Abstract
Stroke is among the three leading causes of death worldwide and the most frequent cause of permanent disability. Brain ischemia induces an inflammatory response involving activated complement fragments. Here we show that i.v. Ig (IVIG) treatment, which scavenges complement fragments, protects brain cells against the deleterious effects of experimental ischemia and reperfusion (I/R) and prevents I/R-induced mortality in mice. Animals administered IVIG either 30 min before ischemia or after 3 h of reperfusion exhibited a 50–60% reduction of brain infarct size and a 2- to 3-fold improvement of the functional outcome. Even a single low dose of IVIG given after stroke was effective. IVIG was protective in the nonreperfusion model of murine stroke as well and did not exert any peripheral effects. Human IgG as well as intrinsic murine C3 levels were significantly higher in the infarcted brain region compared with the noninjured side, and their physical association was demonstrated by immuno-coprecipitation. C5-deficient mice were significantly protected from I/R injury compared with their wild-type littermates. Exposure of cultured neurons to oxygen/glucose deprivation resulted in increased levels of C3 associated with activation of caspase 3, a marker of apoptosis; both signals were attenuated with IVIG treatment. Our data suggest a major role for complement-mediated cell death in ischemic brain injury and the prospect of using IVIG in relatively low doses as an interventional therapy for stroke.
Keywords: C5a, cerebral cortex apoptosis, ischemic stroke, lymphocyte, microglia
Arterial occlusion can be demonstrated in 70–80% of all stroke cases and results in ischemia that leads to dysfunction and death of neurons in the brain regions supplied by the affected blood vessels (1). In addition to cell damage, regional brain ischemia induces an inflammatory response initiated by complement activation and generation of active fragments, such as C3a and C5a anaphylatoxins, C3b, C4b, and iC3b (2). In addition to their direct biological effects, such as increased permeability of microvasculature, smooth muscle contraction and inflammatory cell migration- (3) activated complement fragments stimulate the expression of proinflammatory cytokines in leukocytes that augment the inflammatory response (4). Subsequently, there is up-regulation of endothelial cell adhesion molecules and infiltration of inflammatory cells (5). Expression of C3a and C5a receptors was found to be significantly increased after transient middle cerebral arterial occlusion (MCAO) in the mouse (6). Direct deposits of different complement fragments have been demonstrated in ischemic brain tissue (7, 8) and complement depletion achieved in rats by administration of cobra venom factor 24 h before MCAO resulted in reduced postischemic brain injury in rats (9).
Therapeutic management of stroke is mainly aimed at prevention and reduction of risk factors associated with stroke, such as elevated blood pressure, thrombosis, and carotid artery thickening. Interventional therapy of stroke remains limited to enzymatic thrombolysis by use of the recombinant tissue plasminogen activator -rtPA (10). Even though rtPA has been considered an important step forward in changing the outcome of stroke, its use remains controversial. The primary concern is the short therapeutic window for the use (3 h) and an increased incidence of hemorrhagic stroke if infused beyond that time point (11).
There is an urgent need for novel therapies capable of reducing mortality and permanent neurological deficits in victims of stroke. A therapy that could fulfill these requirements is high-dose i.v. Ig (IVIG), because it has the ability to scavenge activated complement fragments and attenuate complement-mediated injury in (auto) immune conditions and is readily available for off-label uses (12). Here we show that IVIG injected before or after brain ischemia/reperfusion (I/R) injury in mice is highly effective in preventing mortality and attenuating brain damage after transient and permanent occlusion of the middle cerebral artery, even in relatively small doses.
Results
IVIG Treatment Reduces Mortality and Infarction Size and Improves Functional Outcome in Murine Stroke.
To assess the effect of IVIG (Instituto Grifols, Barcelona, Spain) in experimental stroke, we used a mouse model that mimics the most common type of human stroke, transient MCAO (13). If untreated, this stroke procedure is lethal for 18.6% of the animals (Table 1). Infusion of IVIG at 2 g/kg 30 min before or 3 h after MCAO practically eliminated mortality, whereas treatment with control reagents, 5% sorbitol (vehicle or stabilizer for Igs in IVIG preparation) or albumin at 2 g/kg before and after MCAO, had no significant effect on the mortality rate after I/R brain injury [Table 1; supporting information (SI) SI Text].
Table 1.
Mortality rates in mice subjected to I/R brain injury and different treatments
| Treatment | Died | Total no. | Percent |
|---|---|---|---|
| Sham | 0 | 30 | 0.0 |
| I/R | |||
| Alone | 8 | 43 | 18.6 |
| + Vehicle | 5 | 23 | 21.7 |
| + Prealbumin | 4 | 21 | 19.0 |
| + Postalbumin | 4 | 22 | 18.2 |
| + Pre IVIG | 0 | 24 | 0.0 |
| + Post IVIG | 1 | 41 | 2.4 |
An overall χ2 test value (for 6 degrees of freedom) for a (2-by-7) contingency table was 17.84, P < 0.01. Mortality rates in both IVIG groups were significantly different from controls and not different from the sham group. Details of the analysis are in SI Text.
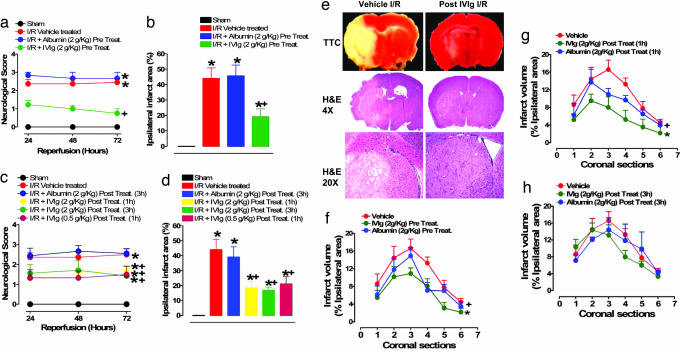
The extent of anatomical and functional brain damage was also greatly reduced by IVIG treatment. When mice were infused with IVIG at a dose of 2 g/kg of body weight 30 min before MCAO, their stroke-induced neurological impairment in the ensuing 3 days of reperfusion was significantly less compared with control mice infused with either vehicle or albumin at 2 g/kg (Fig. 1a). The amount of brain damage induced by I/R (infarct size) was reduced by ≈60% in IVIG-pretreated mice compared with controls (Fig. 1b). Dramatic improvement in functional outcome and reduction in brain damage were also seen in mice treated with IVIG at 2 g/kg 1 and 3 h after the completion of the ischemic period and into the reperfusion period (Fig. 1 c and d). Because the latter high dose of IVIG increases blood viscosity, which could conceivably promote thrombosis (14), we tested a 4-fold lower dose. Poststroke treatment with 0.5 g/kg of IVIG was equally effective in reducing brain damage and improving functional outcome (Fig. 1 c and d).
Fig. 1.
IVIG treatment reduces brain damage and improves functional outcome in a mouse stroke model. (a) A five-point neurological score was applied to the sham (n = 5), vehicle-treated I/R (n = 12), albumin-treated I/R (n = 7), and IVIG- (2 g/kg) pretreated I/R (n = 10) mice; *, P < 0.001 compared with sham value; +, P < 0.01 compared with vehicle treated I/R value. (b) Ischemic infarct sizes 72 h after I/R in vehicle-treated (n = 12), I/R albumin-treated (n = 7), and IVIG-pretreated (n = 10) mice; *, P < 0.001 compared with sham value; +, P < 0.01 compared with vehicle-treated I/R value. (c) Neurological function in mice posttreated with 2 g/kg IVIG either 1 h (n = 8) or 3 h (n = 8) after ischemia or with 0.5 g/kg IVIG 1 h after ischemia (n = 9) and albumin at 2 g/kg (n = 10) 3 h into reperfusion; +, P < 0.05 compared with vehicle-treated mice (n = 8); *, P < 0.01 compared with sham-operated mice (n = 10). (d) The infarct size was significantly reduced in mice treated with 2 g/kg IVIG 3 h (n = 8) or 1 h (n = 7) after stroke as well as with 0.5 g/kg IVIG 1 h after stroke (n = 8) but not in mice treated with 2 g/kg albumin (n = 10) compared with vehicle-treated mice (n = 8); *, P < 0.001 compared with sham value; +, P < 0.05 compared with vehicle-treated mice. (e) Representative images (from brain sections taken from four animals in each treatment group) of 2,3,5-triphenyltetrazolium chloride-stained sections (Top) and hematoxylin/eosin-stained sections at ×4 (Middle) and ×20 (Bottom) magnification from both vehicle-treated I/R mice and mice treated with 2 g/kg IVIG 1 h after stroke. (f) Infarct volume in serial brain sections from mice treated with vehicle (n = 8), IVIG (n = 8), and albumin (n = 10) 30 min before being subjected to PMCAO; +, P < 0.05; *, P < 0.01. (g) The effect of vehicle (n = 8), albumin (n = 9), and IVIG (n = 8) on brain infarct size after administration 1 h after PMCAO; +, P < 0.05; *, P < 0.01. (h) The effect 3 h posttreatment with vehicle (n = 6), albumin (n = 8), and IVIG (n = 10) on infarction size in PMCAO; +, P < 0.05; *, P < 0.01.
Histological analysis of brain sections from the ischemic areas of control (vehicle and albumin-treated) mice subjected to transient MCAO revealed edema, loss of normal tissue architecture, and total necrosis (Fig. 1e, SI Fig. 6). In contrast, brain sections of IVIG-treated animals showed intact tissue architecture and occasional and elective neuronal loss (Fig. 1e).
Permanent occlusion of cortical branches of the MCA resulted in infarction volumes that were evaluated in individual serial coronal sections 1–6 that covered the entire volume of the infarction. IVIG pretreatment as well as 1 h postocclusion therapy at 2 g/kg significantly reduced the size of infarctions in segments 2–5, whereas a 3-h posttreatment with IVIG at the same dose showed a trend toward reduction that reached significance for section 4. Albumin treatment was much less effective, decreasing infarct size only in a single coronal section in pretreatment (section 4) and 1 h posttreatment (section 3) groups (Fig. 1 f–h).
IVIG Crosses the Blood–Brain Barrier (BBB) After a Stroke.
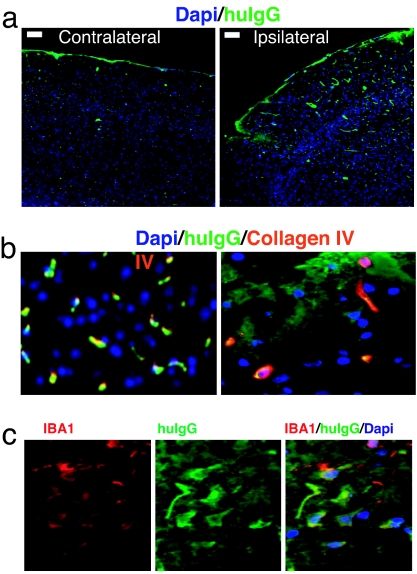
To establish whether the BBB was damaged in the brain tissue affected by the stroke, we quantified the concentration of human IgG in samples of brain tissue taken from mice treated with low-dose IVIG. We found that human IgG levels were higher in samples taken from the infarcted area (12.4 ± 2.1 μg/ml, n = 3) as compared with the corresponding region of the contralateral noninjured brain hemisphere (3.4 ± 1.4 μg/ml, n = 3, P < 0.01). This was confirmed by immunohistochemistry, which showed more intense and extensive staining for human IgG at the site of injury as compared with the contralateral side of the brain (Fig. 2a). Furthermore, dual staining for human IgG and blood vessels (collagen IV) allowed us to visualize the leakage of the BBB and the crossing of IgG into the parenchyma. In the ipsilateral (injured) side, costaining of human IgG, and blood vessels demonstrates intravascular localization of infused IgG as well as its parenchymal deposition, whereas in the contralateral side human IgG was localized exclusively within the blood vessels (Fig. 2b). The parenchymal presence of human IgG was confirmed by colocalization of human IgG and IBA1, a specific glial cell marker (Fig. 2c).
Fig. 2.
The integrity of the BBB is compromised in ischemic brain regions. (a) Staining for human IgG (green fluorescence) in brain sections from the site of infarction and the corresponding site from nonaffected hemisphere. (Scale bars: 100 μM.) (b) Double staining for collagen IV, blood vessel marker (red fluorescence), and human IgG (green fluorescence) reveals the presence of human IgG in both blood vessels and parenchyma at the site of injury and intravascular localization in the contralateral side. (c) Double staining for glial cell marker IBA1 (red fluorescence) and human IgG (green fluorescence) and their colocalization in the ipsilateral side.
Direct and Indirect Role of Complement in Brain I/R Injury.
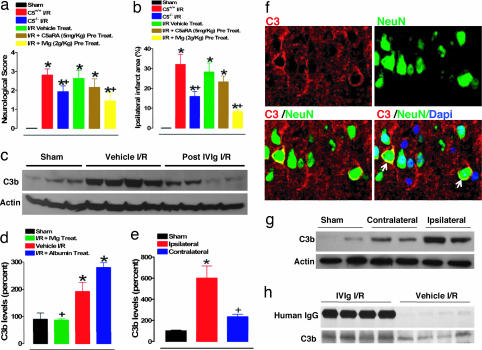
To evaluate the role of complement in this model of stroke, we subjected mice lacking the C5 gene (C5−/−) to I/R brain injury and determined they exhibited improved functional outcome and less brain damage compared with their wild-type littermates (Fig. 3 a and b). Although treatment of mice with a C5aR antagonist did not result in a statistically significant improvement of the stroke outcome, there was a trend toward protection (Fig. 3 a and b). We also measured the levels of the active fragment of the third complement component, C3b, in brain samples of mice subjected to I/R. We found this injury caused an increase of C3b compared with sham-manipulated mice (Fig. 3 c–e) and also that C3b overexpression was confined to the site of injury, because the levels of C3b in samples from the corresponding contralateral side of the brain were much lower than in samples from the ipsilateral (infarcted) side and not significantly different from baseline values found in sham controls (Fig. 3 e and g). IVIG treatment resulted in a highly significant suppression of stroke-induced increases in C3b levels (Fig. 3 c and d). Vehicle (Fig. 3 c and d) and albumin (Fig. 3d and SI Fig. 7a) had no effect on the C3b levels. Double-immunofluorescent staining of brain section from infarcted regions shows up-regulation of intrinsic neuronal C3 at the membrane level (Fig. 3f, SI Fig. 8) and cytoplasmic pattern of C3 expression in nonneuronal brain cells (SI Fig. 8). immunoprecipitation analysis of brain samples from the ischemic hemisphere of four IVIG-treated mice showed that human IgG bound mouse C3b. Immunoprecipitate from identical samples of vehicle-treated mice most likely represents an association of mouse C3b and host IgG (Fig. 3h). Coimmunoprecipitation data support scavenging of complement fragments as the protective mechanism of action of IVIG in this model.
Fig. 3.
Contribution of C5 and C3 complement components to pathophysiology of experimental stroke and attenuation with IVIG. Neurological scores (a) and infarct sizes (b) 24 h after I/R brain injury in wild-type (C5+/+) sham-operated mice (n = 8) and the following groups of mice subjected to I/R stroke: C5+/+ mice (n = 10), C5−/− mice (n = 10), vehicle-treated C5+/+ mice (n = 12), C5a receptor antagonist (C5aRA)-treated C5+/+ mice (n = 8), and IVIG-treated C5+/+ mice (n = 10). C5aRA and IVIG were administered 30 min before onset of ischemia; *, P < 0.001 compared with the sham value; +, P < 0.05 compared with the C5+/+ I/R value. (c) Proteins in cerebral cortex samples from mice subjected to sham surgery (n = 3), vehicle I/R (n = 4), and IVIG-treated at 2 g/kg administered 1 h after stroke (n = 4) were immunoblotted by using anti-C3b and antiactin antibodies. (d) C3b levels determined by densitometric analysis of immunoblots (normalized to actin levels and expressed as a percentage of the mean value for sham-treated mice) in infarcted brain region compared with sham (*, P < 0.01) and vehicle-treated (+, P < 0.01) controls. Quantification of C3b levels (e) and immunoblots (g) in the ischemic area as compared with the corresponding contralateral area; *, P < 0.01 compared with the sham value; +, P < 0.01 compared with the vehicle I/R value. (f) Staining for C3 (red membranous fluorescence), neuronal nuclear marker NeuN (green fluorescence), and double staining including nonneuronal nuclear staining (DAPI) show expression of intrinsic neuronal C3 in the infarcted hemisphere (arrows). (h) Coimmunoprecipitation of human IgG and mouse C3 in the infarcted brain sample.
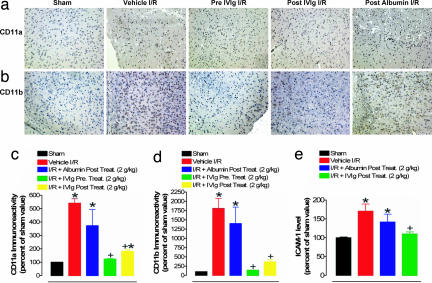
Next, we used immunohistochemistry to measure relative levels of ICAM-1, CD11a, and CD11b in brain sections of I/R mice as well as in those treated with IVIG and control reagents. The I/R-induced increases in expression of these molecules were all significantly attenuated by IVIG but not vehicle and albumin, treatment (Fig. 4 and SI Fig. 7b).
Fig. 4.
IVIG treatment suppresses endothelial cell adhesion, lymphocyte infiltration, and microglial activation after a stroke. Representative images of CD11a (a) and CD11b (b) immunoreactivity and corresponding quantitative analysis (c and d) in the indicated groups of mice. (e) Densitometry analysis of ICAM-1 immunoblots; *, P < 0.001 compared with the sham groups; +, P < 0.001 compared with vehicle-treated I/R value.
IVIG Treatment Does Not Affect Body Temperature or Blood Pressure.
Because changes in body temperature and blood pressure may affect the outcome of stroke, we measured these physiological parameters in IVIG-treated and control animals 15 min before induction of I/R injury (−15-min time point) and at 3, 12, and 24 h after treatment (administered 3 h after the end of 1-h occlusion of MCA). Blood pressure and rectal temperature measurements at these time points showed no significant increase or decrease compared with preocclusion (baseline) values; the same was observed in the vehicle- (SI Table 2) and albumin-treated groups of mice (data not shown). The lack of effects of IVIG on body temperature and blood pressure suggests its beneficial effects in stroke are due mainly to its central actions.
IVIG Directly Protects Cultured Neurons Against Ischemia-Like Conditions.
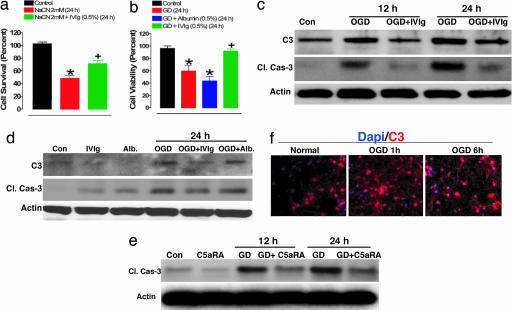
We next used a cell culture model of ischemic neuronal injury in which primary rat cortical neurons were subjected to conditions mimicking in vivo occlusion of a cerebral artery, including lack of oxygen, glucose deprivation (GD) and combined oxygen deprivation (OD) and GD (OGD). Treatment with IVIG significantly protected neurons from death mediated by cyanide-induced OD as well as GD (Fig. 5 a and b), whereas addition of albumin had no effect (Fig. 5b). We next exposed neurons to OGD by using glucose-free medium and incubation in oxygen-free atmosphere and assessed the expression of neuronal C3 in relation to cell survival. OGD caused an increase of C3 levels in cultured neurons within 12 h as compared with control neurons exposed to normal culture conditions (presence of glucose and 95% air/5% CO2). Increased expression of C3 was associated with a parallel increase in levels of cleaved (enzymatically active) caspase-3, a marker of apoptosis (Fig. 5c), and a progressive decrease in neuronal viability (not shown), suggesting the involvement of C3 in apoptotic cell death. Intrinsic neuronal C3 up-regulation was visualized by immunocytochemical staining (Fig. 5f). Treatment with IVIG suppressed the OGD-induced increases in C3 as well as activated caspase-3 levels (Fig. 5c; SI Fig. 9 a–d). Treatment of cultured neurons with albumin did not affect levels of neuronal C3 and caspase-3 and did not protect them from being killed (Fig. 5 b and d; SI Fig. 9 c and d). Treatment of neurons with the C5aR antagonist also suppressed caspase-3 activation induced by GD (Fig. 5e). Taken together, our in vitro data suggest a critical role for complement activation in ischemic neuronal death, and that therapy targeting the intrinsic neuronal complement cascade can directly protect against these events.
Fig. 5.
IVIG protects cultured primary cortical neurons against apoptosis induced by glucose and OD. (a) Cell survival in neurons cultured with vehicle (5% sorbitol) added to the culture medium (control), culture medium containing sodium cyanide (NaCN), and culture medium containing NaCN and supplemented with 0.5% IVIG (NaCN + IVIG); neuronal survival was quantified 12 h later. Values are the mean and SEM (n = 12–18 cultures); *, P < 0.01 compared with the control value; +, P < 0.05 compared with the values for neurons exposed to NaCN. (b) Neurons were incubated for 24 h in the medium with glucose (control), in the medium lacking glucose (GD), and in the medium lacking glucose supplemented with IVIG (GD + IVIG) and albumin (GD + albumin) at 0.5%; cell viability was quantified by using Alamar blue; *, P < 0.01 compared with the control value; +, P < 0.05 compared with the values for neurons exposed to GD. (c) Immunoblot showing kinetics of increase of caspase-3 cleavage product and complement 3 (C3) in neurons exposed to combined oxygen and GD and incubated with IVIG or albumin (d). (e) Immunoblot showing the effect of C5a receptor antagonist (C5aRA) on the level of caspase-3 cleavage product in neurons subjected to GD. (f) C3 staining (red fluorescence) of cultured neurons exposed to OGD shows a signal increase at early time points relative to neurons cultured under normal conditions.
Discussion
Our results provide preclinical evidence supporting the possibility of using IVIG as a stroke therapy targeting the complement system. Pre- and post-IVIG therapy practically eliminated mortality in mice subjected to experimental stroke and reduced the size of brain infarction by 50–60%. Moreover, not only was the infarcted area reduced, but also neurons were spared within this ischemic region, and only occasional cell loss was observed. This had an impact on functional outcome, which was significantly improved over control animals. Because stroke survivors are left with severe disability, our findings imply that the neuron-sparing effect of IVIG could reduce the neurological consequences of stroke in humans. The effect of IVIG was specific for the Ig content, because neither 5% sorbitol (the vehicle or stabilizing solution) nor albumin in equal concentrations had any protective effect in the I/R model, and albumin had only a small beneficial effect in the permanent model. However, it has been reported that albumin, when infused into rats immediately after reperfusion or up to 4 h after onset of MCAO, significantly reduced total brain infarction volumes by ≈60% (15). Similarly, albumin treatment 2 h after the onset of permanent MCAO (PMCAO) resulted in significant reduction of infarction size and neurological deficit (16). It has been suggested that the neuroprotective effect of albumin is mediated by improved local cerebral blood flow to affected regions (17) and by mobilization of polyunsaturated fatty acids as an alternative source of energy in ischemic regions (18). Regardless of the reason (species difference, perhaps) for this discrepancy in effectiveness of albumin in experimental stroke, should both albumin and IVIG prove to be effective in stroke clinical trials, two therapies that are beneficial through different mechanisms would be available.
Our data suggest that the superior efficacy of IVIG is due to its ability to selectively bind complement components. Deficiency of the parent molecule, complement C5, reduces adverse consequences of transient MCAO. Furthermore, levels of the C3b fragment (known to be crucial for continuation of the complement cascade and generation of cell-destroying terminal membrane complex) were increased at the site of injury but not in the contralateral side of the brain. This indicates focal in situ complement activation. Of particular interest is the finding that OGD resulted in an increased expression of the main complement component C3 in cultured primary neurons, which was associated with a parallel increase in caspase-3 activation, a marker of apoptosis. Complement has been implicated in apoptosis (19), but this previously undescribed finding links ischemia with overexpression of neuronal complement cascade components and activation of apoptotic pathway in neurons under conditions mimicking in vivo obstruction of a cerebral artery.
Active complement fragments are known to induce expression of proinflammatory molecules that play a role in magnifying the inflammatory reaction triggered by complement. C5a up-regulates CD11b (5), whereas ICAM-1 and CD11a are up-regulated by iC3b, a further degradation product of C3b (20). We found that IVIG treatments suppressed ischemia-induced increases in the levels of ICAM-1, CD11a, and CD11b, markers of endothelial cell adhesion, lymphocyte infiltration, and microglial activation, respectively, in the ischemic cortex adjacent to the brain infarction. Taken together, our data indicate that, in this model of stroke, complement fragments generated under ischemic conditions presumably led to either direct neuronal necrosis via generation of terminal membrane attack complex mediated by C3b/C5b and/or apoptotic cell death, as indicated by the rise in levels of activated caspase-3 that was linked to increased levels of neuronal C3. Furthermore, complement activation fragments may contribute to neuronal loss by triggering an inappropriate inflammatory reaction that can damage neuronal cells via release of proteases and other mediators of inflammation from cells recruited to the site of infarction.
Complement activation is implicated in current interventional therapeutic approaches for stroke. Recombinant tissue plasminogen activator, a thrombolytic agent used to treat some stroke patients, causes a striking increase in C3a and C5a levels (21). Although reinstitution of blood flow through a recanalized brain artery may improve immediate outcome, complement activation via enzymatic reperfusion could trigger a chronic inflammatory response ultimately leading to restenosis and recurrence of stroke. Similarly, carotid endarterectomy, a procedure aimed at reducing the risk of stroke in patients with advanced plaque buildup in the carotid artery, causes local vascular inflammatory changes with deposition of complement components C3 and C9 in the vascular wall and subsequent incidence of restenosis of 15–20% (22).
The rationale to evaluate the therapeutic effect of IVIG was its capacity to bind and neutralize both small- (12) and large-complement fragments (23) and its well established safety. However, it has been reported that, when infused at high rate and optimal doses (that can reach up to 2 g/kg of body weight), IVIG can substantially increase blood viscosity. In certain at-risk patients with high blood pressure and advanced atherosclerosis, occurrence of thrombotic events was associated with changes in viscosity after high-dose IVIG (14). We used IVIG at a dose that is four times less than the maximal used in humans and found it was equally protective against the effects of I/R injury. This finding eliminates the concern of increased blood viscosity and possible adverse effect due to therapy itself. We believe the effectiveness of low-dose IVIG was related to the higher concentration of infused Ig molecules at the site of injury as compared with the contralateral side of the brain, as shown by direct quantification of human IgG in brain tissue samples from both hemispheres and visualized by immunohistochemistry. It is likely that the focal increase of complement activation attracts more IgG molecules to the site of injury from brain vessels damaged by hypoxia. Despite the low dose that was infused systemically, accumulation of infused Ig at the site of infarction allows scavenging of C3b and other complement fragments to proceed effectively. The long half-life of Ig molecules (up to 21 days) and subsequent prolonged stay at the site of brain injury would be an added safeguard mechanism against further inflammatory responses triggered by possible repetitive episodes of complement activation. In light of the extensive clinical experience with IVIG for other indications, our data justify consideration of the development of clinical trials to evaluate the use of IVIG in human stroke patients.
Methods
Focal Cerebral I/R Injury.
Three-month-old male mice were subjected to transient middle cerebral artery occlusion/reperfusion, as reported (13). A brief description of the model is provided in SI Text.
PMCAO.
PMCAO was performed by following a standard procedure (24), the details of which are provided in SI Text. Both procedures were approved by the National Institute on Aging Animal Care and Use Committee.
Neurological Assessment.
The functional consequences of focal cerebral I/R injury were evaluated by using a five-point neurological deficit score (0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; and 4, unable to walk spontaneously) and were assessed in a blinded fashion.
Quantification of Cerebral Infarction.
Two-millimeter-thick coronal brain sections taken after 72 h of reperfusion were stained with 2% 2,3,5-triphenyltetrazolium chloride and evaluated for infarct size by using standard methodology (25).
Quantification of Human IgG in Brain Tissue Samples.
Brain tissue samples from three IVIG-treated animals were removed from the site of infarction and the corresponding site on the contralateral noninjured side of the brain. Samples were liquefied by sonication, and the concentration of human IgG in each sample was determined after 24-h radial immunodiffusion according to the manufacturer's instructions (The Binding Site, San Diego, CA).
C5aR Antagonist.
The small-molecular-weight C5a receptor antagonist AcF-[OpdChaWR] was synthesized as described (26). The receptor affinity and antagonistic potency were determined on intact human polymorphonuclear leukocytes.
IVIG Preparation.
The IVIG preparation (Flebogamma) used in this work (Instituto Grifols) is a 5% protein solution containing >99% IgG, <0.05 mg/ml IgA, and a trace amount of IgM. Igs are stabilized with 5% sorbitol.
Neuronal Cell Cultures, Chemical Hypoxia, and Neuronal Survival.
Dissociated cell cultures of neocortical fragments were established from 18-day Sprague–Dawley rat embryos as described (27). Approximately 95% of the cells in such cultures were neurons, and the remaining cells were astrocytes. Chemical hypoxia was accomplished by exposing cultures to sodium cyanide (Sigma, St. Louis, MO). Cell survival was evaluated by incubating them for 30 min with DNA-binding Hoechst stain (Roche, Nutley, NJ) and quantified by fluorescence microscopy. Neuronal cell viability was determined by a mitochondrial activity assay using Alamar blue solution (Roche), as described (28).
OGD.
For GD, the cultured neurons were incubated in glucose-free Locke's medium for 24 h. For OGD, neurons were incubated in glucose-free Locke's medium in an oxygen-free chamber with 95% N2/5% CO2 atmosphere for either 12 or 24 h. The OGD procedure we used in the present study involved hypoxia, but not complete anoxia, because we did not purge the culture medium with nitrogen. Therefore, a longer time period of exposure to the OGD (hypoxia/GD) condition is required to kill neurons, compared with anoxia/GD.
Control cultures were incubated in Locke's medium containing 10 mM glucose under a 95% air/5% CO2 atmosphere. Cell survival was quantified as described above.
Immunoblotting and Immunohistochemistry.
Immunostaining of blots and frozen brain sections was performed according to standard protocols. Details related to primary antibodies used are provided in SI Text.
Statistical Analyses.
Statistical comparisons were made by using Student's t test, Fisher's exact test, ANOVA, and Newman–Keuls post hoc tests for pairwise comparisons.
Supplementary Material
Acknowledgments
We thank Dr. Juan Jorquera (Instituto Grifols, Barcelona, Spain) and Instituto Grifols for providing IVIG for this study, Haiyang Zhu for technical assistance, and Craig Morrissette for the help with statistical analysis. This research was supported by the National Institute on Aging Intramural Research Program of the National Institutes of Health (NIH) and NIH Grant HL26441 (to D.N.G.).
Abbreviations
- MCAO
middle cerebral arterial occlusion
- IVIG
i.v. Ig
- I/R
ischemia/reperfusion
- BBB
blood–brain barrier
- GD
glucose deprivation
- OD
oxygen deprivation
- OGD
combined OD and GD
- PMCAO
permanent MCAO.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700506104/DC1.
References
- 1.Adams HP. J Hypertens. 2003;(Suppl 21):S3–S7. doi: 10.1097/00004872-200306005-00002. [DOI] [PubMed] [Google Scholar]
- 2.D'Ambrosio AL, Pinsky DJ, Connolly ES. Mol Med. 2001;7:367–382. [PMC free article] [PubMed] [Google Scholar]
- 3.Ember J, Hugli T. Curr Top Microbiol Immunol. 1989;153:181–208. doi: 10.1007/978-3-642-74977-3_10. [DOI] [PubMed] [Google Scholar]
- 4.DeBoer JP, Wolbink GJ, Thijs LG, Boars JW, Wagstaff J, Hack CE. Immunopharmacology. 1992;24:135–148. doi: 10.1016/0162-3109(92)90019-9. [DOI] [PubMed] [Google Scholar]
- 5.Foreman KF, Glovsky MM, Warner RI, Horvath SJ, Ward PA. Inflammation. 1996;20:1–8. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- 6.Van Beek J, Bernandin M, Petit E, Gasque P, Nouvelot A, Mackenzie ET, Fontaine M. Exp Neurol. 1992;161:117–125. doi: 10.1006/exnr.1999.7273. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg PJ, Öhman J, Lehto T, Wuorimaa T, Meri S, Karjalainen-Lindsberg ML, Paetau A, Carpén O, Kaste M. Ann Neurol. 1996;40:587–596. doi: 10.1002/ana.410400408. [DOI] [PubMed] [Google Scholar]
- 8.Nishino H, Czurko A, Fukuda A, Hida H, Hashitani T, Karadi Z, Lenard L. Brain Res Bull. 1994;35:51–55. doi: 10.1016/0361-9230(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa F, Gordon LE, Feldhoff FW, Lassiter HA. Neurosci Lett. 2005;380:48–53. doi: 10.1016/j.neulet.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Al-Buhairi AR, Jan MM. Saudi Med J. 2002;23:13–19. [PubMed] [Google Scholar]
- 11.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. J Am Med Assoc. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 12.Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, Szebeni J, Alving CR, Carroll M, Berkower I, et al. Nat Med. 2003;9:431–4438. doi: 10.1038/nm836. 13. [DOI] [PubMed] [Google Scholar]
- 13.Bederson JB, Pitts LH, Tsuiji M. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 14.Orbach H, Katz U, Sherer Y, Shoenfeld Y. Clin Rev Allergy Immunol. 2005;29:173–184. doi: 10.1385/CRIAI:29:3:173. [DOI] [PubMed] [Google Scholar]
- 15.Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Stroke. 2001;32:553–560. doi: 10.1161/01.str.32.2.553. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Belayev L, Zhao W, Busto R, Belayev A, Ginsberg MD. Eur J Pharmacol. 2001;428:193–201. doi: 10.1016/s0014-2999(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 17.Huh PW, Belayev L, Zhao W, Busto R, Saul I, Ginsberg MD. Brain Res. 1998;804:105–113. doi: 10.1016/s0006-8993(98)00674-x. 31. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez de Turco EB, Belayev L, Liu Y, Busto R, Parkins N, Bazan NG, Ginsberg MD. J Neurochem. 2002;83:515–524. doi: 10.1046/j.1471-4159.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- 19.Fishelson Z, Attali G, Mevorach D. Mol Immunol. 2001;38:207–219. doi: 10.1016/s0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- 20.Marks RM, Todd RF, Ward PA. Nature. 1989;339:314–317. doi: 10.1038/339314a0. [DOI] [PubMed] [Google Scholar]
- 21.Bennett WR, Yawn DH, Migliore PJ, Young JS, Pratt CM, Raizner AE, Roberts R, Bolli R. J Am Coll Cardiol. 1987;10:627–632. doi: 10.1016/s0735-1097(87)80206-1. [DOI] [PubMed] [Google Scholar]
- 22.Szeplaki G, Varga L, Laki J, Dosa E, Madsen HO, Prohaszka Z, Szabo A, Acsady G, Selmeci L, Garred P, et al. Thromb Haemost. 2006;96:529–534. [PubMed] [Google Scholar]
- 23.Basta M. Clin Exp Immunol. 1996;104:21–25. [PubMed] [Google Scholar]
- 24.Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Ann Neurol. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 26.Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. J Med Chem. 1999;42:1965–1974. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- 27.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White MJ, Dicaprio MJ, Greenberg DA. J Neurosci Methods. 1996;70:195–200. doi: 10.1016/s0165-0270(96)00118-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.