing transgene expression.
Fig. 4.
Telomeric repeats cause DNA methylation-dependent transgene silencing. (A) Clones with a silenced uninsulated transgene were incubated with TSA, nicotinamide (NAM), and/or 5-azadC, as is reported in Materials and Methods. (B) As an additional example, clone 805 is shown that is sensitive to TSA. Its behavior is representative of several independent clones, like the 861 and 862 clones. Clone 805 was treated as above, and the fluorescence mean (Upper) and the percentage of positive cells (Lower) are shown.
Enrichment of Histone Methylation Open Marks over the Telomeric Insulated Transgene.
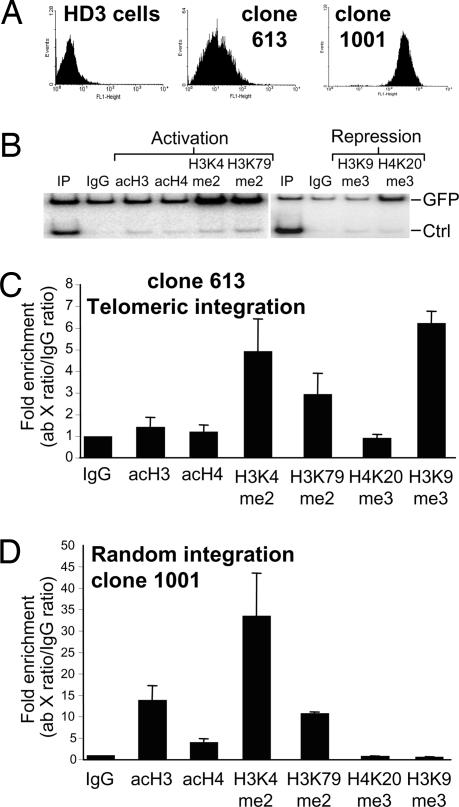
To understand the mechanisms involved in the telomeric transgene protection by the cHS4, we evaluated the epigenetic marks on the insulated transgene. Histone acetylation and methylation have been suggested as primary mechanisms to counteract CPE in interstitial integration sites (6, 21). Thus, we analyzed whether the insulator constitutively recruits such activities in telomeric regions. Using ChIP, we analyzed the transgene histone modification profile, looking specifically at open chromatin marks: global acetylation of histones H3 and H4, histone H3 lysines 4 and 79 methylation, and repressive chromatin marks H3K9me3 and H4K20me3 (Fig. 5B). We compared these marks on the telomeric insulated transgene (613) and against the randomly integrated transgene line (1001) (Fig. 5 C and D). Notably, we found that histone methylation marks corresponding to an open conformation, particularly H3K4me2 and H3K79me2, were significantly enriched in the insulated telomeric transgene. Unexpectedly, we did not detect histone acetylation over the telomeric transgene. In contrast, we found enrichment for H3K9me3. For uninsulated telomeric lines we found a reproducible loss of active marks and enrichment of H3K9me3 and H4K20me3 after 100 days of continuous culture (SI Fig. 8). The coexistence of “open” and “closed” histone marks has been recently proposed as a “bivalent chromatin” conformation, present at genomic loci that need to be rapidly activated or repressed (22, 23). To demonstrate the coexistence of “bivalent” histone marks we performed a ChIP–re-ChIP experiment (SI Fig. 9). We found no colocalization of open and repressive marks (SI Fig. 9). Instead, there is a mix of a silent and low-expressing cell population, with a chromatin conformation that is capable of counteracting telomeric silencing but not variegation. As expected, in random integrants we found enrichment of histone acetylation and histone methylation marks (Fig. 5C) (6, 13). Interestingly, no enrichment of H3K9me3 was observed on randomly integrated insulated transgenes (Fig. 5D). In both insulated transgenes, the H4K20me3 repressive histone mark is not enriched (Fig. 5 C and D). Our results suggest that TPE is counteracted by an active histone methylation of the transgene and not histone acetylation. This contrasts with previous reports that demonstrated the importance of histone acetylation in insulation-mediated protection of random integrants from CPE (13, 24). We propose that insulators may adapt themselves to block different chromatin propagation signals in distinct insertional epigenome environments.
Fig. 5.
Histone modifications over cHS4-insulated transgenes. (A) FACS profiles of the lines with telomeric (613) and random (1001) integration, respectively. (B) Duplex PCRs were used to evaluate the relative enrichment of open chromatin marks. (C and D) ChIP assays were performed with insulated telomeric (C) and random (D) integrants. Relative enrichment of histone modification was plotted, and the average enrichment values of at least two independent immunoprecipitations are shown. Each PCR was performed at least twice, and standard error is presented.
USF Is Not Involved in the Protection Against TPE.
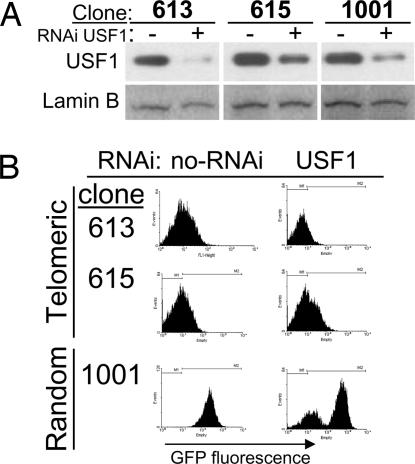
The cHS4 insulator has been shown to protect against transgene silencing by recruiting chromatin remodeling activities via USF1/2 (13). To evaluate the contribution of USF in protecting a transgene against TPE, we knocked down USF1 expression using five RNAi-expressing vectors against this protein (13). Telomeric and random integrants were stably transfected and maintained under antibiotic selection for 3 weeks. USF1 knockdown was assessed by Western blot, demonstrating a significant reduction in the protein level after 3 weeks of selection (Fig. 6A). Thereafter, we evaluated transgene expression by FACS. Our results showed that, in the absence of USF1, the cHS4 maintained the ability to protect transgene expression against telomeric gene silencing (Fig. 6B). As shown previously, a reduction on USF1 levels causes a loss of transgene expression on an insulated and random integrated vector (Fig. 6B) (13). In summary, our results show that the cHS4 can protect transgenes against TPE through a USF-independent mechanism and suggest that the barrier function of this insulator can adapt to different chromatin environments.
Fig. 6.
Protection against TPE by the cHS4 does not require USF. Stable clones with the core cHS4-insulated transgene both in random or telomeric regions were stably transfected with a mixture of five USF1-specific RNAi-expressing plasmids (13). (A) Knockdown of USF1 was addressed by Western blotting. (B) RNAi-expressing subclones were maintained for 3 weeks, and GFP expression was analyzed by FACS. Representative FACS profiles of three transfections are shown.
Discussion
The domain hypothesis of the eukaryotic genome organization postulates that the genome is partitioned into euchromatin and heterochromatin but also into a number of independent functional and transcriptional units called domains (25). Chromatin insulators emerged as epigenetic regulatory elements present in some domains that contribute to their formation, maintenance, and topology in the nucleus (10). With the aim to better understand the capacity of insulators to delimitate opposing chromatin conformations, we tested the ability of the chicken β-globin core cHS4 insulator to protect a transgene against TPE. Our results demonstrate that the core cHS4 insulator is able to maintain sustained transgene expression over >100 days of continuous cell culture when integrated into telomeric regions. Reactivation experiments showed that transgenes integrated into telomeric regions were significantly reactivated by a DNA methylation inhibitor, but not with histone deacetylase inhibitors. Furthermore, chromatin conformation over the telomeric insulated transgenes showed enrichment of open chromatin histone methylation marks like H3K4me2 and H3K79me2. Contrary to previous reports, no enrichment of histone acetylation was seen. These results suggest that the acquisition of specific chromatin configuration is mediated through insulator action in response to the integration site.
In yeast, TPE has been associated with type III histone deacetylases. However, in tumor cell lines, telomeric-associated silencing could be reversed by TSA, an inhibitor for histone deacetylases type I and II, but not with DNA methylation inhibitors (14, 26). Transgenic mice showed direct evidence that TPE requires DNA methylation (27). Our reactivation studies correlate well with this last finding and support DNA methylation as the main source of telomeric silencing. The presence of the cHS4 can decode the DNA methylation-dependent repressive program generating an isolated active domain in the telomere. We hypothesized that such decoding could be generated by recruitment of specific machinery to the cHS4, like USF, Sp1, CCCTC-binding factor (CTCF), and many other nuclear factors and cofactors. However, USF knockdown experiments presented here suggest that this transcription factor is not playing a role in protection against TPE (Fig. 6). On the other hand, for a few genes on the inactive X chromosome, CTCF binding sites and associated sequences are able to facilitate expression of those genes (28). Thus, we cannot rule out an active contribution of CTCF in the protection against TPE.
It has been reported that the mechanism by which the cHS4 insulator protects against CPE is largely based on the USF-dependent recruitment of the histone acetyltransferase p300/CBP-associated factor (PCAF) and the H3K4-specific histone methyltransferase SET7/9 (13). Our results demonstrate the enrichment of H3K4me2 and H3K79me2 exclusively. The latter suggests the possible participation of SET7/9, the chicken Dot1 homologue. Furthermore, we cannot exclude the contribution of other HMTases (29, 30). Participation of Dot1 is a particularly attractive model, because contradictory results originally suggested the involvement of the H3K79me2 on gene silencing at telomeres, but recent evidence associates this histone mark with active transcription regions (29, 30). Our results confirm that this mark is also present in transcribed regions, as previously suggested.
The cHS4 insulator incorporates a multifactor-associated complex or complexes with varied compositions or even with the capacity to acquire distinct conformational changes that allow shielding of transgenes from different sources of euchromatin and heterochromatin. We propose that, in the context of telomeric and subtelomeric transgene insertions, the insulator adapts to epigenetic environments counteracting the heterochromatin expansion over the transgene domain and favoring open histone methylation marks like H3K4me2 and H3K79me2. Moreover, the insulator is not protecting against chromosomal variegation; instead, it seems to increase the probability of long-term gene expression from a chromatin repressive environment.
Materials and Methods
Cell Culture and Generation of Stable Lines.
Cultures of the chicken erythroblast cell line LCCHD3 were maintained as previously described (31). Individual integrants were obtained by using a semisolid media (Methocel Fluka, Buchs, Switzerland) with neomycin at 0.9 mg/ml (10). After 7 days of selection, individual clones were isolated and their expression was evaluated by FACS (at day 0). Transgene expression was evaluated twice a month until completion of 100 days of continuous cell culture. For reactivation assays, histone deacetylase inhibitors TSA (Sigma, St. Louis, MO) and nicotinamide were used at 2.5 ng/ml and 10 mM, respectively, for 24 h. The DNA methylation inhibitor 5-azadC (Sigma) was used at 2 μM for 48 h. For knockdown assay, clones containing the telomeric and random insulated transgene were stably transfected with a mixture of five different USF1-specific RNAi-expressing vectors in the same conditions as described above (kindly provided by Suming Huang, University of Florida, Gainesville, FL) (13). Selection was done with 0.9 mg/ml hygromycin for 3 weeks.
Antibodies.
Antibodies against acetylated histone H3 and acetylated histone H4 (06-599 and 06-866) were obtained from Upstate Biotechnolgy (Lake Placid, NY). The antibody against H3K4me2 (ab7766) was purchased from Abcam (Cambridge, MA). Antibody against H3K79me2 was kindly provided by Fred van Leeuwen (Nederlands Kanker Instituut–Antoni van Leeuwenhoek Ziekenhuis, Amsterdam, The Netherlands). Antibodies against the trimethylated versions of histone H3 on K9 and K20 were kindly provided by Thomas Jenuwein (Research Institute of Molecular Pathology, Vienna, Austria).
Plasmids.
The chicken αD globin gene promoter was cloned into the BamHI site of the commercial vector pEGFP-1 (Clontech) and named pGαD3. Two copies of the core insulator cHS4 were introduced into the EcoRI and SalI sites to flank the 5′ end of the transgene. To flank the 3′ end, a second multiple cloning site was introduced into the AflII site located downstream of the GFP-coding sequence, which containing the sites BstEII, NheI, PacI, MluI, and AscI. The core cHS4 sequence was cloned into the PacI site to generate the plasmid pLCC. To target the transgene into the telomere, 1.6 kb of telomeric repeats (kindly provided by Titia de Lange, The Rockefeller University, New York, NY) was cloned into the BglII site located upstream of the flanked transgene generating uninsulated and insulated plasmids named pTRαD3 and pTLCC, respectively.
Telomere Purification.
Telomere purification was performed essentially as described in refs. 15–17. HD3 cells' genomic DNA was digested overnight with DraIII restriction enzyme. Bound telomeres were eluted from the beads, and three rounds of purification were carried out to enrich the telomeric genomic fraction.
In Situ Hybridization.
GFP probe was produced by labeling the LCC plasmid with digoxigenin–UTP by nick translation. Telomeric repeats were identified by using a biotinylated (TTAGGG)7 oligonucleotide. HD3 cells were swollen with 75 mM KCl for 20 min at room temperature and fixed with standard methanol–acetic acid treatment. Nuclei were spread onto slides, and genomic DNA was denatured by using a solution with 70% formamide/2× SSC at 72°C for 2 min. The samples were dehydrated with incubation in ethanol at 70%, 90%, and 100% for 2 min each. Probes were hybridized at 37°C for at least 24 h as reported (15). For visualizing telomeric repeats, the probe was identified with Alexa Fluor 568-labeled streptavidin. Transgene was visualized with a FITC-labeled mouse monoclonal antibody, anti-digoxigenin, and the signal was amplified with a goat antibody anti-mouse IgG. Slides were counterstained with DAPI. A Nikon C800 microscope with the appropriated filters was used to evaluate the slides. The microphotographs were taken on a CCD camera and analyzed with Photoshop CS (Version 8; Adobe Systems, San Jose, CA).
ChIP Assays.
ChIP was performed and analyzed as was described (32). PCR duplex was used for evaluating histone mark enrichment. GFP sequence was amplified with the primers EGFP1 (forward, 5′-ACATGAAGCAGCACGACTTC-3′) and EGFP2 (reverse, 5′-TGCTCAAGGTAGTGGTTGTC-3′). For active chromatin marks, duplex PCR was performed with the primers Hete15bF (forward, 5′-GCAAAGTCATTGCCTGGTGC-3′) and Hete15bR (reverse, 5′-GCATTTGGTTAAAATGTTTATGC-3′), which amplify a heterochromatin genomic region located upstream of the β-globin domain. For negative marks, primers used were AP120-3 (forward, 5′-GACGTGGGCAGCAGATAGC CTCG-3′) and AP120-4 (reverse, 5′-GCCGGACCCCAATGGTGCCAG-3′), which amplify the 3′ enhancer of the chicken α-globin domain. Enrichment was calculated by using the following equation: Ab test (GFP signal/control signal)/IgG (GFP signal/control signal). ChIP–re-ChIP experiment is described in SI Fig. 9's legend and SI Methods.
Supplementary Material
Acknowledgments
We thank Georgina Guerrero for her excellent technical assistance, Mark Groudine and Jessica Halow for critical reading of the manuscript, and members of the F.R.-T. laboratory for constant scientific discussions and suggestions. We thank L. Ongay, G. Codiz, and M. Mora (Unidad de Biología Molecular, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México) for DNA sequencing and access to the FACS facility. This work was supported by the Dirección General de Asuntos del Personal Académico–Universidad Nacional Autónoma de México Grants IN203200, IX230104, IN209403, and IN214407; Consejo Nacional de Ciencia y Tecnología Grants 33863-N and 42653-Q; and the Third World Academy of Sciences Grant 01-055. H.R.-A. and M.F.-M. were the recipients of a fellowship from Consejo Nacional de Ciencia y Tecnología.
Abbreviations
- CPE
chromosomal position effect
- TPE
telomeric position effect
- TSA
trichostatin A
- 5-azadC
5-aza-2′-deoxycytidine
- USF
upstream stimulatory factor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704999104/DC1.
References
- 1.Blasco MA. Nat Rev Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalo S, Jaco I, Fraga MF, Chen T, Li E, Esteller M, Blasco MA. Nat Cell Biol. 2006;8:416–424. doi: 10.1038/ncb1386. [DOI] [PubMed] [Google Scholar]
- 3.Perrod S, Gasser SM. Cell Mol Life Sci. 2003;60:2303–2318. doi: 10.1007/s00018-003-3246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakimoto BT. Cell. 1998;93:321–324. doi: 10.1016/s0092-8674(00)81159-9. [DOI] [PubMed] [Google Scholar]
- 5.Recillas-Targa F, Valadez-Graham V, Farrell CM. BioEssays. 2004;26:796–807. doi: 10.1002/bies.20059. [DOI] [PubMed] [Google Scholar]
- 6.Pikaart MJ, Recillas-Targa F, Felsenfeld G. Genes Dev. 1998;12:2852–2862. doi: 10.1101/gad.12.18.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG, Gaszner M, Felsenfeld G. Proc Natl Acad Sci USA. 2002;99:6883–6888. doi: 10.1073/pnas.102179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetze S, Baer A, Winkelmann S, Nehlsen K, Sebler J, Maasss K, Bode J. Mol Cell Biol. 2005;25:2260–2272. doi: 10.1128/MCB.25.6.2260-2272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess-Beusse B, Farrell C, Gaszner M, Litt M, Mutskov V, Recillas-Targa F, Simpson M, West A, Felsenfeld G. Proc Natl Acad Sci USA. 2002;99:16433–16437. doi: 10.1073/pnas.162342499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capelson M, Corces VG. Biol Cell. 2004;96:617–629. doi: 10.1016/j.biolcel.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Ling JQ, Li T, Hu JF, Vu TH, Chen HL, Qui XW, Cherry AM, Hoffman AR. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- 12.Kurukuti S, Tiwari VK, Tavoosidana G, Pugacheva E, Murrell A, Zhao Z, Lobanenkov V, Reik W, Ohlsson R. Proc Natl Acad Sci USA. 2006;103:10684–10689. doi: 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AG, Huang S, Gaszner M, Litt MD, Felsenfeld G. Mol Cell. 2004;16:453–463. doi: 10.1016/j.molcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Baur JA, Zou Y, Shay JW, Wright WE. Science. 2001;292:2075–2077. doi: 10.1126/science.1062329. [DOI] [PubMed] [Google Scholar]
- 15.Nanda I, Schrama D, Feichtinger W, Haaf T, Schartle, Schmid M. Chromosome. 2002;111:215–227. doi: 10.1007/s00412-002-0206-4. [DOI] [PubMed] [Google Scholar]
- 16.Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Genes Dev. 1997;11:2801–2809. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baur JA, Wrigth WE, Shay JW. Methods Mol Biol. 2004;287:121–136. doi: 10.1385/1-59259-828-5:121. [DOI] [PubMed] [Google Scholar]
- 18.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 19.Sauve AA, Schramm VL. Biochemistry. 2003;42:9249–9256. doi: 10.1021/bi034959l. [DOI] [PubMed] [Google Scholar]
- 20.Pruitt K, Zinn RL, Ohm JE, McGarvey KM, Kang SH, Watkins DN, Herman JG, Baylin SB. PLoS Genet. 2006;2:344–352. doi: 10.1371/journal.pgen.0020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutskov V, Felsenfeld G. EMBO J. 2004;23:138–149. doi: 10.1038/sj.emboj.7600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meisner A, Wering M, Plath K, et al. Cell. 2006;125:315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 23.McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Cancer Res. 2006;66:3541–3549. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 24.Litt MD, Simpson M, Recillas-Targa F, Prioleau MN, Felsenfeld G. EMBO J. 2001;20:2224–2235. doi: 10.1093/emboj/20.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razin SV, Farrell CM, Recillas-Targa F. Int Rev Cytol. 2003;226:63–125. doi: 10.1016/s0074-7696(03)01002-7. [DOI] [PubMed] [Google Scholar]
- 26.Koering CE, Pollice A, Zibella MP, Bauwens S, Pusieux A, Brunori M, Brun L, Martins L, Sabatier L, Pulitzer JF, Gilson E. EMBO Rep. 2002;3:1055–1061. doi: 10.1093/embo-reports/kvf215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedram M, Sprung CN, Gao Q, Lo AW, Reynolds GE, Murnane JP. Mol Cell Biol. 2006;26:1865–1878. doi: 10.1128/MCB.26.5.1865-1878.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippova GN, Cheng MK, Moore JM, Truong JP, Hu YJ, Nguyen DK, Tsuchiya KD, Disteche CM. Dev Cell. 2005;8:31–42. doi: 10.1016/j.devcel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Lacoste N, Utley RT, Hunter JM, Poirier GG, Côté J. J Biol Chem. 2002;277:30421–30424. doi: 10.1074/jbc.C200366200. [DOI] [PubMed] [Google Scholar]
- 30.Schübeler D, MacAlpine DM, Scalzo D, Wirbelauer C, Kooperberg C, van Leeuwen F, Gottschling DE, O'Neill LP, Turner BM, Delrow J, et al. Genes Dev. 2004;18:1263–1271. doi: 10.1101/gad.1198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rincón-Arano H, Recillas-Targa F. Methods Mol Biol. 2004;267:435–450. doi: 10.1385/1-59259-774-2:435. [DOI] [PubMed] [Google Scholar]
- 32.Sawado T, Igarashi K, Groudine M. Proc Natl Acad Sci USA. 2001;98:10226–10231. doi: 10.1073/pnas.181344198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.