Abstract
Ca2+ influx into presynaptic nerve terminals activates synaptic vesicle exocytosis by triggering fast synchronous fusion and a slower asynchronous release pathway. In addition, a brief rise in Ca2+ after consecutive action potentials has been correlated with a form of short-term synaptic plasticity with enhanced vesicle fusion termed facilitation. Although the synaptic vesicle protein Synaptotagmin 1 (Syt1) has been implicated as the Ca2+ sensor for synchronous fusion, the molecular identity of the Ca2+ sensors that mediate facilitation and asynchronous release is unknown. To test whether the synchronous Ca2+ sensor, Syt1, or the asynchronous Ca2+ sensor is involved in facilitation, we analyzed whether genetic elimination of Syt1 in Drosophila results in a concomitant impairment in facilitation. Our results indicate that Syt1 acts as a redundant Ca2+ sensor for facilitation, with the asynchronous Ca2+ sensor contributing significantly to this form of short-term plasticity. We next examined whether other members of the Drosophila Syt family functioned in Ca2+-dependent asynchronous release or facilitation in vivo. Genetic elimination of other panneuronally expressed Syt proteins did not alter these forms of exocytosis, indicating a non-Syt Ca2+ sensor functions for both facilitation and asynchronous release. In light of these findings, the presence of two presynaptic Ca2+ sensors can be placed in a biological context, a Syt1-based Ca2+ sensor devoted primarily to baseline synaptic transmission and a second non-Syt Ca2+ sensor for short-term synaptic plasticity and asynchronous release.
Keywords: exocytosis, synapse, synaptic vesicle, Drosophila, synaptic plasticity
Fast communication at synapses depends on Ca2+-regulated neurotransmitter release at presynaptic nerve terminals (1). Ca2+ acts upon presynaptic Ca2+-binding proteins that transduce Ca2+ signals into a fusion of synaptic vesicles and a release of neurotransmitters. Three lines of evidence indicate the existence of two kinetically distinct Ca2+ sensors at most synapses, with one mediating a rapid, synchronous component of transmitter release, and a second underlying a slower, asynchronous component of fusion. First, the kinetics of evoked neurotransmitter release exhibit a biphasic decay that can be fitted by a double-exponential curve, revealing fast and slow components of release (2). Second, the divalent cation Sr2+ has been reported to have differential effects on the fast and slow components of neurotransmitter release (2, 3), although differential Sr2+ influx may also contribute to this effect (4). Third, in both mice and Drosophila mutants lacking the synaptic vesicle Ca2+-binding protein, Synaptotagmin 1 (Syt1), the fast synchronous component of release is abolished, whereas slow asynchronous release is enhanced (5–7). Taken together, kinetic, pharmacological, and genetic evidence support a two-Ca2+ sensor model for neurotransmitter release, where: (i) Syt1 functions as the synchronous Ca2+ sensor and suppresses release through the asynchronous pathway, and (ii) a residual Ca2+ sensor remaining in syt1-null mutants mediates asynchronous release. Although the asynchronous Ca2+ sensor is kinetically distinct from Syt1, neither its molecular identity nor its functional significance at synapses is known. It has been widely postulated that other isoforms of the large Syt family may mediate asynchronous release.
Like baseline synaptic transmission, Ca2+-regulated neurotransmitter release is also required for a form of short-term synaptic plasticity termed “facilitation.” Facilitation is an enhancement in synaptic transmission resulting from prior synaptic activity that lasts on a millisecond time scale (8). When observed with pairs of stimuli in which the second postsynaptic response is larger than the first, the phenomenon is termed “paired-pulse facilitation.” This form of short-term synaptic plasticity has been correlated with elevated residual Ca2+ in presynaptic terminals after an action potential (8). The molecular target on which Ca2+ ions act to mediate facilitation is unknown, although it was originally postulated that both evoked release and facilitation would share a common Ca2+ sensor (9). We tested this model by determining the role of the Drosophila panneuronal Syt proteins in paired-pulse facilitation and basal synaptic transmission. Our results indicate that a non-Syt Ca2+ sensor mediates asynchronous release and contributes to presynaptic facilitation.
Results
Both Syt1 and the Asynchronous Ca2+ Sensor Contribute to Presynaptic Facilitation.
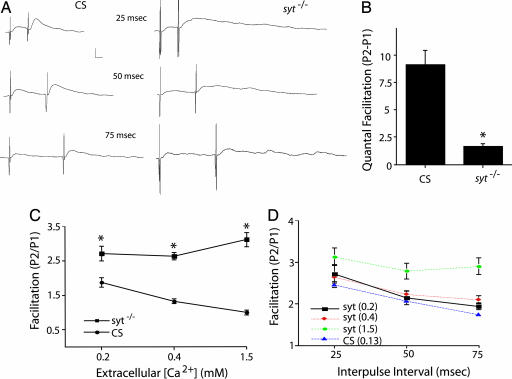
To investigate whether the synchronous Ca2+ sensor, Syt1, is involved in facilitation, we examined paired-pulse facilitation by performing intracellular recordings from Drosophila third-instar larval neuromuscular junctions (NMJs) of wild-type animals and syt1-null mutants (sytAD4/sytN13). Evoked neurotransmitter release in the absence of Syt1 is characterized by a dramatic reduction in the overall number of vesicle fusion events, compared with controls, and a shift from fast fusion to a slower and prolonged asynchronous release of vesicles (Fig. 1A). Given the reduced baseline synaptic transmission in syt1 mutant animals, we examined facilitation in wild-type controls at a reduced extracellular Ca2+ concentration of 0.13 mM, which gave a comparable evoked response to syt1-null mutants in 1.5 mM extracellular Ca2+. As shown in Fig. 1, a severe impairment of baseline synaptic transmission in the absence of the synchronous Ca2+ sensor did not result in a concomitant impairment of paired-pulse facilitation as measured by the ratio between two postsynaptic responses. Indeed, paired-pulse facilitation is enhanced in syt1 mutants compared with wild type across all extracellular Ca2+ levels examined (Fig. 1C), likely reflecting a depletion of readily releasable vesicles in controls and the enhanced function of the asynchronous Ca2+ sensor previously demonstrated in syt1 mutants (6, 7). Similar to wild type, the extent of facilitation in syt1-null mutants also decreases with increasing interpulse interval (Fig. 1D), indicating that the kinetics of facilitation is unaltered in the absence of Syt1. As observed in syt1-null mutants in response to a single action potential, release during paired-pulse stimulation remained asynchronous compared with controls.
Fig. 1.
Syt1 plays a redundant role in paired-pulse facilitation. Intracellular recordings of paired-pulse facilitation from Drosophila third-instar larval NMJs at muscle 6 of wild-type Canton S (CS) and syt1null mutants. (A) Representative traces at 1.5 mM extracellular Ca2+ in sytAD4/sytN13 and at 0.13 mM extracellular Ca2+ in wild type. (Calibration bar: 5 mV/5 ms.) (B) Quantification of quantal facilitation as measured by the difference between the numbers of quanta released in two postsynaptic responses at 25-ms interpulse intervals and 0.2 mM extracellular Ca2+. The number of muscles examined and the average resting potentials (avg. RP) in millivolts ± SEM for each genotype were as follows: CS (n = 4; avg. RP = −62.88 ± 3.68) and syt1 (n = 6; avg. RP = −59 ± 2.24). Data points are mean ± SEM; statistical significance (*, P < 0.05) was determined by Student's t test. (C) Quantification of facilitation, as measured by the ratio between two postsynaptic responses, at 25-ms interpulse intervals in control and syt1null mutants at 0.2, 0.4, and 1.5 mM extracellular Ca2+. Data points are mean ± SEM; statistical significance (*, P < 0.05) was determined by Student's t test. The numbers of muscles examined for each genotype were as follows: CS (0.2 mM Ca2+, 6; 0.4 mM Ca2+, 15; and 1.5 mM Ca2+, 8) and syt1 (0.2 mM Ca2+, 9; 0.4 mM Ca2+, 27; and 1.5 mM Ca2+, 17). Average muscle resting membrane potentials for each genotype were as follows: CS (0.2 mM Ca2+, 54.58 ± 1.34; 0.4 mM Ca2+, 59.48 ± 0.61; and 1.5 mM Ca2+, 62.38 ± 1.23) and syt1 (0.2 mM Ca2+, 56.83 ± 1.74; 0.4 mM Ca2+, 56.74 ± 0.69; and 1.5 mM Ca2+, 61.19 ± 1.37). (D) Quantification of facilitation, as measured by the ratio between two postsynaptic responses, at 25-, 50-, and 75-ms interpulse intervals in control at 0.13 mM extracellular Ca2+ and in syt1null mutant animals at 0.2, 0.4, and 1.5 mM extracellular Ca2+. The numbers of muscles examined for each genotype were as follows: CS (25 ms, 19; 50 ms, 19; and 75 ms, 18), syt1 at 0.2 mM Ca2+ (25 ms, 9; 50 ms, 7; and 75 ms, 8), syt1 at 0.4 mM Ca2+ (25 ms, 27; 50 ms, 24; and 75 ms, 22), and syt1 at 1.5 mM Ca2+ (25 ms, 17; 50 ms, 14; and 75 ms, 11). Average muscle resting membrane potentials for each genotype were as follows: CS (25 ms, 56.08 ± 0.54; 50 ms, 55.77 ± 0.53; and 75 ms, 56 ± 0.63), syt1 at 0.2 mM Ca2+ (25 ms, 56.83 ± 1.74; 50 ms, 57.18 ± 2.62; 75 ms, 57.75 ± 2.76), syt1 at 0.4 mM Ca2+ (25 ms, 56.74 ± 0.69; 50 ms, 56.46 ± 0.79; and 75 ms, 57.31 ± 0.81), and syt1 at 1.5 mM Ca2+ (25 ms, 61.19 ± 1.37; 50 ms, 60.71 ± 1.59; 75 ms, 58.56 ± 1.68).
Although facilitation persists in syt1-null mutants, binding of residual Ca2+ to Syt1 also might contribute independently from the asynchronous Ca2+ sensor to promote facilitation. Given the larger overall amount of release observed in controls compared with syt1 mutants, we tested whether Syt1 functions cooperatively with the asynchronous Ca2+ sensor to promote facilitation. For this experiment, we measured paired-pulse facilitation as the difference between quantal content for the two postsynaptic responses at identical 0.2 mM Ca2+ concentrations in both control and mutant animals. The syt1-null mutants showed a 5.6-fold reduction in the absolute number of vesicles released during the second pulse, compared with controls at identical extracellular Ca2+ (Fig. 1B). These results indicate that both the asynchronous Ca2+ sensor and Syt1 can mediate presynaptic facilitation of neurotransmitter release. As such, both proteins are likely to sense residual Ca2+ that occurs during paired stimuli.
Generation of Mutants Lacking Syt4 and Syt7.
Given that the asynchronous Ca2+ sensor contributes to facilitation, we tested whether the two remaining Drosophila panneuronal Syt proteins, Syt4 and Syt7 (10), function in presynaptic facilitation. It has been hypothesized that the asynchronous Ca2+ sensor at presynaptic terminals would be another member of the Syt family (11). Syt proteins form a family of C2 domain-containing proteins with seven members in Drosophila and >14 members in mammals (12). Among the family members in Drosophila, Syt4 and Syt7 are candidates for asynchronous Ca2+ sensors because they are the only isoforms of the family besides Syt1 to be panneuronally expressed and present in motor neurons (10).
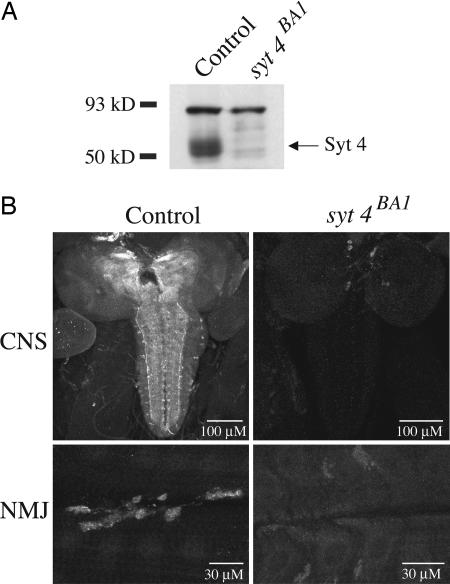
A P element (P{EPgy2}SytIVEY12073) inserted 100 bp upstream of the first exon of syt4 was used to generate syt4 mutations as previously described (13). To determine whether imprecise excisions removed the Syt4 protein, we performed Western blot analysis and immunohistochemistry using a Syt4 polyclonal antibody (10). Western blot analysis of adult head extract isolated from one such deletion, syt4BA1, indicated that the Syt4 protein was absent (Fig. 2A). Furthermore, immunostaining of syt4BA1 revealed a loss of anti-Syt4 immunoreactivity in both the CNS and muscles in wandering third-instar larvae (Fig. 2B), compared with precise excision controls (syt4pre1).
Fig. 2.
Generation of animals lacking Syt4. (A) Western blot of adult head extracts from control (precise excision) and syt4BA1 mutants. One fly-head equivalent of protein was loaded into each lane and blotted by using the α-Syt4 polyclonal antibody. Although several nonspecific bands remain and serve as loading controls, the syt4BA1 head extract is missing the abundant band corresponding to the predicted molecular weight of Syt4. (B) Immunostaining for Syt4 at larval brains (Upper) and at muscles 6 and 7 of the third-instar larval NMJ (Lower) in control and syt4BA1 mutants. Control and mutants were imaged by using identical confocal settings.
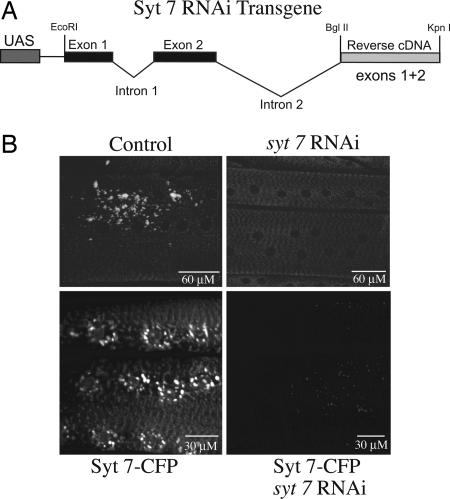
Because of the lack of P-element insertions in the vicinity of the syt7 locus on the fourth chromosome, we generated syt7 transgenic RNAi lines that express a genomic-cDNA syt7 RNAi fusion under control of the Gal4/UAS system (Fig. 3A). To test whether expression of the construct reduced Syt7 protein levels in vivo, we performed immunostaining experiments by using the Syt7 polyclonal antibody (12). As shown in Fig. 3B, muscle-specific expression of the syt7 RNAi construct with the myosin heavy chain (Mhc)-Gal4 driver reduced the levels of native Syt7 protein found on post-Golgi vesicles to below detection. To further validate the syt7 RNAi construct, we constructed transgenic lines that express a syt7–cyan fluorescent protein [(CFP) fused to the C-terminal of syt7] transgene under the control of an upstream activating sequence (UAS). When driven by Mhc-Gal4 driver, overexpression of this transgene mimicked the endogenous expression pattern of Syt7, with localization to a post-Golgi vesicle compartment that costained with lysotracker (Fig. 3B). We then tested for the ability of the syt7 RNAi construct to reduce the expression of the syt7-CFP transgene when coexpressed within muscles. CFP fluorescence measured by confocal microscopy in muscles 6 and 7 in larvae carrying the syt7 RNAi transgene was dramatically depleted compared with controls (Fig. 3B), suggesting that the RNAi transgene could efficiently target and eliminate syt7.
Fig. 3.
Generation of animals lacking Syt7. (A) Syt7 knockdown construct. The initial genomic region of the syt7 locus, including exons and introns 1 and 2, was fused to the reverse cDNA coding for exons 1 and 2, maintaining the splice acceptor and donor sites in the two introns. (B) (Lower) The third-instar larval muscles from white and Mhc-Gal4;syt7 RNAi larvae stained with the α-Syt7 polyclonal antibody. (Upper) Muscle-specific expression of a syt7–CFP transgene with or without coexpression of the syt7 RNAi transgene. Larvae coexpressing the two transgenes have dramatically reduced Syt7–CFP levels in muscles compared with control siblings.
Paired-Pulse Facilitation Remains Intact in syt4 and syt7 Loss-of-Function Animals.
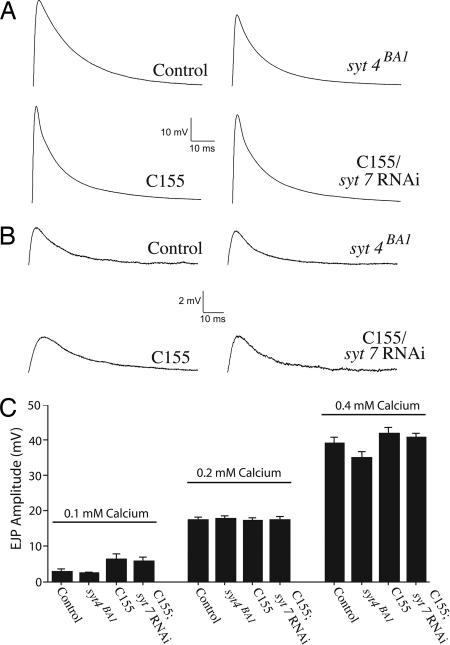
To test the hypothesis that Syt4 or Syt7 may function in synaptic vesicle fusion, we performed electrophysiological analysis on loss-of-function syt4 and syt7 animals. We first investigated whether loss of Syt4 or Syt7 caused defects in quantal content by measuring evoked excitatory junctional potentials (EJPs) at the third-instar NMJ at 0.2 and 0.4 mM extracellular Ca2+. As shown in Fig. 4 A–C, we did not observe any statistical differences in evoked EJP amplitudes between syt4 or syt7 animals (syt4BA1 and C155; syt7 RNAi) and controls (syt4pre1 and C155), indicating that robust neurotransmission is still intact in these mutants. Next, we investigated the possibility that loss of Syt4 or Syt7 may have more subtle effects on quantal content by measuring evoked EJPs at a low 0.1 mM extracellular Ca2+ concentration. Again, we did not detect any changes in the EJP amplitudes in syt4 or syt7 mutants (Fig. 4 B and C). These results indicate that loss of Syt4 or Syt7 does not affect basal synaptic transmission at the Drosophila third-instar NMJ.
Fig. 4.
Evoked neurotransmitter release in animals lacking Syt4 and Syt7. (A) Representative traces of evoked EJP at 0.4 mM extracellular Ca2+ in control and syt4BA1 mutants, as well as control (C155) and C155; syt7 RNAi knockdown animals. (B) Representative traces of evoked EJP at 0.1 mM extracellular Ca2+ in control and syt4BA1 mutants, as well as control (C155) and C155; syt7 RNAi knockdown animals. (C) Quantification of evoked EJP amplitudes in control and syt4null mutants, as well as control (c155) and C155; syt7 RNAi knockdown animals at 0.1, 0.2, and 0.4 mM extracellular Ca2+. Data points are mean ± SEM. The numbers of muscles examined and the average resting potential (avg. RP) in millivolts ± SEM for each genotype were as follows: control (0.1 mM Ca2+, n = 10, avg. RP = −64 ± 1.27; 0.2 mM Ca2+, n = 14, avg. RP = −60.3 ± 1.18; 0.4 mM Ca2+, n = 9, avg. RP = −69 ± 2), syt4null (0.1 mM Ca2+, n = 9, avg. RP = −68 ± 2.33; 0.2 mM Ca2+, n = 18, avg. RP = −59.43 ± 0.92; 0.4 mM Ca2+, n = 9, avg. RP = −66 ± 1.67), C155 (0.1 mM Ca2+, n = 8, avg. RP = −62 ± 2.12; 0.2 mM Ca2+, n = 30, avg. RP = −59 ± 0.7; 0.4 mM Ca2+, n = 9, avg. RP = −75 ± 2), and C155; syt7 RNAi (0.1 mM Ca2+, n = 8, avg. RP = −62 ± 1.41; 0.2 mM Ca2+, n = 26, avg. RP = −59.02 ± 0.82; 0.4 mM Ca2+, n = 9, avg. RP = −73 ± 1).
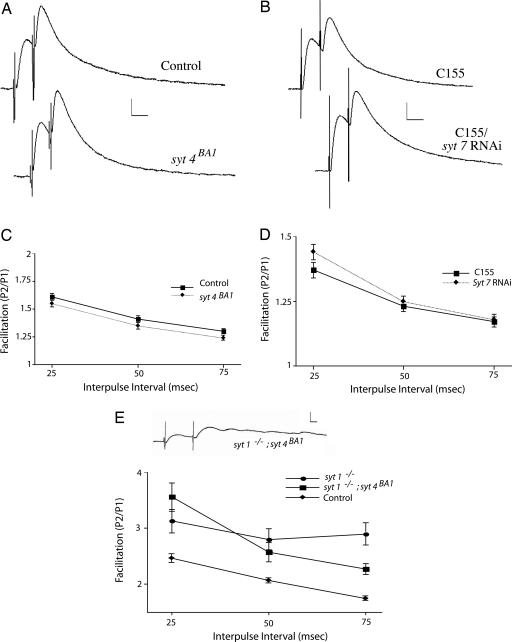
Having identified paired-pulse facilitation as an assay for the asynchronous Ca2+ sensor, we tested the possibility that syt4 or syt7 may function as the asynchronous sensor at synapses. We measured paired-pulse facilitation at larval NMJs in syt4-null mutants and syt7 RNAi knockdown animals (syt7 RNAi driven by the panneuronal driver C155) at a 0.2 mM extracellular Ca2+ concentration. As shown in Fig. 5 A–D, the amount of facilitation measured at 25-, 50-, and 75-ms interpulse intervals was statistically the same for all genotypes tested, indicating that neither syt4 nor syt7 contributes to paired-pulse facilitation.
Fig. 5.
Paired-pulse facilitation remains intact in syt4null mutants, syt7 RNAi knockdown animals, and syt1;syt4 double mutants. (A) Representative traces of facilitation at 0.2 mM extracellular Ca2+ in syt4BA1 mutants and control animals. (Calibration bar: 5 mV/20 ms; interpulse interval: 25 ms.) (B) Representative traces of facilitation at 0.2 mM extracellular Ca2+ in C155; syt7 RNAi knockdown and control (C155) animals. (Calibration bar: 5 mV/20 ms; interpulse interval: 25 ms.) (C) Quantification of facilitation at 25-, 50-, and 75-ms interpulse intervals in control and syt4null mutant animals at 0.2 mM extracellular Ca2+. The numbers of muscles examined and the average resting potential (avg. RP) in millivolts ± SEM for each genotype were as follows: control (25 ms, n = 12, avg. RP = −59.84 ± 1.31; 50 ms, n = 12, avg. RP = −58.54 ± 1.56; and 75 ms, n = 12, avg. RP = −59.46 ± 1.46) and syt4null (25 ms, n = 16, avg. RP = −59.88 ± 0.83; 50 ms, n = 16, avg. RP = −60.08 ± 0.86; and 75 ms, n = 15, avg. RP = −59.3 ± 0.8). (D) Quantification of facilitation at 25-, 50-, and 75-ms interpulse intervals in control (C155) and in C155; syt7 RNAi knockdown animals at 0.2 mM extracellular Ca2+. The number of muscles examined and the avg. RP for each genotype were as follows: control (25 ms, n = 26, avg. RP = −58.49 ± 0.65; 50 ms, n = 25, avg. RP = −58.07 ± 0.69; and 75 ms, n = 24, avg. RP = −58.41 ± 0.76) and C155; syt7 RNAi (25 ms, n = 20, avg. RP = −59.01 ± 0.96; 50 ms, n = 24, avg. RP = −59.34 ± 0.85; and 75 ms, n = 24, avg. RP = −59.3 ± 0.83). (E) A representative trace from syt1; syt4 double mutants in 1.5 mM extracellular Ca2+. (Calibration bar: 5 mV/5 ms.) Quantification of facilitation at 25-, 50-, and 75-ms interpulse intervals in control (CS) at 0.13 mM extracellular Ca2+ and in syt1; syt4 double mutants at 1.5 mM extracellular Ca2+ is shown. For comparison, facilitation in the syt1-null mutant alone at 1.5 mM extracellular Ca2+ is shown. The number of muscles examined and avg. RP for each genotype were as follows: CS (25 ms, n = 19, avg. RP = −56.08 ± 0.54; 50 ms, n = 19, avg. RP = −55.77 ± 0.53; and 75 ms, n = 18, avg. RP = −56 ± 0.63) and syt1; syt4 (25 ms, n = 11, avg. RP = −60.98 ± 1.15; 50 ms, n = 11, avg. RP = −60.32 ± 0.97; and 75 ms, n = 10, avg. RP = −59.7 ± 1.16).
Asynchronous Release and Paired-Pulse Facilitation Remain Intact in syt1;syt4 Double Mutants.
To further test the hypothesis that syt4 may encode the asynchronous Ca2+ sensor, we generated syt1; syt4 double-mutant animals and performed intracellular recordings from larval NMJs to examine paired-pulse facilitation and evoked release. Like syt1 mutants alone, syt1; syt4 double mutants displayed robust asynchronous release, indicating that Syt4 is not the residual asynchronous Ca2+ sensor remaining in syt1-null mutants. To examine facilitation and reduce any possible effects of reduced baseline synaptic transmission in syt1; syt4 double-mutant animals, we recorded from wild-type controls at a reduced 0.13 mM extracellular Ca2+ concentration, which gave a comparable response to syt1; syt4 double mutants in 1.5 mM extracellular Ca2+. As shown in Fig. 5E, paired-pulse facilitation remains intact in syt1; syt4 double mutants, with enhanced facilitation as observed in syt1 mutants alone (Fig. 1C). Similar to syt1 mutants alone, the release observed during paired-pulse stimulation in syt1; syt4 double mutants remained asynchronous compared with wild-type controls. These results confirm that Syt4 is not a residual asynchronous Ca2+ sensor and does not contribute to facilitation. We attempted similar experiments on syt1; syt7 double mutants. Although these larvae hatched and exhibited uncoordinated and slow movements, similar to syt1 mutant animals alone, they died during the first instar larval stage, preventing a detailed physiological analysis at the third-instar NMJ. However, the residual locomotion observed in double mutants suggests that Syt7, like Syt4, is not required for the asynchronous neurotransmitter release driving residual locomotion observed in syt1 mutants.
Discussion
Although the synaptic vesicle protein Syt1 has been implicated as the Ca2+ sensor for synchronous synaptic vesicle fusion, the molecular identity of the Ca2+ sensors that mediate facilitation and asynchronous release is unknown. It was originally postulated that both evoked release and facilitation would share the same Ca2+ sensor (9). We took a genetic approach to determine whether evoked release and facilitation are mediated by the same Ca2+ sensor, Syt1. Here we demonstrate that facilitation persists in Drosophila syt1 mutants, although the magnitude of facilitation is reduced. Our findings indicate that Syt1 acts as a redundant Ca2+ sensor, with a second asynchronous Ca2+ sensor to mediate facilitation. We also addressed whether other Syt family members act as the asynchronous Ca2+ sensor for facilitation. Syt proteins have two well characterized Ca2+-binding motifs known as C2 domains, which participate in multiple Ca2+-dependent interactions, including lipid (14) and SNARE complex binding (15). The distinct Ca2+ affinities for individual Syt isoforms have made them attractive candidates for mediating asynchronous release.
Among the Syt family, two strong candidates for the asynchronous Ca2+ sensor are Syt4 and Syt7, which are conserved through evolution (12) and highly expressed in the nervous system (10, 16). Syt7 localizes to the plasma membrane of PC12 cells when overexpressed, whereas endogenous Syt7 has been reported to be concentrated at presynaptic active zones of central synapses, where it was hypothesized to act as a plasma membrane Ca2+ sensor in synaptic vesicle exocytosis (17). Syt7 also exhibits the slowest disassembly kinetics of Ca2+–Syt membrane complexes (11), making it an attractive candidate for the asynchronous sensor. Similar to Syt7, several studies have suggested that Syt4 might function during synaptic vesicle exocytosis. Up-regulation of syt4 in PC12 cells alters fusion pore dynamics (18) and has been reported to influence the choice between “kiss and run” and full fusion (19).
To test whether Syt4 or Syt7 is the asynchronous Ca2+ sensor, we generated loss-of-function mutants in Drosophila and characterized their synaptic physiology at the NMJ. Neither null mutations in syt4 nor RNAi knockdown of syt7 in neurons alters basic synaptic transmission at the Drosophila NMJ or paired-pulse facilitation. These results are consistent with our earlier in vivo localization studies in Drosophila, which indicated that Syt4 localizes postsynaptically and that Syt7 fails to target to larval NMJ synapses (10). If neither Syt4 nor Syt7 participates in synaptic vesicle exocytosis in vivo, what are their endogenous functions? Recent experiments in our laboratory indicate that Syt4 regulates the Ca2+-dependent exocytosis of retrograde signals from the postsynaptic compartment, triggering changes in presynaptic release properties and structural plasticity (13). The localization of Syt7 to a putative lysosomal compartment raises the possibility that Syt7 is required for a more ubiquitous role in vesicular trafficking, which is important for plasma membrane repair processes, similar to its proposed role in mammals (20).
Because Syt4 and Syt7 do not encode the asynchronous Ca2+ sensor, it is difficult to conceive how any of the remaining four Drosophila Syt proteins encoded in the genome could supply this function. Syt-α and Syt-β are exclusively expressed in subsets of putative neurosecretory cells and are not present in motor neurons (10). Similar to mammals, the final two Syt proteins encoded in the Drosophila genome, Syt12 and Syt14, have conserved only 2 and 3 of the 10 Ca2+-coordinating aspartic acid residues within their C2 domains, respectively, indicating that these Syt proteins most likely function in Ca2+-independent trafficking pathways. In addition, their mRNAs are expressed at levels below detection in Drosophila embryos, and antisera to the proteins do not detect Syt12 or Syt14 protein at NMJ synapses (10). Our results complement a recent study that defined the protein content of mammalian synaptic vesicles (21), revealing Syt1 as the major synaptic vesicle Syt isoform. Besides Syt1, the authors also detected Syt2, -5, -12, and -17 by mass spectrometry on some synaptic vesicles. Syt2, -5, and -17 are mammalian-specific isoforms with no invertebrate homologs. Similar to Drosophila Syt12, mammalian Syt12 lacks most of the Ca2+-coordinating aspartate residues in its C2 domains and does not bind Ca2+ in vitro (22).
In conclusion, our genetic studies in Drosophila argue against the idea that any member of the Syt family encodes the asynchronous Ca2+ sensor, making a simplistic two-Syt Ca2+ sensor model of neurotransmitter release unlikely. While eliminating Syt1 as the only Ca2+ target for facilitation, our results indicate that the asynchronous Ca2+ sensor plays a significant role in this form of presynaptic plasticity. Although kinetically distinct in its Ca2+ binding, experimental evidence demonstrating the functional significance of the asynchronous Ca2+ sensor is lacking. The present study suggests a function for the asynchronous Ca2+ sensor in the biology of short-term synaptic plasticity. In light of these findings, the presence of two presynaptic Ca2+ sensors can now be placed in a biological context: a Syt1 Ca2+ sensor devoted to baseline synaptic transmission and a second asynchronous Ca2+ sensor that contributes to short-term presynaptic plasticity.
Materials and Methods
Drosophila Genetics.
Drosophila melanogaster was cultured on standard medium at 25°C. syt4pre1 is a precise excision used as a control for syt4BA1. The syt7 RNAi is a transgenic RNAi knockdown for syt7. The C155 elav-GAL4 driver was used for neuronal expression of transgenes.
Generation of Syt7 Mutants by Transgenic RNAi.
An ≈3.2-kb genomic fragment consisting of the first two exons and introns (including all splice donor and acceptor sites) of the syt7 locus was PCR-amplified from genomic DNA and ligated into the pUAST vector. The reverse cDNA sequence encoding the first two exons (≈300 bp) of syt7 was then PCR-amplified from a cDNA library and directionally cloned into a pUAST-Syt7 genomic clone.
Electrophysiological Analysis.
Electrophysiological analysis of wandering third-instar larva was performed in Drosophila saline [70 mM NaCl/5 mM KCl/4 mM MgCl2/10 mM NaHCO3/5 mM trehalose/115 mM sucrose/5 mM Hepes-NaOH (pH 7.2)] modified from HL3 by using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA) at 22°C as previously described (23). Ca2+ concentrations varied with experiment and are indicated in the figure legends. Evoked EJPs were recorded intracellularly from muscle fiber 6 or 7 of segments A3–A5 under current clamp. Paired-pulse facilitation was measured by determining the peak amplitude response occurring within a 20-ms interval after stimulation. Stimulation pairs with no response to the first stimulus were not included in the analysis. All error bars indicate SEM. Statistical significance (P < 0.05) was determined by Student's t test.
Immunostaining and Western Blot Analysis.
Immunostaining was performed on third-instar larvae at wandering stage after rearing at 25°C as described previously (24). The dilution of primary antibodies was as follows: Syt1 (1:1,000), Syt4 (1:500), and Syt7 (1:1,000). Immunoreactive proteins were visualized on a Zeiss Pascal Confocal by using fluorescent secondary antibodies (Molecular Probes, Eugene, OR; Chemicon, Temecula, CA; Jackson ImmunoResearch, West Grove, PA). Western blots were done by using standard laboratory procedures. All antibodies were used at a 1:1,000 dilution.
Acknowledgments
This work was supported by National Institutes of Health grants and the Packard Foundation (J.T.L.).
Abbreviations
- CFP
cyan fluorescent protein
- CS
Canton S
- EJP
excitatory junctional potential
- Mhc
myosin heavy chain
- NMJ
neuromuscular junction
- UAS
upstream activating sequence.
Footnotes
The authors declare no conflict of interest.
References
- 1.Katz B. The Release of Neural Transmitter Substances. Liverpool: Liverpool Univ Press; 1969. [Google Scholar]
- 2.Goda Y, Stevens CF. Proc Natl Acad Sci USA. 1994;1994:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustine GJ, Eckert R. J Physiol (London) 1984;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu-Friedman MA, Regehr WG. J Neurosci. 2000;20:4414–4422. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 6.Yoshihara M, Littleton JT. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- 7.Nishiki T, Augustine GJ. J Neurosci. 2004;24:6127–6132. doi: 10.1523/JNEUROSCI.1563-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zucker RS, Regehr WG. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 9.Del Castillo J, Katz B. J Physiol (London) 1954;124:574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adolfsen B, Saraswati S, Yoshihara M, Littleton JT. J Cell Biol. 2004;166:249–260. doi: 10.1083/jcb.200312054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER. Proc Natl Acad Sci USA. 2005;102:5210–5214. doi: 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adolfsen B, Littleton JT. Cell Mol Life Sci. 2001;58:393–402. doi: 10.1007/PL00000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- 14.Brose N, Petrenko AG, Sudhof TC, Jahn R. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 15.Davis AF, Bai J, Fasshauer D, Wolowick MJ, Lewis JL, Chapman ER. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 17.Sugita S, Han W, Butz S, Liu X, Fernandez-Chacon R, Lao Y, Sudhof TC. Neuron. 2001;30:459–473. doi: 10.1016/s0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 18.Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- 19.Wang CT, Lu JC, Bai J, Chang PY, Martin TF, Chapman ER, Jackson MB. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Maximov A, Shin OH, Liu X, Sudhof TC. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P, Saraswati S, Guan Z, Watkins CJ, Wurtman RJ, Littleton JT. J Neurosci. 2004;24:4518–4529. doi: 10.1523/JNEUROSCI.0542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieckhof GE, Yoshihara M, Guan Z, Littleton JT. J Biol Chem. 2003;278:41099–41108. doi: 10.1074/jbc.M306417200. [DOI] [PubMed] [Google Scholar]