Abstract
The maintenance of immune tolerance to apoptotic cells (AC) within an inflammatory milieu is vital to prevent autoimmunity. To investigate this, we administered syngeneic AC i.v. into mice carrying a cohort of ovalbumin (OVA)-specific transgenic T cells (DO11.10) along with OVA peptide and complete Freund's adjuvant, observing a dramatic increase in OVA-specific IL-10 secretion. Activated splenic B cells responded directly to AC, increasing secretion of IL-10, and this programming by AC was key to inducing T cell-derived IL-10. We went on to ask whether AC are able to modulate the course of autoimmune-mediated, chronic inflammation. AC given up to 1 month before the clinical onset of collagen-induced arthritis protected mice from severe joint inflammation and bone destruction. Antigen-specific CD4+ T cells again secreted significantly more IL-10, associated with a reduced titer of pathogenic anti-collagen II antibodies. Inhibition of IL-10 in vivo reversed the beneficial effects of AC. Passive transfer of B cells from AC-treated mice provided significant protection from arthritis. These data demonstrate that AC exert a profound influence on an adaptive immune response through the generation of CD19+ regulatory B cells, which in turn are able to influence the cytokine profile of antigen-specific effector T cells.
Keywords: apoptosis, IL-10, regulation, T cells
Apoptosis is the programmed and physiological death of unwanted cells (1), and the uptake of apoptotic cells (AC) by antigen-presenting cells (APC) is generally agreed to have a profound effect on the immune system (2). The ability of dendritic cells (DC) to activate naïve T cells depends on a process of maturation, involving increases in MHC class II and costimulatory molecules among others (3). It has been shown that AC can influence this maturation; thus, immature DC that capture AC are unable to mature into potent immunostimulatory cells (4–6). However, this modulation of DC maturation/function is overridden in the immunostimulatory environment prevalent after immunization or infection (driven by microbial antigen and T cell signals, e.g., CD40) (7–9). In addition to DC, B cells act as APC, contributing to T cell clonal expansion and differentiation at a surprisingly early stage (10). As well as providing extra and essential antigen presentation capacity in the early phase of the response, B cells influence the T cell response by performing an important regulatory role in normal and autoimmune responses (11–13). At present there is no information concerning the effect of AC on B cell function.
We were interested to know whether AC are able to influence the outcome of an immune response when given alongside a commonly used maturation stimulus, complete Freund's adjuvant (CFA). If so, they may play an important role in the resolution of inflammation when danger signals are still present. We found that AC administered with CFA in vivo induced a population of antigen-specific IL-10-secreting CD4+ T cells. Their induction depended on AC interacting directly with activated B cells, and the secretion of IL-10 by B cells was required to induce these regulatory T cells both in vivo and in vitro. The fact that AC are able to influence this important APC within an inflammatory environment suggests that they play an important role in preventing an excessive inflammatory response and may play a key role in the resolution phase of an adaptive immune response where activated B cells and an influx of AC coexist.
Results
CD4 T Cells Stimulated in the Presence of AC Secrete More IL-10.
Naïve DO11.10 T cells were injected i.v. into BALB/c mice along with AC or PBS, and the mice were immunized s.c. with ovalbumin (OVA) emulsified in CFA (OVA/CFA). After 7 days CD4+ T cells were purified from these mice and restimulated in vitro with naïve BALB/c splenocytes as APC. Supernatants collected after 3 days of culture revealed that CD4+ T cells from AC-treated mice produced significantly more IL-10 and IFN-γ than controls (Fig. 1 a and b), whereas the levels of IL-4 were similar in both groups (Fig. 1c). To further investigate the cellular basis of the IFN-γ and IL-10 secretion we analyzed OVA-specific (CD4+ KJ-126+) cells by intracellular cytokine staining (Fig. 1d). Effector T cells produced predominantly either IL-10 or IFN-γ. We could not detect any IL-4 by intracellular staining (data not shown). Levels of TNF-α, MCP-1, IL-6, and TGF-β were unchanged between groups (data not shown). Hence, AC treatment in vivo in the presence of adjuvant induces distinct populations of antigen-specific CD4+ T cells that make either IFN-γ or IL-10 upon secondary antigen stimulation.
Fig. 1.
T cells generated in the presence of AC. DO11.10 T cells were transferred into BALB/c mice, immunized with OVA/CFA, and treated with AC or vehicle alone on days 0, 1, and 2. CD4+ T cells harvested on day 7 from the spleens and draining lymph nodes were restimulated with naïve BALB/c splenocytes in the presence of increasing doses of OVA peptide. IL-10 (a), IFN-γ (b), and IL-4 (c) in the supernatants was measured after 72 h. Intracellular cytokine staining was carried out on OVA-specific CD4+KJ126+ T cells (d). Data (mean ± SEM) are representative of three separate experiments with five mice per group for each experiment. **, P < 0.005; ***, P < 0.0007.
B Cells Exposed to AC in Vivo Induce an IL-10-Secreting T Cell Population.
We then asked whether AC have a differential effect on APC populations in vivo when given with adjuvant. BALB/c mice were immunized with OVA/CFA and given AC i.v. On day 7 the spleens and draining lymph nodes were harvested and CD11c+ and CD19+ cells were isolated. These APC were pulsed with OVA peptide and used to stimulate naïve CD4+ OVA-specific DO11.10 transgenic T cells. Proliferation of T cells was equally effective with all groups of APC (Fig. 2 a and b). The amount of IL-10 generated by the T cells stimulated with DC was also equivalent (Fig. 2c), whereas CD19+ B cells from AC-treated mice induced significantly more IL-10 from DO.11.10 T cells than the PBS-treated B cells (Fig. 2d). Thus, i.v. AC in association with OVA/CFA affects B cells and DC differently such that B cells from AC-treated mice induce naïve OVA-specific DO.11.10 T cells to secrete IL-10 when stimulated in vitro.
Fig. 2.
B cells exposed to AC induce a population of IL-10-secreting OVA-specific T cells. CD11c+ DC and CD19+ B cells from BALB/c mice that had been given CFA/OVA and AC or PBS i.v. 7 days earlier were pulsed with OVA peptide and used as APC to stimulate naïve DO11.10 OVA-specific T cells. Cells were pulsed with tritiated thymidine for the last 12 h of a 72-h culture, and thymidine incorporation was measured (a and b). IL-10 in the supernatants was measured after 72 h in the CD11c+ DC and in the CD19+ B cell cultures (c and d). Data (mean ± SEM) are representative of three separate experiments with five mice per group in each experiment. *, P < 0.03; **, P < 0.009.
AC Interact Directly with B Cells to Induce IL-10-Secreting Effector T Cells.
We next asked whether B cells interacted directly with AC to alter B and T cell function. B cells purified from the spleens of BALB/c mice were sorted into marginal zone (MZ) or follicular (FO) B cell subsets. They were then pulsed with OVA peptide and used to stimulate naïve DO.11.10 T cells. AC at 106 per well were added for the duration of the culture period (72 h), after which supernatants were collected and analyzed for cytokine secretion (Fig. 3 a–c). This showed that both subsets of activated B cells interacted directly with AC and induced T cells to secrete significantly more IL-10, which was greater from cultures containing MZ B cells and AC compared with FO B cells. T cells cultured alone with AC did not secrete cytokines (data not shown). In contrast to the results obtained with T cells stimulated in vivo in the presence of CFA, IFN-γ secretion was equivalent and only a small increase in IL-4 was seen in the AC-treated groups. Using OVA-pulsed CD19+ B cells cocultured with AC and DO11.10 T cells, we confirmed by intracellular cytokine staining that the increase in IL-10 secretion was a product of both B and T cell production (Fig. 3 d and e). Finally, we asked whether B cell production of IL-10 was absolutely required to induce T cell-derived IL-10. When total wild-type (C57BL/6) B cells and AC were used to stimulate OVA-specific OTII T cells a significant increase of IL-10 was seen, which was completely abrogated when IL-10-deficient B cells were used as APC (Fig. 3f). These results confirmed that the production of IL-10 by activated B cells that have interacted directly with AC was required to induce a distinct population of IL-10-secreting T cells but not the IFN-γ-producing population. The in vitro results with OTII mice on a C57BL/6 background were entirely similar to those with DO11.10 (on a BALB/c background).
Fig. 3.
B cells interact directly with AC, inducing regulation through IL-10. Splenocytes from BALB/c mice were separated into MZ and FO B cell subsets and cultured with naïve DO11.10 T cells in the presence of increasing doses of OVA peptide with or without added AC for 3 days after which supernatants were collected and analyzed for secreted IL-10 (a), IFN-γ (b), and IL-4 (c). Intracellular cytokine staining for IL-10 was performed on purified whole CD19+ B cells (d) and CD4+ T cells (e). To establish the requirement for B cell secretion of IL-10 to induce T cell IL-10 secretion, IL-10 wild-type and IL-10-deficient whole CD19+ B cells were used to stimulate OTII OVA-specific T cells on the C57BL/6 background with and without added AC for 72 h before analysis for secreted IL-10 (f). Data are means ± SEM. *, P < 0.05; **, P < 0.02; ***, P < 0.0001.
AC Protect Mice from Autoimmune Disease.
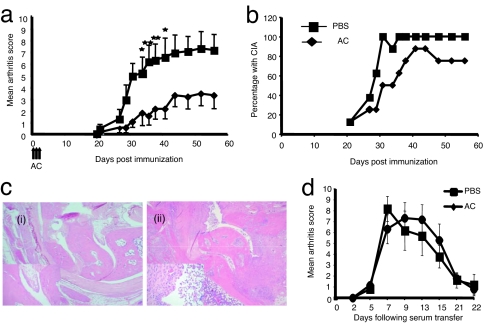
Given evidence that i.v. AC might influence B cell presentation to CD4+ T cells of a model antigen, we went on to ask whether AC could affect a CD4+ T cell-dependent autoimmune disease, collagen-induced arthritis (CIA). In control, type II collagen (CII)/CFA-immunized DBA-1 mice (treated with PBS), clinical disease was apparent from day 21 and became steadily worse until the end of the experiment (Fig. 4a). This was in marked contrast to the AC-treated mice that displayed a significantly lower mean arthritis score, which was a result of reduced disease severity in the AC-treated mice, because the incidence of disease was similar in both groups (Fig. 4b). Histological analysis of joints from arthritic mice revealed minimal cellular infiltrate within the synovium of AC-treated mice (Fig. 4ci), whereas the control, vehicle-treated mice showed a marked synovial thickening and pannus formation with associated cartilage and bone destruction (Fig. 4cii). We conclude that AC were able to protect mice from severe autoimmune-mediated inflammation and bone destruction.
Fig. 4.
AC protect mice from CIA. (a) CIA was induced in male DBA-1 mice. The AC group (diamonds) were given an i.v. injection of AC on days 0, 1, and 2, whereas the control group received vehicle only (squares). Clinical disease became apparent by day 20 and increased progressively thereafter (mean ± SEM). *, P < 0.05; **, P < 0.02. (b). The majority of mice given AC at the time of immunization also developed arthritis by day 42, but the severity was significantly less than controls. (c) Ankle joints from mice with CIA at day 56 after immunization with CII/CFA. (ci) Sections through the ankle joint of treated mice show essentially normal joints with no evidence of joint destruction, synovitis, or effusion (hematoxylin and eosin staining). [Magnification: ×100 (×400 for Inset).] (cii) Sections through the ankle joint of control mice show a marked destructive active chronic inflammatory synovitis characterized by synovial hyperplasia, formation of pannus-like tissue, and a fibrinulo-purulent exudate within the joint space (hematoxylin and eosin staining). [Magnification: ×100 (×400 for Inset).] Data are representative of eight separate experiments with between seven and nine mice per group for each experiment. (d) C57BL/6 mice (aged 6–8 weeks) were injected i.v. with AC on days −20, −19, and −18 (AC group, diamonds) whereas the control group received vehicle only (squares). At time −20 days they also received 50 μl of CFA i.d. At time 0 and +2 days they were given 100 μl of K/BxN serum i.p. and then were observed for the onset of clinical arthritis. Data are representative of two separate experiments with six mice per group in each experiment.
AC Do Not Affect Tissue Injury Induced by Passive Transfer of Serum Containing Autoantibodies to Glucose-6-Phosphate Isomerase.
AC have been shown in vitro and in vivo to elicit profound suppression of proinflammatory cytokine production by macrophages stimulated through their Toll-like receptors (2). Macrophages are important for the effector phase of joint destruction in CIA (14). However, AC given after the onset of clinical arthritis in the CIA model had no protective effect on limiting the severity of disease (data not shown). This indicated that the mechanism of protection seen in CIA required AC to be present during the phase of induction of autoimmunity rather than the effector phase of clinically apparent joint inflammation. To confirm this we used a passive antibody transfer model of arthritis (K/BxN), which bypasses the immune induction phase. Transfer of anti-G6PI antibodies (serum) from these mice engenders an acute arthritis in recipient mice that resolves spontaneously after 18–21 days (15). To directly reproduce the timing of administration of AC and CFA before the onset of joint inflammation, we injected CFA intradermally at the base of the tail on day 0; gave AC on days 0, 1, and 2; and then 21 days later induced arthritis using K/BxN serum. Fig. 4d shows that the arthritis score in both groups was not significantly different, suggesting that i.v. AC do not inhibit proinflammatory cytokine secretion by innate effector cells directly.
AC Reduce the Level of Pathogenic Autoantibodies in CIA.
We next investigated the effects of AC on the induction of the adaptive autoimmune response. Blood samples were collected from day 14 after immunization with CII and assayed for anti-CII antibodies in the various IgG subclasses. The levels of total anti-CII IgG (Fig. 5a) and IgG2a (Fig. 5b) were significantly reduced in the mice treated with AC. This was paralleled in the IgG1 subclass (measured at day 56, AC mean group titer was 1/1,990, and PBS mean group titer was 1/6,550), which indicated a reduction of anti-CII antibodies across the board and not simply a skewing from Th1 to Th2 response in AC-treated mice.
Fig. 5.
Coadministration of AC with antigen reduces the level of pathogenic autoantibodies but not CD4+ proliferation. Blood was collected by tail bleed on the days indicated after immunization with CII/CFA. Antibody levels of total IgG (a) and IgG2a (b) were determined and compared with control sera from arthritic mice. On days 11 and 50 after immunization spleens (c) and peripheral lymph nodes (d) were harvested from mice treated with AC (diamonds) and controls treated with vehicle alone (squares). CD4+ T cells were isolated and stimulated with native collagen and syngeneic splenocytes as APC. Cells were pulsed with tritiated thymidine 72 h later and harvested after a further 18 h of culture. Data (mean ± SEM) are representative of four experiments with seven to nine mice per group in each experiment. Differences between groups were ascertained with Student's unpaired t test.
CII-Specific T Cells from AC-Treated Mice Proliferate Normally but Secrete Higher Levels of IL-10.
To ask whether priming was affected by the administration of AC, splenocyte or lymph node populations were taken 11 and 50 days after immunization and restimulated with graded doses of CII. CII-specific T cells proliferated normally (Fig. 5 c and d), but T cells from AC-treated mice secreted significantly more IL-10 and IFN-γ than the T cells from control mice at all doses of CII tested (Fig. 6 a and b). Other cytokines, including IL-4, IL-6, MCP-1, TGF-β, and TNF-α, showed no significant differences (data not shown). Thus, as with the DO11.10 model, a significant increase in IL-10 and IFN-γ secretion is seen when T cells are isolated from mice that have been treated with AC and immunized in the presence of adjuvant.
Fig. 6.
Collagen-specific T cells secrete more IL-10 when AC have been administered. (a) Fourteen days after immunization with CII/CFA spleens and peripheral lymph nodes were harvested, and CD4+ T cells were isolated and restimulated with native collagen and syngeneic splenocytes. After 72 h of culture supernatants were tested for IL-10 (a) and IFN-γ (b) cytokine content. (c) Mice were given a blocking anti-IL-10 monoclonal antibody (SXC-1) by i.p. injection at the time of immunization with CII/CFA and AC (open diamonds) or with CII/CFA and PBS control (open squares) and weekly thereafter for 14 days. As indicated they were also given AC (filled diamonds) or vehicle only (filled squares) on days 0, 1, and 2 after immunization. (d) IgG2a levels from arthritic mice were determined from each group. Differences between groups were ascertained with Student's unpaired t test. Data are means ± SEM. *, P < 0.05.
The Protective Effect of AC in CIA Depends on IL-10.
Our data thus far have shown that AC induce activated B and T cells to secrete IL-10 and that B cell-derived IL-10 is essential to induce T cell IL-10 in vitro. To investigate whether IL-10 was important in providing protection from CIA in AC-treated mice, we blocked IL-10 in vivo during disease induction by giving anti-IL-10 at the time of immunization with CII/CFA and weekly thereafter, for 2 weeks. Whereas AC-treated mice made less IgG2a anti-collagen antibody (Fig. 6d), when AC treatment was combined with anti-IL-10 blockade the level of IgG2a anti-CII antibodies was restored to the same level as the PBS- and anti-IL-10-treated group (Fig. 6d). Although we did not note an increased disease severity in the mice treated with anti-IL-10, the protection from severe clinical arthritis seen when AC are given at the time of immunization was lost (Fig. 6c). We concluded that the protective effect of AC in vivo is mediated by IL-10.
CD4+ T Cells from AC-Treated Mice Showed No Enrichment of FoxP3-Positive T Cells.
To investigate whether the T cell-derived IL-10 in AC-treated mice was the result of an expansion of a CD25+FoxP3+CD4+ T cell population we stained splenocytes on day 21, the time after which clinical disease becomes apparent. There was no significant difference in the percentage of CD25+FoxP3+CD4+ T cells among CD4+ cells in the spleen in the treated mice or the controls (12.7 ± 0.3% versus 12.3 ± 0.44% in AC-treated and PBS-treated mice, respectively). Similar results were obtained when lymph node cells were analyzed (data not shown).
Adoptive Transfer of Spleen Cell Populations from AC-Treated Mice Protects from CIA.
To test whether populations of IL-10-secreting effector cells were able to transfer protection from CIA, whole spleen cell populations (Fig. 7a), purified splenic CD4+ T cells (Fig. 7b), or CD19+ B cells (Fig. 7d) from AC- or PBS-treated and CII/CFA-immunized mice were transferred to naïve DBA1 mice before arthritis induction. Mice that received AC-treated splenocytes or CD19+ B cells had a significantly reduced disease severity compared with those that received CD4+ T cells. Levels of anti-CII IgG2a were similar in both groups that received CD4+ T cells (Fig. 7c) but were again found to be reduced in mice that had received AC-treated B cells (Fig. 7e).
Fig. 7.
Passive transfer of protection from CIA using splenocyte-derived populations. Single-cell suspensions of whole splenocyte populations from AC and CII/CFA or PBS and CII/CFA-immunized mice were isolated from mice on day 21. Twenty million were injected i.v. into naïve mice on day 0, which were then immunized with CII/CFA (a). CD4+ T cells (b) or CD19+ B cells (d) from AC- or PBS-treated mice were injected i.v. into naïve DBA1 mice before immunization with CII/CFA. Tail bleed was taken on the days indicated, and CII-specific IgG2a levels were measured from mice given CD4+ T cells (c) or CD19+ B cells (e). Data are means ± SEM. *, P < 0.05.
Discussion
The data presented in this paper indicate that, in two different model systems and three different strains of mice, the administration of AC was able to affect splenic B cells such that they secreted IL-10 and enhanced secretion of the immunosuppressive cytokine IL-10 from antigen-specific effector CD4+ T cells. In addition, they inhibited the severity of inflammatory arthritis and the generation of pathogenic autoantibody despite immunization in the presence of a powerful adjuvant, CFA. Thus, administration of AC or the passive transfer of B cells from AC-treated mice protected the mice from CIA. AC themselves did not secrete immunosuppressive cytokines, such as IL-10 or TGF-β, and were unable to affect the progression of a passively induced arthritis. This demonstrates that AC are able to directly affect B cell function and cytokine production inducing regulatory B cells.
Previous studies on the antiinflammatory role of AC have focused on their capacity to inhibit macrophage proinflammatory cytokine (e.g., TNF-α) generation (16). If the AC were acting directly by generating immunosuppressive cytokines or indirectly by switching off proinflammatory cytokine production from inflammatory macrophages then we would have expected to see a diminution in joint swelling in the passive arthritis induced with K/BxN serum.
In the CIA model we found that injecting AC by the i.v. or i.p. route was protective because both deliver AC to the spleen. The injection of AC s.c. in the presence of adjuvants or DC has been shown to induce autoantibodies in susceptible mice (17). Clearly, if the chosen route of administration does not deliver AC to the spleen such that they can interact with B cells, protection is unlikely to be elicited. We also found that populations of late AC (see Materials and Methods) were as protective as early AC (as used in the experiments).
CFA is a strong maturation stimulus to DC and has been used for many years to promote Th1 antigen-specific responses. In the AC-treated mice, both an IFN-γ and a separate IL-10-secreting population of CD4+ T cells were generated. IL-10 is a multifunctional cytokine with the ability to inhibit activation and effector function of T cells, monocytes, and macrophages, ultimately terminating inflammatory responses (18). Our data support the generation of a population of antigen-specific Tr1 regulatory T cells driven by IL-10-producing regulatory B cells. The blockade of IL-10 at the time AC were given restored the anti-CII antibody response to normal levels and abrogated protection from CIA. In contrast to published data (19, 20) we did not see an exacerbation of arthritis as has been reported in IL-10-deficient mice. This may reflect that fact that blocking anti-IL-10 was given only during the induction phase of arthritis and not during the effector phase.
We were also intrigued to see an augmented IFN-γ response (both in the OVA transgenic models and in mice protected from arthritis) from T cells isolated from mice immunized in vivo with CFA, despite the attenuated IgG2a response. Studies of CIA development in genetically susceptible mice in which IFN-γ or the IFN-γ receptor has been deleted reveal that arthritis develops faster and is more severe (21–23). In addition, mice on the C57BL/6 background that are normally resistant to the induction of CIA develop acute arthritis when IFN-γ is genetically deleted. Thus, as well as protecting mice through the generation of IL-10, the heightened IFN-γ response in AC-treated mice seen after administration of CFA may actually serve to further protect the mice from excessive inflammation. We do not see an increase in T cell-derived IFN-γ when B cells are used to stimulate T cells in vitro in the presence of AC, suggesting that in vivo AC may provoke additional functions in the presence of adjuvant.
We were unable to demonstrate an increase in numbers of CD4+CD25+FoxP3+ T cells 21 days after AC infusion in DBA1 mice. Protection from autoimmune arthritis or the generation of pathogenic antibody could not be transferred passively with AC-treated CD4+ effector T cells given on the day of immunization with CII/CFA. This was despite the fact that they secreted significantly more IL-10 than control T cells. It is possible that IL-10-secreting T cells have a prominent role to play in controlling the disease at the site of inflammation, which was not tested in our experimental system because we gave the IL-10-secreting T cells 21 days before the onset of arthritis along with CII/CFA.
In contrast to the findings with passively transferred T cells, purified splenic B cells from AC-treated CII/CFA-immunized mice mimicked the effect seen after i.v. administration of AC. This indicates that AC were able to induce a stable population of regulatory CD19+ B cells. Both FO and MZ B cells are able to interact with AC and induce IL-10 secretion, although the latter population induces more IL-10. MZ B cells are found at the border of the red and white pulp of the spleen, where blood flow slows as it enters the marginal sinus (24). One could speculate that they interact with AC here both because of their favorable position in the spleen and by use of anti-self receptors (25). Regulatory B cells have been shown to exert a protective effect on a number of models of autoimmune-mediated injury, although the means by which they are generated in vivo has remained unknown (11–13).
In summary, we have demonstrated that CD19+ B cells from animals receiving i.v. AC transfer protection from CIA. Our data indicate that B cells respond directly to AC secreting IL-10 and that the secretion of IL-10 by the B cells is vital to induce T cell-mediated IL-10 secretion and immunoregulation. These IL-10-secreting populations are stable for up to 40 days after administration of AC and CII/CFA. They regulate the generation of autoantibodies and skew the cytokine profile of effector T cells toward an immunosuppressive phenotype. This indicates that the correct recognition and response to AC by activated B cells may play a decisive role in influencing the generation of regulatory populations within an inflammatory milieu that molds the outcome of an autoimmune insult. It is possible that a failure in the ability of B cells to respond to AC may be an important contributor to the breakdown in tolerance that is seen in autoimmune diseases.
Materials and Methods
Mice.
DO.11.10 mice TcR transgenic mice (H2Ad-restricted, OVA peptide323–339-specific) (26) and BALB/c, NOD, and KRN TcR transgenic mice (NOD-derived Ag7-restricted, glucose-6-phosphate isomerase-specific) (27) were bred and maintained under specific pathogen-free conditions in the Animal Facilities at the University of Edinburgh. Six- to 8-week-old male DBA1 mice, purchased from Harlan (Bicester, U.K.) were used at 8–9 weeks of age. DO.11.10, OTII, C57BL/6, and BALB/c mice were used at 6–12 weeks of age and were sex- and age-matched within experiments. NOD and KRN mice were bred, and arthritic progeny were killed at 60 days of age; their serum was collected and pooled. All experiments were covered by a Project License granted by the Home Office under the Animal (Scientific Procedures) Act 1986. Locally, this license was approved by the University of Edinburgh Ethical Review Committee.
Immunizations and Transfers.
A total of 5 × 106 purified CD4+ DO11.10 T cells were injected i.v. into syngeneic BALB/c mice, followed by immunization with 50 μl of OVA peptide323–339 (1 mg/ml) emulsified in an equal volume of CFA s.c. into each hind leg. A total of 20 × 106 syngeneic apoptotic thymocytes were given i.v. on days 0, 1, and 2, whereas the control group received vehicle alone (PBS). CD4+ T cells were isolated by magnetic sorting on day 7 from spleens and lymph nodes. For the splenocyte transfers spleens were removed on day 21 after CII/CFA with or without AC. Single-cell suspensions were depleted of red cells with red cell lysis buffer (Sigma–Aldrich, St. Louis, MO). Purified CD4+ and CD19+ cells from splenocytes were prepared by magnetic sorting. Cells with purity >95% by flow cytometry were used at the time of transfer.
Generation of AC.
Thymi were removed from 6-week-old syngeneic mice, teased into single-cell suspensions, and incubated in IMDM (GIBCO) supplemented with 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 20 mM 2-mercaptoethanol, and 10% FCS (complete IMDM). Apoptotic murine thymocytes were generated for use in these experiments by incubating cells for 6 h before infusion. Cells were stained for exposed phosphatidylserine on AC by using annexin V and run on FACS. This gave an average of 43% AC and <5% postapoptotic cells when stained with propidium iodide. In earlier experiments (data not shown) AC that had been cultured overnight and were thus at a more advanced stage of apoptosis and necrosis (90% annexin V-positive and 25% propidium iodide-positive) were equally protective. However, after 18 h of culture significantly fewer AC were recovered from culture for injection. AC have also been injected both i.v. and by the i.p. route. Neither the stage of apoptosis nor the route of administration affected the protection seen. For the sake of uniformity we have chosen to inject AC by the i.v. route and after 6 h of culture. For the in vitro experiments 1 × 106 AC were added to B cells after 6 h of culture but then left in the wells for the duration of the assay.
Induction of CIA.
CII from chicken sternal cartilage (Sigma–Aldrich), dissolved at 4 mg/ml in PBS containing 0.1 M acetic acid at 4°C overnight, was emulsified in an equal volume of CFA (Sigma–Aldrich) with added Mycobacterium tuberculosis H37RA (Difco, Detroit, MI), giving a final Mycobacterium content of 1.5 mg/ml of emulsion. A total of 100 μl of this emulsion was injected intradermally at the base of the tail on day 0. For mice given anti-IL-10 (SXC1 hybridoma cell line) 500 μg of purified antibody was given by the i.p. route weekly starting from day 0 for 3 weeks.
The first signs of arthritis appeared between days 21 and 35 with a prevalence of 60–90% of immunized mice. Each arthritic limb was scored by an investigator who was blinded to the treatment regime as follows: 0, normal; 1, erythema or swelling in a single digit; 2, erythema and swelling in two or more joints; 3, swelling of whole paw including hock joint. The total score of all four joints was added and taken as a measure of the degree of arthritis with a maximum possible score of 12 (i.e., 4 × 3) for all four limbs combined. For groups the mean of this score was taken.
Induction of K/BxN Arthritis.
Pooled arthritic serum from KRN T cell transgenic × NOD mice was generated from 60-day-old mice as previously described (27, 28). A total of 100 μl was injected i.p. into recipient C57BL/6 mice on days 0 and 2. Arthritis was seen in all mice 48 h after the last injection. The mice were scored in the same way as the CIA model.
Serum Anti-Collagen Antibody Measurement.
Chicken CII was dissolved overnight in Tris buffer [0.05 M Tris containing 0.2 M NaCl (pH 7.4)], blocked with 1% BSA and 5% sucrose, and then incubated with serial dilutions of test sera. The same reference sample consisting of pooled arthritic sera was included on each plate. Bound total IgG, IgG1, or IgG2a was detected by incubation with HRP-conjugated rabbit anti-mouse IgG, IgG1, or IgG2a, respectively (Zymed, San Francisco, CA), followed by Sure-Blue TMB reserve substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Optical density was measured at 450 nm. A reference titer based on the reference sample was calculated.
T Cell Proliferation.
Viable single-cell suspensions were prepared from spleens or lymph nodes using lympholyte-M (Cedarlane, Ontario, Canada). Cells were cultured in complete IMDM at 5 × 106 cells per milliliter with various concentrations of CII for 72 h in 200 μl using a 96-well round-bottomed plate (Nunc, Uxbridge, U.K.). During the last 18 h of culture, cells were pulsed with 1 μCi of tritiated thymidine (Amersham, Little Chalfont, U.K.), then harvested and counted in a scintillation counter. For experiments with DC or B cells, 1 × 104 cells were pulsed with various doses of OVA323–329 (Albachem Laboratories, Edinburgh University, Edinburgh, U.K.) and incubated with 1 × 105 purified DO11.10 CD4+ cells. All experimental conditions were performed in triplicate.
Assessment of CD4+CD25+Foxp3+ T Cell Populations.
Eight mice per treatment group were killed 21 days after immunization with CII/CFA and administration of AC or PBS. Splenocytes were surface-stained with anti-CD4 and anti CD25 (BD Pharmingen, San Diego, CA), fixed, and permeabilized before staining with anti-mouse Foxp3 (FJK-16; eBioscience, Insight Biotechnology, Wembley, U.K.) according to the manufacturer's instructions. Cells were then analyzed by flow cytometry.
Histology.
Knee and ankle joints were separated mid-tibia from hind limbs and dissected to remove skin and outer muscle. After fixation in 4% paraformaldehyde, they were demineralized for 2 weeks in 14% EDTA, followed by routine processing and paraffin embedding. Sagittal sections (4-μm) were cut and stained with hematoxylin and eosin for evaluation of inflammation, bone erosion, and cartilage destruction. Sections were analyzed blind by a histopathologist (D.S.).
Cytokine Quantification.
Viable splenocytes were separated from red blood cells by using lympholyte-M and cultured for 72 h in 96-well plates (Nunc) at a density of 5 × 106 cells per milliliter (200 μl per well) with various concentrations of CII. Supernatants were analyzed for production of cytokines by a sandwich ELISA according to the manufacturer's instructions (R & D Systems, Abingdon, U.K.). For experiments with CD11c+ DC or CD19+ B cells 1 × 104 cells were pulsed with various doses of OVA323–329 and incubated with 1 × 105 purified CD4+ cells in a total of 200 μl. For experiments using mice on the C57BL/6 background 2 × 105 B cells and 105 CD4 T cells were cocultured with and without 106 AC. Supernatants were collected after 72 h of culture. All experiments were performed in triplicate.
Magnetic Sorting of Cells.
CD11c+ cells were labeled with CD11c (N418) microbeads as per the manufacturer's instructions (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetically separated by using an LS column (MACS). Purity was typically >95% positive. CD4+ and CD19+ B cells from single-cell suspensions of spleens or lymph nodes were separated by using CD4 and CD19 microbeads, respectively, from the same source. In some experiments CD19+ B cells were further sorted by FACS into FO and MZ B cells after staining with anti-CD21 and anti-CD23.
Intracellular Cytokine Staining.
Cells from either primary or restimulation assays were taken on day 3 of culture and resuspended at 2 × 106 per milliliter in fresh medium in 24-well plates with added PMA (20 ng/ml) and ionomycin (1 μg/ml). After 1 h brefeldin (Sigma–Aldrich) at 10 μg/ml was added to block the secretion of cytokines, and the cells were cultured for a further 3.5 h after which the cells were surface-stained, fixed, and permeabilized (eBioscience) before intracellular cytokine staining.
Statistics.
Data are expressed, when appropriate, as mean and SEM. Significance was assessed by using unpaired t tests, and P values <0.05 were considered significant.
Acknowledgments
We thank Kris Houlberg for help with the serum transfer experiments. This work was supported by grants from the Arthritis Research Campaign (to M.G.) and the Wellcome Trust (to D.G. and J.S.).
Abbreviations
- CII
type II collagen
- CIA
collagen-induced arthritis
- MZ
marginal zone
- FO
follicular
- AC
apoptotic cell
- OVA
ovalbumin
- CFA
complete Freund's adjuvant
- DC
dendritic cell
- APC
antigen-presenting cell.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Savill J, Fadok V. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Dransfield I, Gregory C, Haslett C. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 4.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 5.Gallucci S, Lolkema M, Matzinger P. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 6.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Ann NY Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Reis e Sousa C. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 10.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 11.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 12.Mauri C, Gray D, Mushtaq N, Londei M. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. J Exp Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmdahl R, Mo J, Nordling C, Larsson P, Jansson L, Goldschmidt T, Andersson M, Klareskog L. Clin Exp Rheumatol. 1989;7(Suppl 3):S51–S55. [PubMed] [Google Scholar]
- 15.Benoist C, Mathis D. Arthritis Res. 2000;2:90–94. doi: 10.1186/ar73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bondanza A, Zimmermann VS, Dell'Antonio G, Cin ED, Balestrieri G, Tincani A, Amoura Z, Piette JC, Sabbadini MG, Rovere-Querini P, Manfredi AA. Arthritis Rheum. 2004;50:1549–1560. doi: 10.1002/art.20187. [DOI] [PubMed] [Google Scholar]
- 18.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 19.Finnegan A, Kaplan CD, Cao Y, Eibel H, Glant TT, Zhang J. Arthritis Res Ther. 2003;5:R18–R24. doi: 10.1186/ar601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson AC, Hansson AS, Nandakumar KS, Backlund J, Holmdahl R. J Immunol. 2001;167:3505–3512. doi: 10.4049/jimmunol.167.6.3505. [DOI] [PubMed] [Google Scholar]
- 21.Rosloniec EF, Latham K, Guedez YB. Arthritis Res. 2002;4:333–336. doi: 10.1186/ar432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. J Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 23.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, Boissier MC, Fournier C. J Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 24.Lopes-Carvalho T, Kearney JF. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin F, Kearney JF. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 26.Murphy KM, Heimberger AB, Loh DY. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 27.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 28.Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, Marchal P, Duchatelle V, Degott C, van Regenmortel M, et al. J Exp Med. 2002;195:1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]