Abstract
Forest ecosystems are important sinks for rising concentrations of atmospheric CO2. In previous research, we showed that net primary production (NPP) increased by 23 ± 2% when four experimental forests were grown under atmospheric concentrations of CO2 predicted for the latter half of this century. Because nitrogen (N) availability commonly limits forest productivity, some combination of increased N uptake from the soil and more efficient use of the N already assimilated by trees is necessary to sustain the high rates of forest NPP under free-air CO2 enrichment (FACE). In this study, experimental evidence demonstrates that the uptake of N increased under elevated CO2 at the Rhinelander, Duke, and Oak Ridge National Laboratory FACE sites, yet fertilization studies at the Duke and Oak Ridge National Laboratory FACE sites showed that tree growth and forest NPP were strongly limited by N availability. By contrast, nitrogen-use efficiency increased under elevated CO2 at the POP-EUROFACE site, where fertilization studies showed that N was not limiting to tree growth. Some combination of increasing fine root production, increased rates of soil organic matter decomposition, and increased allocation of carbon (C) to mycorrhizal fungi is likely to account for greater N uptake under elevated CO2. Regardless of the specific mechanism, this analysis shows that the larger quantities of C entering the below-ground system under elevated CO2 result in greater N uptake, even in N-limited ecosystems. Biogeochemical models must be reformulated to allow C transfers below ground that result in additional N uptake under elevated CO2.
Keywords: global change, net primary production
Terrestrial ecosystems, and particularly forests, exchange large quantities of carbon with the atmosphere each year; ≈15% of the atmospheric pool of CO2 is exchanged between plants and the atmosphere annually (1, 2). Globally, trees represent 80% of plant biomass (3–5) and 50–60% of annual net primary production (NPP) in terrestrial ecosystems (6, 7). Given their large contribution to terrestrial productivity and carbon (C) storage, forest ecosystems are important sinks for anthropogenic emissions of CO2 to the earth's atmosphere (8, 9).
Previous research (10) shows that enhanced rates of forest NPP under free-air CO2 enrichment (FACE) were similar among four forest sites differing in species composition, climate, and soil fertility (Table 1). At one of these sites (Duke FACE), spatial variation in soil nitrogen (N) availability correlated with increasing rates of forest NPP under present-day and elevated concentrations of atmospheric CO2 (11, 12). This observation raised the question of how forest trees acquired N to support high rates of forest NPP under elevated CO2, and whether responses were consistent among the four forest FACE experiments. Indeed, because N availability often limits primary productivity through its effect on photosynthesis (13) and on the synthesis of proteins required for the construction and maintenance of living tissue, some combination of increased N uptake from the soil and more efficient use of the N already assimilated by trees is necessary to sustain the high rates of forest NPP observed under elevated CO2.
Table 1.
Characteristics of the four FACE experiments
| Name | Rhinelander | Duke | ORNL | POP-EUROFACE |
|---|---|---|---|---|
| Location | Rhinelander, WI | Durham, NC | Oak Ridge, TN | Tuscania (Viterbo), Italy |
| Latitude, longitude | 45°40′N, 89°37′W | 35°58′N, 79°05′W | 35°54′N, 84°20′W | 42°22′N, 11°48′E |
| Mean annual precipitation, mm | 810 | 1,140 | 1,390 | 818* |
| Mean annual temperature, °C | 4.9 | 15.5 | 14.2 | 14.1 |
| Growing season,† days | 150 | 200 | 190 | 247 |
| Soil classification (US) | Alfic Haplorthod | Ultic Hapludalf | Aquic Hapludult | Pachic Xerumbrept |
| Soil texture | Sandy loam | Clay loam | Silty clay loam | Loam and silt loam |
| Total soil N, g·kg−1 | 1.20 | 0.79 | 1.12 | 1.1–1.4 |
| Overstory vegetation | Populus tremuloides Michx., Acer saccharum Marsh., Betula papyrifera Marsh | Pinus taedaL. | Liquidambar styraciflua L. | Populus alba L. P. nigra L. P. x euramericana Dode Guinier |
| Peak leaf area index,‡ m2·m−2 | 2.7–3.4 | 3.4 | 5.5 | 4.6–7.4 |
| Year planted | 1997 | 1983 | 1988 | 1999 |
| Year of treatment initiation | 1997 | 1997 | 1998 | 1999 |
Data are taken from ref. 10.
*The POP-EUROFACE experiment used irrigation to avoid drought, so inferences regarding precipitation should be avoided for this site.
†For deciduous stands, the growing season is the duration that trees have leaves; for the evergreen system, it is the period of active stem growth.
‡Values of leaf area index are expressed as leaf area per ground area. Values for the Duke FACE site are projected, and values for the hardwood sites are one-sided.
Nitrogen-use efficiency (NUE) measures the amount of biomass produced per unit of N taken up from the soil (14), defined as
where NPP and Nuptake are measured in units of dry matter production or N taken up from soil per square meter of land surface per unit of time (i.e., g of DM·m−2·yr−1 or g of N·m−2·yr−1). Furthermore, NUE can be decomposed into two processes of considerable ecological interest (15, 16): (i) the rate at which dry matter is produced per unit of N in tree biomass per unit of time (i.e., N productivity, g of DM·g of N−1·yr−1), and (ii) the amount of time N is available for use in C fixation before it is recycled into the soil system [i.e., the mean residence time of N in biomass in years (MRT)]. Thus, NUE can be rewritten as
 |
where NPP and Nuptake are as in Eq. 1 and Ncontent is the mass of N in biomass per square meter of land surface (g of N·m−2). It is useful to estimate both N productivity and the MRT of N in biomass because a change in the magnitude of either or both quantities has important implications for the cycling of C and N under elevated CO2.
The objective of this study was to determine the relative importance of increases in the uptake of N from the soil and increases in NUE as processes supporting higher rates of NPP under elevated CO2 compared with present-day concentrations of atmospheric CO2 among the four forest FACE experiments. To meet this objective, we assembled plot-specific data on plant tissue N concentrations (foliage, wood, fine roots, above-ground litterfall, and fine root turnover) and NPP from these experiments. We calculated the rate of N uptake from the soil, NUE, the N content of biomass, N productivity, and the MRT of N in biomass under present-day and elevated concentrations of atmospheric CO2.
Results
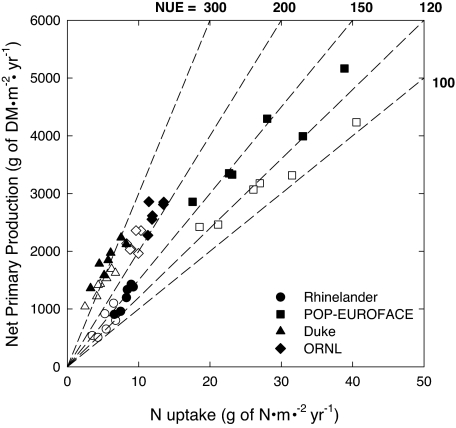
The four FACE sites varied in the relationship between NPP and N uptake (Eq. 1 and Fig. 1). Both NPP and N uptake were substantially higher at POP-EUROFACE compared with the other three sites. NPP at POP-EUROFACE was higher in elevated CO2, but N uptake was not; hence, the data points for plots from elevated and present-day CO2 concentrations align along different NUE isopleths, suggesting greater NUE in elevated CO2. By contrast, the data points for present-day and elevated CO2 from the Rhinelander, Duke, and Oak Ridge National Laboratory (ORNL) FACE sites generally plotted along the same NUE isopleth because NPP and N uptake were increased to a similar degree under elevated CO2. NUE in the two established stands (Duke and ORNL) was greater than that of the developing stands (Rhinelander and POP-EUROFACE). These relationships will now be explored in a statistical framework.
Fig. 1.
Correlation between N uptake (g of N·m−2·yr−1) and NPP (g·m−2·yr−1) at each of the four forest FACE sites under present-day (open symbols) and elevated (filled symbols) CO2. The data points represent the mean values in present-day and elevated CO2 in different years and in different species (POP-EUROFACE) or species assemblages (Rhinelander FACE). The dashed lines across the plot are isopleths of differing NUE ranging from 100 to 300.
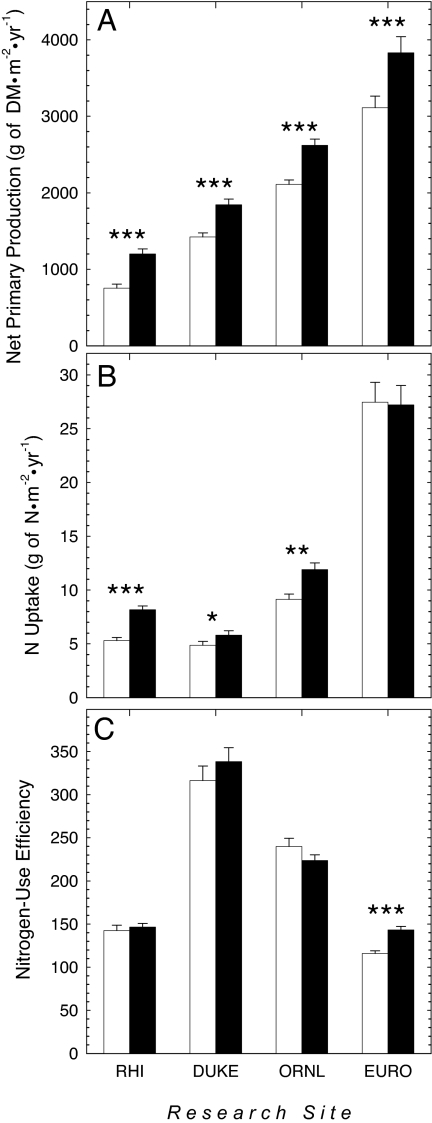
Compared with NPP at present-day CO2 concentrations, NPP was significantly higher under elevated CO2 (Fig. 2A) (10). The average annual uptake of N from the soil increased significantly under elevated CO2 at the Rhinelander, Duke, and ORNL FACE sites but not at the POP-EUROFACE site (Fig. 2B). By contrast, NUE was significantly higher under elevated CO2 at the POP-EUROFACE site but not at the Rhinelander, Duke, or ORNL FACE sites (Fig. 2C).
Fig. 2.
Responses of NPP (g of DM·m−2·yr−1) (A), N uptake (g of N·m−2 ·yr−1) (B), and NUE (g of DM·g of N−1) (C) to forest growth under present-day and elevated CO2 at the Rhinelander (RHI), Duke, ORNL, and POP-EUROFACE (EURO) sites. The error bar corresponds to one standard error of the mean. Significant differences between CO2 treatments within a site are given by *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
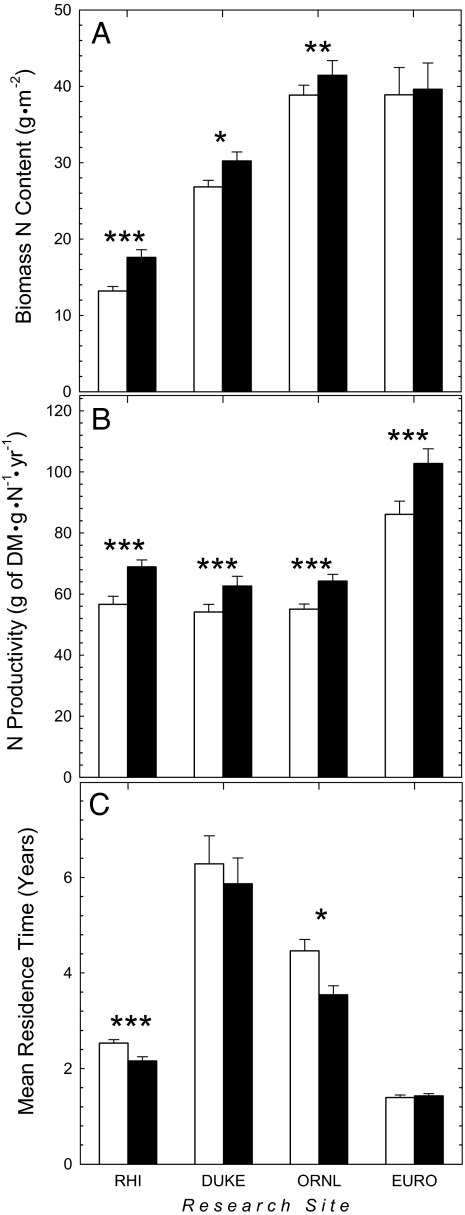
The content of N in biomass was significantly higher under elevated CO2 than at present-day concentrations of atmospheric CO2 at the Rhinelander, Duke, and ORNL FACE sites but not at the POP-EUROFACE site (Fig. 3A). At all sites, N productivity was significantly higher under elevated CO2 (Fig. 3B). At the Rhinelander, Duke, and ORNL FACE sites, the increase in N productivity occurred because elevated CO2 stimulated forest NPP (Fig. 2A) to a greater degree than the content of N in biomass (Fig. 3A). At the POP-EUROFACE site, the increase in N productivity was only due to greater forest NPP under elevated CO2 (Fig. 2A); there was no effect of elevated CO2 on the N content of biomass at this site (Fig. 3A).
Fig. 3.
Responses of N content (A), N productivity (B), and MRT (C) of N in biomass to forest growth under present-day and elevated CO2 at the Rhinelander (RHI), Duke, ORNL, and POP-EUROFACE (EURO) sites. The error bar corresponds to one standard error of the mean. Levels of statistical significance for within-site responses to CO2 treatment are the same as those in Fig. 2.
The MRT of N in biomass at the Rhinelander and ORNL FACE sites was significantly lower under elevated CO2 than under present-day CO2 concentrations (Fig. 3C). A similar trend was observed at the Duke FACE site under elevated CO2, but the effect was not statistically significant (Fig. 3C). There was no effect of elevated CO2 on the MRT of N in biomass at the POP-EUROFACE site.
Discussion
Forest ecosystems are important sinks for rising concentrations of atmospheric CO2. In a previous data synthesis of four forest FACE experiments (10), forest NPP increased on average by 23 ± 2% when the forests were grown under atmospheric concentrations of CO2 predicted for the latter half of this century. Because N often limits temperate forest productivity (17–19), some combination of increased uptake of N from the soil and more efficient use of the N already assimilated by trees is necessary to sustain the high rates of forest NPP observed under elevated CO2 (i.e., rearranging Eq. 1, NPP = Nuptake × NUE). In this data synthesis, we show that increases in forest productivity under elevated CO2 at the Rhinelander, Duke, and ORNL FACE sites were supported by significant increases in the quantity of N taken up from the soil, not by increases in NUE (Fig. 2B). By contrast, the increase in forest productivity under elevated CO2 at the POP-EUROFACE site was supported by an increase in NUE, not greater N uptake from the soil. Thus, increased uptake of N from the soil was the more typical mechanism supporting high rates of forest NPP under elevated CO2.
During the decomposition of soil organic matter (SOM), microorganisms release N to the soil solution, often in mineral form (“mineralization”), as well as take up N from the soil solution (“immobilization”), with the difference between mineralization and immobilization assumed to represent N availability to plants. Given that the rate of N mineralization did not increase significantly more than the rate of N immobilization in the soils under elevated CO2 at the Rhinelander, Duke, and ORNL FACE sites (20–23), greater uptake of N from the soil in response to forest growth under elevated CO2 was unexpected. This was particularly true of the Duke and ORNL FACE sites where tree growth is demonstrably N-limited [supporting information (SI) Table 2] (11). In N-limited ecosystems, the rate at which N is converted to available forms is slow relative to the rate of N uptake by trees, and as a consequence it is assumed that there is little or no additional capacity of soils to supply N to forest trees (24–29). Indeed, most biogeochemical models predict increases in NUE in response to high rates of forest NPP under elevated CO2 in N-limited ecosystems (26, 27, 30) and suggest that enhanced rates of N uptake under elevated CO2 can only occur where N availability exceeds demand under present-day concentrations of atmospheric CO2 (28, 30). Thus, the response of N uptake and NUE in these young temperate forests exposed to FACE is the opposite of that predicted by the current generation of biogeochemical models (26–28, 30).
A variety of mechanisms have been proposed to explain greater uptake of N by plants growing under elevated CO2. The most common explanation is that significant increases in fine root production under elevated CO2 allow trees to explore more of the soil volume for available N [“root exploration” (22, 31–35)]. The underlying assumption for this model is that N is being mineralized in excess of microbial demand in the soil and that this supply of N is only available to trees growing under elevated CO2 because of a more extensive fine root network. The root-exploration hypothesis is attractive because it reconciles the contradiction between the observation that the rate of N mineralization does not increase under elevated CO2 (20–22) but that more N is taken up by trees under elevated CO2 (Fig. 2B). If the root-exploration hypothesis is correct, then enhanced NPP of forests under elevated CO2 decreases the degree to which N limits forest productivity.
Increases in fine root production are often associated with increases in C allocation to mycorrhizal fungi and root exudation, processes that are also thought to increase plant access to soil N under elevated CO2 (36–38). Mycorrhizal fungi play a crucial role in N cycling through the release of enzymes involved in the decomposition of SOM (39–41), the capture of organic and inorganic forms of N from the soil (42, 43), and the transfer of N to host plants (44, 45). Similarly, the addition of C substrates to the soil stimulates the decomposition of SOM, often as a result of an increase in microbial activity (reviewed in ref. 46). Because SOM contains N, the delivery of plant-derived substrates can also stimulate a more rapid mineralization of N from SOM (47–51). Rapid plant growth under elevated CO2 is associated with greater hyphal length and fungal activity in the soil and an increase in the degree to which fine roots are colonized by ecto- and arbuscular mycorrhizal fungi (36, 52, 53). Moreover, enhanced NPP under elevated CO2 increases the quantity of C entering soil (54, 55) by increasing fine root production and turnover (56–58) and root exudation (59, 60), processes that increase the metabolism of organic substrates by soil microbial communities (61–65) and soil respiration (66–68).
In contrast to the Rhinelander, Duke, and ORNL sites, N uptake was not significantly greater under elevated CO2 at the POP-EUROFACE site (Fig. 2B), a response that is likely explained by the growing conditions and land-use history of this site. The POP-EUROFACE site was irrigated during exposure to elevated CO2, so there were few constraints of water on tree growth throughout the summer growing season (Table 1) (69). Similarly, the POP-EUROFACE site was located on former agricultural land where soil N availability was high and not limiting to tree growth (SI Table 2) (35). As a result, additional uptake of soil N was not required to support high rates of NPP under elevated CO2 (Fig. 2B). Rather, the N already assimilated by the trees at the POP-EUROFACE site was used more efficiently to support high rates of forest NPP under elevated CO2 (Fig. 2C) (70).
Although NUE did not change at all sites, the analysis of the two components of NUE, N productivity and the MRT of N in biomass, provides important insights into how these forests used assimilated N. Elevated CO2 increased N productivity at all sites (Fig. 3B), indicating that more C was fixed per unit of N in biomass per unit time under elevated CO2 than under present-day concentrations of atmospheric CO2. This response is likely to have occurred as a result of the significant increase in photosynthetic NUE under elevated CO2 at the four FACE sites (71–74). Despite the increase in N productivity under elevated CO2, NUE did not increase at the Rhinelander, Duke, and ORNL sites because elevated CO2 stimulated NPP and N uptake from the soil (Fig. 2B) to similar degrees, whereas N content in biomass increased less (Fig. 3A). The smaller increase in N content is explained by the more rapid turnover of the plant N pool at these sites [i.e., shorter MRT (Fig. 3C)]. The decline in the MRT of N in these stands was associated with the allocation of N to plant pools that turn over rapidly and have high N concentrations [i.e., leaves and fine roots (SI Table 3)]. Consequently, the gains in N productivity were offset by the declines in the MRT of N in biomass, resulting in no change in NUE under elevated CO2 (Eq. 2 and Fig. 2C). Notably, the decrease in the MRT of N in biomass, and the increased production of ephemeral tissues with high N concentrations, reinforces a requirement for greater N uptake under elevated CO2 at the Rhinelander, Duke, and ORNL FACE sites (Fig. 2B). In contrast, at the POP-EUROFACE site, where >60% of the N taken up annually was allocated to the production of wood (SI Table 3), MRT was not altered by elevated CO2, and NUE and N productivity increased similarly.
In biogeochemical models, trees take up N from the “available” pool in the soil (26–28, 30). The available pool of N is defined as the amount of inorganic N in soil solution, a pool that increases or decreases in size through time based on microbial activity and the amount of N needed for microbial growth and maintenance. The consequence of this model construction is that increases in forest NPP under elevated CO2 result in a significant increase in NUE in N-limited ecosystems. In contrast to the predictions of biogeochemical models, this data synthesis documents greater tree N uptake in N-limited ecosystems and increases in NUE in ecosystems that were not limited by N availability. It is likely that the combination of increasing fine root production, enhanced rates of SOM decomposition due to increased root exudation, and increased allocation of C to mycorrhizal fungi explains the ability of forest trees to take up more N from the soil under elevated CO2. Site-specific studies must now quantify the importance of these different processes. Regardless of the specific mechanism, this analysis demonstrates that larger quantities of C entering the below-ground system under elevated CO2 result in greater N uptake, even in N-limited ecosystems. To accurately forecast the response of forest ecosystems to rising concentrations of atmospheric CO2, biogeochemical models must be reformulated to allow C transfers below ground (52) that result in additional N uptake under elevated CO2.
Materials and Methods
This research synthesized data from four, temperate-forest FACE experiments. Data from the Duke and ORNL sites were collected from experiments initiated in established monoculture plantations. At the time measurements began, the loblolly pine (Pinus taeda) trees at the Duke FACE site were 13 years old, and the sweetgum (Liquidambar styraciflua) trees at the ORNL FACE site were 10 years old. Data from the Rhinelander FACE site were obtained from two communities composed of Populus tremuloides and of P. tremuloides interplanted with Betula papyrifera. Data from the POP-EUROFACE site were collected from three genotypes of Populus spp. that were initiated from cuttings planted into bare ground.
Each experiment used FACE technology to expose 20- to 30-m-diameter plots to high concentrations of atmospheric CO2. The Duke, Rhinelander, and ORNL FACE sites used similar technology, where pure CO2 was mixed with a turbulent air stream, distributed through a plenum, and released from the ground surface to the top of the tree canopy via vertical vent pipes (75). The POP-EUROFACE site used pure CO2 delivered at supersonic velocity directly into the FACE plots on the upwind side of each plot (69). The target concentration for atmospheric CO2 in the enriched plots at the Rhinelander and ORNL FACE sites was 550 μl/liter, at the POP-EUROFACE site was 560 μl/liter, and at the Duke site was present-day + 200 μl/liter, which are concentrations predicted for the year 2050 (76). The control plots at the Rhinelander, Duke, and ORNL sites were treated identically to the CO2-enriched plots with the exception that air at current atmospheric CO2 concentrations (376 μl/liter) was delivered through vent pipes. No fumigation infrastructure was built around the control plots at the POP-EUROFACE site. Detailed descriptions of the experimental methods for CO2 exposure at each site are presented for Duke, ORNL, Rhinelander, and POP-EUROFACE in refs. 75, 77, 78 and 79, and 69, respectively.
Extensive description of the methods used to calculate NPP can be found in ref. 10. In brief, NPP was measured in units of dry matter per square meter of land surface per day, as the sum of biomass increments (Iwood + Ileaf + Icoarse root + Ifine root) plus inputs by major detrital pools (Dleaves + Dfine roots). Although methods used to estimate NPP differed slightly from site to site, increments in biomass pools were estimated by means of allometric scaling of diameter growth measurements (Duke in ref. 80, ORNL in ref. 77) or destructive harvests (Rhinelander in ref. 81, POP-EUROFACE in ref. 82). The turnover of leaves was measured from litter collected in litter baskets (83–85). Fine root production and turnover were estimated from analysis of minirhizotron images and in-growth cores (56–58, 86), or from published rates of Populus fine root production and mortality (31) that were applied to allometrically determined peak fine root biomass estimates at the Rhinelander FACE site (81).
The concentration of N in biomass increments and turnover was measured on an element analyzer. At the Rhinelander and POP-EUROFACE sites, the concentration of N in foliage, wood, and coarse and fine root increments was measured on samples from the destructive harvests. At the Duke and ORNL FACE sites, the concentration of N in wood increments was taken from tree cores collected from 5 to 10 trees per FACE plot. The concentration of N in above-ground turnover (leaves, twigs, bark) was measured from subsamples of litter. We assumed that N was not retranslocated before fine root senescence (87). The concentration of N in coarse roots was assumed to be the same as that in wood.
The concentration data were multiplied by the appropriate biomass data to calculate the N content of the different pools (g·m−2) and fluxes (g·m−2·yr−1). From these values, the rate of N uptake from the soil (g·m−2·yr−1) was calculated as the sum of (i) the N content of the wood (i.e., branches, bole, and coarse roots) produced in the current year, (ii) the N content in the canopy produced in the current year minus the amount of N resorbed from the canopy in the previous year, and (iii) the content of N in the roots produced in the current year (33, 87). The N content of biomass (g·m−2) was calculated as the sum of (i) the N content in wood, (ii) the canopy content of N at peak mass, and (iii) the N content of fine roots at peak biomass. Seasonal maxima in foliage and fine roots were determined annually at each site based on repeated analysis of foliar biomass and fine root production. NUE was calculated as NPP divided by N uptake (Eq. 1). N productivity was calculated as NPP divided by the N content of biomass (Eq. 2). The MRT of N in biomass was calculated as the N content of biomass divided by N uptake (Eq. 2).
We used two-way ANOVA to test for site-specific changes in the pools and fluxes of N in response to forest growth under present-day and elevated CO2, with year as a random variable. In this article, we only interpreted the main effect of CO2 but provide P values for both effects and their interaction in SI Table 4. The data from this synthesis activity can be downloaded from the Carbon Dioxide Information Analysis Center website (http://public.ornl.gov/face/synthesis.shtml).
Supplementary Material
Acknowledgments
We thank John Pastor and an anonymous reviewer for insightful comments on a previous version of this manuscript. The Duke, ORNL, and Rhinelander FACE experiments were supported by the U.S. Department of Energy Office of Science, Biological and Environmental Research. POP-EUROFACE was supported by EU-POPFACE (ENV4-CT97-0657), EU-EUROFACE (EVR1-CT-2002-40027), the Center of Excellence “Forest and Climate” (Italian Ministry of University and Research), and Italy–USA Bilateral Project on Climate Change of the Italian Ministry of Environment. The synthesis activity was supported by the Research Foundation–Flanders Scientific Research Network on Impact of Global Change on Terrestrial Ecosystems and by the National Science Foundation Research Coordination Network on Terrestrial Ecosystem Response to Atmospheric and Climatic Change. A.C.F. and R.B.J. acknowledge ancillary support from the U.S. National Science Foundation (Grants DEB0236356 and DEB0235425). B.G. and M.L. acknowledge the Fund for Scientific Research–Flanders (Belgium). C.M.I. acknowledges the support of a Department of Energy Global Change Education Program Fellowship.
Abbreviations
- NPP
net primary production
- FACE
free-air CO2 enrichment
- NUE
nitrogen-use efficiency
- MRT
mean residence time of N in biomass in years
- ORNL
Oak Ridge National Laboratory
- SOM
soil organic matter.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706518104/DC1.
References
- 1.Amthor JS. Global Change Biol. 1995;1:243–274. [Google Scholar]
- 2.Schlesinger WH. Biogeochemistry: An Analysis of Global Change. New York: Academic; 1997. [Google Scholar]
- 3.Whittaker RH, Likens GE. Brookhaven Symp Biol. 1973:281–302. [PubMed] [Google Scholar]
- 4.Atjay GL, Ketner P, Duvigneaud P. In: The Global Carbon Cycle. Bolin B, Degens ET, Kempe S, Ketner P, editors. Vol. 13. Chichester, UK: Wiley; 1979. pp. 129–181. SCOPE Report. [Google Scholar]
- 5.Olson JS, Watts JA, Allison LJ. Carbon in Live Vegetation of Major World Ecosystems. Oak Ridge, TN: Oak Ridge National Laboratory; 1983. Report ORNL-5862. [Google Scholar]
- 6.Houghton RA, Skole DL. In: Earth as Transformed by Human Action. Turner BI II, editor. Cambridge, UK: Cambridge Univ Press; 1990. [Google Scholar]
- 7.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 8.Pacala SW, Hurtt GC, Baker D, Peylin P, Houghton RA, Birdsey RA, Heath L, Sundquist ET, Stallard RF, Ciais P, et al. Science. 2001;292:2316–2320. doi: 10.1126/science.1057320. [DOI] [PubMed] [Google Scholar]
- 9.Janssens IA, Freibauer A, Ciais P, Smith P, Nabuurs GJ, Folberth G, Schlamadinger B, Hutjes RWA, Ceulemans R, Schulze ED, et al. Science. 2003;300:1538–1542. doi: 10.1126/science.1083592. [DOI] [PubMed] [Google Scholar]
- 10.Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, et al. Proc Natl Acad Sci USA. 2005;102:18052–18056. doi: 10.1073/pnas.0509478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schafer KVR, McCarthy H, Hendrey G, McNulty SG, Katul GG. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- 12.Finzi AC, DeLucia EH, Hamilton JG, Richter DD, Schlesinger WH. Oecologia. 2002;132:567–578. doi: 10.1007/s00442-002-0996-3. [DOI] [PubMed] [Google Scholar]
- 13.Field C. Oecologia. 1983;56:341–347. doi: 10.1007/BF00379710. [DOI] [PubMed] [Google Scholar]
- 14.Vitousek P. Am Nat. 1982;119:553–572. [Google Scholar]
- 15.Berendse F, Aerts R. Funct Ecol. 1987;1:293–296. [Google Scholar]
- 16.Pastor J, Bridgham SD. Oecologia. 1999;118:50–58. doi: 10.1007/s004420050702. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell HL, Chandler RF. Black Rock Forest Bulletin No. 11. Cornwall, NY: Cornwall Press; 1939. [Google Scholar]
- 18.Miller HG. Forestry. 1981;54:158–167. [Google Scholar]
- 19.Magill AH, Aber JD, Berntson GM, McDowell WH, Nadelhoffer KJ, Melillo JM, Steudler P. Ecosystems. 2000;3:238–253. [Google Scholar]
- 20.Sinsabaugh RL, Saiya-Corka K, Long T, Osgood MP, Neher DA, Zak DR, Norby RJ. Appl Soil Ecol. 2003;24:263–271. [Google Scholar]
- 21.Finzi AC, Schlesinger WH. Ecosystems. 2003;6:444–456. [Google Scholar]
- 22.Holmes WE, Zak DR, Pregitzer KS, King JS. Global Change Biol. 2003;9:1743–1750. [Google Scholar]
- 23.Holmes WE, Zak DR, Pregitzer KS, King JS. Ecosystems. 2006;9:1–10. [Google Scholar]
- 24.Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM. Bioscience. 1989;39:378–386. [Google Scholar]
- 25.Vitousek PM, Howarth RW. Biogeochemistry. 1991;13:87–115. [Google Scholar]
- 26.Comins HN, McMurtrie RE. Ecol Appl. 1993;3:666–681. doi: 10.2307/1942099. [DOI] [PubMed] [Google Scholar]
- 27.Vegetation/Ecosystem Modeling and Analysis Project (VEMAP) Global Biogeochem Cycles. 1995;9:407–437. [Google Scholar]
- 28.Luo YQ, Reynolds JF. Ecology. 1999;80:1568–1583. [Google Scholar]
- 29.Luo Y, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, et al. Bioscience. 2004;54:731–739. [Google Scholar]
- 30.Rastetter EB, Agren GI, Shaver GR. Ecol Appl. 1997;7:444–460. [Google Scholar]
- 31.Pregitzer KS, Zak DR, Maziasz J, DeForest J, Curtis PS, Lussenhop J. Ecol Appl. 2000;10:18–33. [Google Scholar]
- 32.Zak DR, Pregitzer KS, Curtis PS, Vogel CS, Holmes WE, Lussenhop J. Ecol Appl. 2000;10:34–46. [Google Scholar]
- 33.Norby RJ, Iversen CM. Ecology. 2006;87:5–14. doi: 10.1890/04-1950. [DOI] [PubMed] [Google Scholar]
- 34.Finzi AC, Moore DJP, DeLucia EH, Lichter J, Hofmockel KS, Jackson RB, Kim HS, Matamala R, McCarthy HR, Oren R, et al. Ecology. 2006;87:15–25. doi: 10.1890/04-1748. [DOI] [PubMed] [Google Scholar]
- 35.Liberloo M, Calfapietra C, Lukac M, Godbold D, Luos ZB, Polle A, Hoosbeek MR, Kull O, Marek M, Raines C, et al. Global Change Biol. 2006;12:1094–1106. [Google Scholar]
- 36.Treseder KK. New Phytol. 2004;164:347–355. doi: 10.1111/j.1469-8137.2004.01159.x. [DOI] [PubMed] [Google Scholar]
- 37.Phillips RP. New Phytol. 2007;173:664–667. doi: 10.1111/j.1469-8137.2007.02006.x. [DOI] [PubMed] [Google Scholar]
- 38.Parrent JL, Morris WF, Vilgalys R. Ecology. 2006;87:2278–2287. doi: 10.1890/0012-9658(2006)87[2278:canaae]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Leake JR, Read DJ. New Phytol. 1990;116:123–128. doi: 10.1111/j.1469-8137.1990.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 40.Chalot M, Brun A. FEMS Microbiol Rev. 1998;22:21–44. doi: 10.1111/j.1574-6976.1998.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 41.Hodge A, Campbell CD, Fitter AH. Nature. 2001;413:297–299. doi: 10.1038/35095041. [DOI] [PubMed] [Google Scholar]
- 42.Read DJ, Perez-Moreno J. New Phytol. 2003;157:475–492. doi: 10.1046/j.1469-8137.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 43.Hobbie JE, Hobbie EA. Ecology. 2006;87:816–822. doi: 10.1890/0012-9658(2006)87[816:nisfap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Abuzinadah RA, Read DJ. New Phytol. 1986;103:507–514. [Google Scholar]
- 45.Smith SE, Read DJ. Mycorrhizal Symbiosis. San Diego: Academic; 1997. [Google Scholar]
- 46.Kuzyakov Y, Friedel JK, Stahr K. Soil Biol Biochem. 2000;32:1485–1498. [Google Scholar]
- 47.Clarholm M. Soil Biol Biochem. 1985;17:181–187. [Google Scholar]
- 48.Kuikman PJ, Jansen AG, Vanveen JA. Soil Biol Biochem. 1991;23:193–200. [Google Scholar]
- 49.Asmar F, Eiland F, Nielsen NE. Biol Fertility Soils. 1994;17:32–38. [Google Scholar]
- 50.Zagal E. Plant Soil. 1994;160:21–31. [Google Scholar]
- 51.Trueman RJ, Gonzalez-Meler MA. Global Change Biol. 2005;11:1258–1271. [Google Scholar]
- 52.Rillig MC, Field CB, Allen MF. Oecologia. 1999;119:572–577. doi: 10.1007/s004420050821. [DOI] [PubMed] [Google Scholar]
- 53.Rillig MC, Hernandez GY, Newton PCD. Ecol Lett. 2000;3:475–478. [Google Scholar]
- 54.Palmroth S, Oren R, McCarthy HR, Johnsen KH, Finzi AC, Butnor JR, Ryan MG, Schlesinger WH. Proc Natl Acad Sci USA. 2006;103:19362–19367. doi: 10.1073/pnas.0609492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoosbeek MR, Li YT, Scarascia-Mugnozza GE. Plant Soil. 2006;281:247–254. [Google Scholar]
- 56.Matamala R, Schlesinger WH. Global Change Biol. 2000;6:967–979. [Google Scholar]
- 57.Pritchard SG, Rogers HH, Davis MA, Van Santen E, Prior SA, Schlesinger WH. Global Change Biol. 2001;7:829–837. [Google Scholar]
- 58.Norby RJ, Ledford J, Reilly CD, Miller NE, O'Neill EG. Proc Natl Acad Sci USA. 2004;101:9689–9693. doi: 10.1073/pnas.0403491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rouhier H, Billes G, Elkohen A, Mousseau M, Bottner P. Plant Soil. 1994;162:281–292. [Google Scholar]
- 60.Cheng WX, Johnson DW. Plant Soil. 1998;202:167–174. [Google Scholar]
- 61.Janssens IA, Crookshanks M, Taylor G, Ceulemans R. Global Change Biol. 1998;4:871–878. [Google Scholar]
- 62.Phillips RL, Zak DR, Holmes WE, White DC. Oecologia. 2002;131:236–244. doi: 10.1007/s00442-002-0868-x. [DOI] [PubMed] [Google Scholar]
- 63.Hoosbeek MR, Lukac M, van Dam D, Godbold DL, Velthorst EJ, Biondi FA, Peressotti A, Cotrufo MF, de Angelis P, Scarascia-Mugnozza G. Global Biogeochem Cycles. 2004;18:GB1040. [Google Scholar]
- 64.Finzi AC, Sinsabaugh RL, Long TM, Osgood MP. Ecosystems. 2006;9:215–226. [Google Scholar]
- 65.Chung HG, Zak DR, Lilleskov EA. Oecologia. 2006;147:143–154. doi: 10.1007/s00442-005-0249-3. [DOI] [PubMed] [Google Scholar]
- 66.Andrews JA, Schlesinger WH. Global Biogeochem Cycles. 2001;15:149–162. [Google Scholar]
- 67.King JS, Hanson PJ, Bernhardt E, DeAngelis P, Norby RJ, Pregitzer KS. Global Change Biol. 2004;10:1027–1042. [Google Scholar]
- 68.Bernhardt ES, Barber JJ, Pippen JS, Taneva L, Andrews JA, Schlesinger WH. Biogeochemistry. 2006;77:91–116. [Google Scholar]
- 69.Miglietta F, Peressotti A, Vaccari FP, Zaldei A, De Angelis P, Scarascia-Mugnozza G. New Phytol. 2001;150:465–476. [Google Scholar]
- 70.Calfapietra C, De Angelis P, Gielen B, Lukac M, Moscatelli MC, Avino G, Lagomarsino A, Polle A, Ceulemans R, Scarascia-Mugnozza G, et al. Tree Physiol. 2007;27:1153–1163. doi: 10.1093/treephys/27.8.1153. [DOI] [PubMed] [Google Scholar]
- 71.Calfapietra C, Tulva I, Eensalu E, Perez M, De Angelis P, Scarascia-Mugnozza G, Kull O. Environ Pollut (Amsterdam, The Netherlands) 2005;137:525–535. doi: 10.1016/j.envpol.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 72.Crous KY, Ellsworth DS. Tree Physiol. 2004;24:961–970. doi: 10.1093/treephys/24.9.961. [DOI] [PubMed] [Google Scholar]
- 73.Sholtis JD, Gunderson CA, Norby RJ, Tissue DT. New Phytol. 2004;162:343–354. [Google Scholar]
- 74.Takeuchi Y, Kubiske ME, Isebrands JG, Pregtizer KS, Hendrey G, Karnosky DF. Plant Cell Environ. 2001;24:1257–1268. [Google Scholar]
- 75.Hendrey GR, Ellsworth DS, Lewin KF, Nagy J. Global Change Biol. 1999;5:293–309. [Google Scholar]
- 76.Houghton RA, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA. Climate Change 2001: The Scientific Basis. Cambridge, UK: Cambridge Univ Press; 2001. Third Assessment Report. [Google Scholar]
- 77.Norby RJ, Todd DE, Fults J, Johnson DW. New Phytol. 2001;150:477–487. [Google Scholar]
- 78.Karnosky DF, Mankovska B, Percy K, Dickson RE, Podila GK, Sober J, Noormets A, Hendrey G, Coleman MD, Kubiske M, et al. Water Air Soil Pollut. 1999;116:311–322. [Google Scholar]
- 79.Karnosky DF, Pregitzer KS, Zak DR, Kubiske ME, Hendrey GR, Weinstein D, Nosal M, Percy KE. Plant Cell Environ. 2005;28:965–981. [Google Scholar]
- 80.Hamilton JG, DeLucia EH, George K, Naidu SL, Finzi AC, Schlesinger WH. Oecologia. 2002;131:250–260. doi: 10.1007/s00442-002-0884-x. [DOI] [PubMed] [Google Scholar]
- 81.King JS, Kubiske ME, Pregitzer KS, Hendrey GR, McDonald EP, Giardina CP, Quinn VS, Karnosky DF. New Phytol. 2005;168:623–635. doi: 10.1111/j.1469-8137.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 82.Calfapietra C, Gielen B, Galema ANJ, Lukac M, De Angelis P, Moscatelli MC, Ceulemans R, Scarascia-Mugnozza G. Tree Physiol. 2003;23:805–814. doi: 10.1093/treephys/23.12.805. [DOI] [PubMed] [Google Scholar]
- 83.Finzi AC, Allen AS, DeLucia EH, Ellsworth DS, Schlesinger WH. Ecology. 2001;82:470–484. [Google Scholar]
- 84.Norby RJ, Sholtis JD, Gunderson CA, Jawdy SS. Oecologia. 2003;136:574–584. doi: 10.1007/s00442-003-1296-2. [DOI] [PubMed] [Google Scholar]
- 85.Cotrufo MF, De Angelis P, Polle A. Global Change Biol. 2005;11:971–982. [Google Scholar]
- 86.Lukac M, Calfapietra C, Godbold DL. Global Change Biol. 2003;9:838–848. [Google Scholar]
- 87.Johnson DW, Cheng W, Joslin JD, Norby RJ, Edwards NT, Todd DE. Biogeochemistry. 2004;69:379–403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.