Abstract
Colonies of social wasps, ants, and bees are characterized by the production of two phenotypes of female offspring, workers that remain at their natal nest and nonworkers that are potential colony reproductives of the next generation. The phenotype difference includes morphology and is fixed during larval development in ants, honey bees, and some social wasps, all of which represent an advanced state of sociality. Paper wasps (Polistes) lack morphological castes and are thought to more closely resemble an ancestral state of sociality wherein the phenotype difference between workers and nonworkers is established only during adult life. We address an alternative hypothesis: a bias toward the potential reproductive (gyne) phenotype among Polistes female offspring occurs during larval development and is based on a facultatively expressed ancestral life history trait: diapause. We show that two signatures of diapause (extended maturation time and enhanced synthesis and sequestration of a hexameric storage protein) characterize the development of gyne offspring in Polistes metricus. Hexameric storage proteins are implicated in silencing juvenile hormone signaling, which is a prerequisite for diapause. Diverging hexamerin protein dynamics driven by changes in larval provisioning levels thereby provide one possible mechanism that can cause an adaptive shift in phenotype bias during the Polistes colony cycle. This ontogenetic basis for alternative female phenotypes in Polistes challenges the view that workers and gynes represent behavior options equally available to every female offspring, and it exemplifies how social insect castes can evolve from casteless lineages.
Keywords: caste development, hexameric storage proteins, juvenile hormone, life-history pleiotropy, social behavior
Social insects often show pronounced differences in behavior, physiology, and morphology between castes of workers (functionally sterile helpers) and reproductives (1). To learn how these phenotypes developed from originally casteless ancestors is considered a key to understanding insect social evolution (2). In most social insect taxa, castes represent alternative intra-specific phenotypes that are environmentally, not genetically, controlled. Therefore, they must represent trait ensembles governed by highly pleiotropic physiological regulators that are sensitive to environmental variables (3). These regulators may have evolved de novo, but it is more likely that they governed alternative or sequential stages in the life cycle of solitary ancestors, as proposed for juvenile hormone and vitellogenin (yolk precursor) that have conserved reproductive roles in solitary insects yet also regulate highly social behavior in essentially sterile worker honey bees (4). However, deeper understanding of caste origins may be more readily accessible by study of less-advanced social insects, such as Polistes paper wasps, in which offspring can be biased into worker and reproductive roles without absolute caste determination.
We proposed that nutrient-sensitive diapause physiology in Polistes underlies a phenotype bias established during larval ontogeny (5). The bias is hypothesized to be driven by larval provisioning and diverges onto two developmental pathways commonly recognized as workers of the current generation and potential reproductives of the next generation (gynes). Diapause, a major alternative life history syndrome of many insects, likely reflects the action of highly pleiotropic regulators including juvenile hormone (6, 7). Diapause is characterized by prolonged development and lifespan, resistance to cold and stress, delayed reproduction, and energy and nutrient sequestration (6, 8) (often as hexameric storage proteins, e.g., see refs. 9 and 10). Nondiapause is associated with the opposite of these. In insects, diapause expression is facultative (7), but in some species not all individuals diapause (not all experience the diapause-inducing cue). Some species show gradients of diapause expression that correspond to environmental variables, as exemplified by some sweat bees (11, 12), a solitary wasp (13), and Drosophila melanogaster (14–16), whereas in others diapause reflects nutritional differences among either larvae (e.g., the comma butterfly; see ref. 17) or adults (e.g., the boll weevil; see ref. 10). Expression of the diapause syndrome is conditional on low circulating levels of juvenile hormone (6, 7, 18) and responds to selection in D. melanogaster (14).
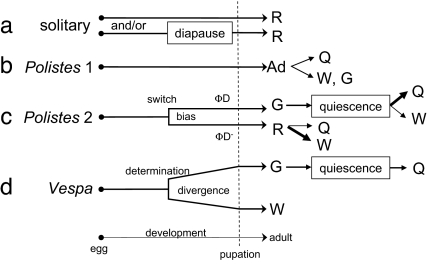
Although nondiapause and diapause phenotypes reflect adaptation to local ambient environments in most insects (14), there are indications that the regulatory networks that underlie diapause provide the foundation for a major developmental switch: the divergence of alloparental worker and potentially reproductive gyne castes in some social Hymenoptera (Fig. 1). Larval diapause correlates with gyne potentiation in some ant species (19). Adult diapause (18) (i.e., reproductive diapause; see ref. 6), which often coincides with behavioral quiescence (20), has been speculated to underlie characteristics of gyne offspring in the paper wasp genus Polistes (21–24), a social insect that lacks discrete morphological castes (25). However, the occurrence of early gynes (26) and late workers (27) in Polistes (5) argues against the role of conventional ambient variables, such as photoperiod or temperature, as diapause cues in this taxon. Instead, experimental evidence points to nourishment: low vs. high food levels during rearing in a common laboratory environment led to putative worker and gyne phenotypes, respectively, as confirmed by differential survival of a cold test (28, 29).
Fig. 1.
Caste dynamics in solitary and independent-founding Vespidae. Female solitary vespid wasps (a) develop directly from egg to reproductive adult (R), or larvae pass an inclement season in developmental diapause before emerging as reproductive adult. Some solitary vespids have only one of these pathways; some have both (61, 62). Vespa (d) exemplifies independent-founding Vespinae. Caste (G, gyne; W, worker) in Vespa is determined by the end of the third larval instar (63), and morphological divergence occurs in fourth and fifth instars (29). Gynes emerge in reproductive diapause, pass an inclement season (winter) in quiescence, and become foundresses and then queens (Q) the following favorable season. Polistes females (b, Polistes 1) seem to be totipotent as adults (Ad) (64), among which queen/worker castes can be determined by inter-adult dominance interactions (65) that influence ovarian activity (66–68). In the present work, however, Polistes is proposed (c, Polistes 2) to exhibit a physiological switch during development leading to adult phenotypes expressing either reproductive diapause (ΦD) or nondiapause (ΦD−). ΦD− females are reproductive (R); although most (thick arrow) become workers that direct maternal behavior toward siblings in the presence of a reproducing female (most often their mother), some can become queens (5, 29); none live through the inclement (cold/dry) season. ΦD phenotype females emerge as gynes (G) in reproductive diapause; thus they do not show maternal behavior but pass the inclement season in quiescence. Some (thin arrow) may become workers (W) as subdominants in foundress associations the next year; many become queens (Q), either as solitary foundresses or as the dominant co-foundress. The developmental switch, thereby, creates a caste bias in Polistes but does not determine caste outcomes.
Support for the hypothesis of a diapause-driven caste bias in Polistes can be drawn from Polistes biology. P. dominulus workers live an average of 30–40 days (30), whereas gynes, which become the next generation's foundresses and queens, live 250–500 days (31). P. exclamans gynes survived a cold test at 4.5-fold worker survival (32), and delayed reproduction is the hallmark of gynes, which do not reproduce until colony initiation the next year (33). Abundant fat has been used to distinguish gynes from workers of P. exclamans (32, 34), and storage proteins have been found in gynes but not workers of several Polistes species (35). These characteristics show clear correspondence to nondiapause and adult diapause (18) phenotypes of insects.
However, despite the fundamental importance of understanding the mechanistic basis for social behavior (36), and despite the recent hypothesis that diapause physiology underlies the worker/gyne dichotomy in Polistes (5), the proposition that the gyne phenotype reflects diapause established before adult emergence has never been tested. Therefore, we sought two physiological signatures of the diapause syndrome, development time and developmental storage protein synthesis and sequestration, in developing immatures of Polistes metricus. This investigation constitutes a strong inference test (37, 38) of the hypothesis that Polistes offspring do, indeed, differentiate into nondiapause- and diapause-derived phenotypes before the adult stage.
To test the hypothesis, we documented duration of development and quantified dynamic storage protein levels in specimens from naturally established single-foundress nests at two phases of the P. metricus colony cycle in Missouri, an earlier phase when larvae are reared only by the single foundress and typically emerge to become workers, and a later phase when larvae are reared by the foundress plus workers and typically emerge to become gynes (25). Larval duration is prolonged by low provisioning level in P. metricus, and nourishment generally is better during the later phase of the colony cycle (28). Thus, to avoid confounding effects of provisioning variation, we studied only the nonfeeding ontogenetic stages, from cocoon spinning to adult emergence, which provide unambiguous measures of inherent developmental rate and storage protein physiology.
Results and Discussion
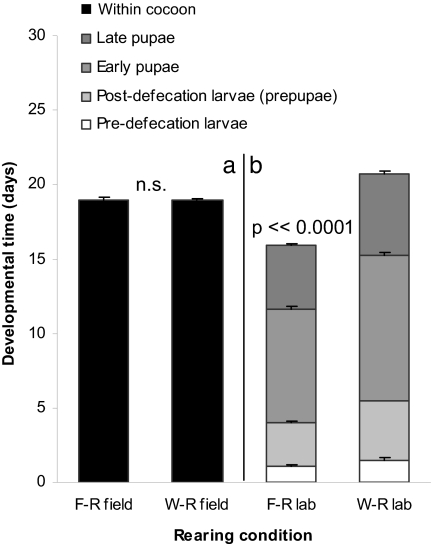
We examined development under natural conditions in the field and under constant conditions in the laboratory. We found that cocoon duration in the field was equal, at 19 days, for both sets of female offspring [Student's t = 0.27, df = 42, P = 0.7; Fig. 2a and supporting information (SI) Table 1]. However, temperature is a positive regulator of developmental rates in insects (39), and there were significant differences in rearing temperature between worker and gyne offspring in the field (nested ANOVA, F1,83 = 14.69, P ≪ 0.0001). To control for temperature effects, newly cocooned individuals were reared in the laboratory. When development proceeded under similar temperatures [least significant difference (LSD) post hoc test, P = 0.5; SI Table 2], there was a significant 5-day difference between the two sets of offspring (Student's t = 17.2, df = 28, P ≪ 0.0001). Foundress-reared (primarily worker-destined) females developed from cocoon spinning to the adult molt in 15.9 days, and worker-reared (primarily gyne-destined) females did so in 20.8 days (Fig. 2b and SI Table 3).
Fig. 2.
Duration of development for female P. metricus. Duration was measured from cocoon spinning to emergence in the field (a) and from cocoon spinning (in the field) to adult molt in the laboratory (b) for P. metrius females reared by a solitary foundress (F-R) or by nestmate workers (W-R). Bars are means with standard errors. Developmental stages for laboratory reared specimens are defined in Fig. 2. Mean temperatures (°C ± SE) during rearing were: F-R field = 23.0 ± 0.5; W-R field = 26.1 ± 0.6; F-R lab = 24.4 ± 0.4; W-R lab = 24.0 ± 0.1.
Thus, we show that gyne offspring are characterized by extended developmental time, a signature of diapause (7). This trait can be masked by variation in ambient conditions: The more rapidly maturing foundress-reared (primarily worker-destined) offspring generally develop under significantly lower temperatures in the field (LSD post hoc test, P < 0.04), and the more slowly maturing worker-reared (primarily gyne-destined) offspring generally develop under significantly warmer ambient conditions later in the season (LSD post hoc test, P < 0.0005). A closer look at the protracted development in worker-reared offspring shows that it reflects a lengthening of all developmental stages that are passed within the cocoon (one-way ANOVA, F7,112 = 492, P ≪ 0.001, LSD post hoc tests P < 0.05; Figs. 2 and 3). Overall, the inherent developmental rates of Polistes offspring are consistent with the hypothesis that the worker and gyne forms are derived from nondiapause and diapause phenotypes, respectively.
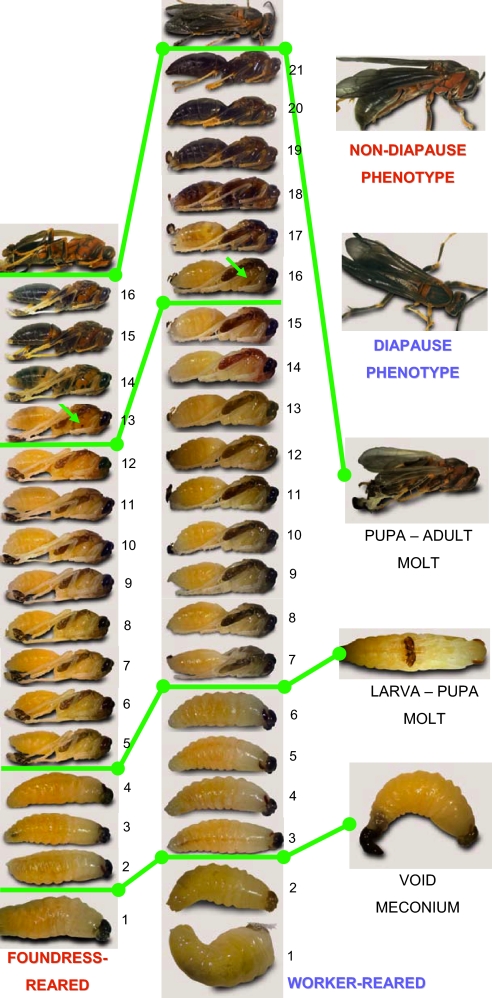
Fig. 3.
Development of foundress-reared and worker-reared individuals under constant conditions. (Left) Foundress-reared individuals. (Center) Worker-reared individuals. Left and Center consist of serial photos of a single specimen taken at 24-hour intervals from cocoon spinning to adulthood; development days from cocoon spinning to the last day of pupation are numbered for each specimen. Pupae are ≈15 mm in length. The cocoon is spun by a fifth-instar larva that has completed feeding and growth, which is the bottom photo in Left and Center. The first developmental marker (green bar; photo at Right) is that the larva voids its larval fecal waste (the meconium) and in doing so becomes a “prepupa.” The next marker (green bar; photo at Right) is that the prepupa sheds its cuticle in the molt to become a pupa. The appearance of a spot of pigmentation on the thorax (arrows) divides the pupal stage into early and late phases (green bar). The pupa then sheds its cuticle in the molt (green bar; photo at Right) to become an adult. The resultant adults (photos in the top of Right) are not visibly different. Larval, prepupal, and early and late pupal durations all were extended in worker-reared vs. foundress-reared females (see also Fig. 1).
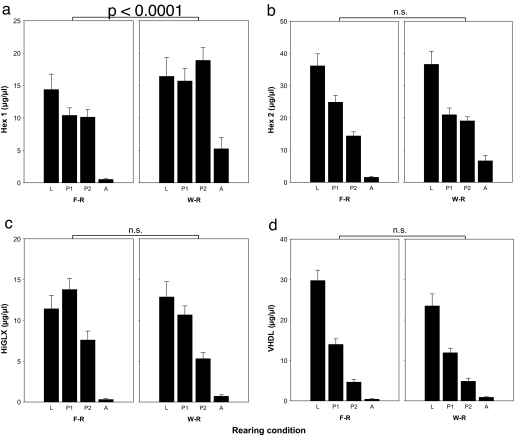
Next we looked at storage protein dynamics. Gel electrophoresis separation and densitometric quantification of hemolymph proteins from known-age females (SI Table 4) spanning development from cocoon spinning to adult emergence revealed presence of four storage proteins as previously identified from whole-tissue preparations of P. metricus pupae (35): hexamerin 1 and 2 (Hex 1, Hex 2), high glutamine/glutamic acid protein (HiGLX), and very high density lipoprotein (VHDL) (SI Table 5). Quantities differed significantly as a function of both developmental stage (multivariate ANOVA, F12,408 = 20.50, P ≪ 0.0001) and whether larvae were foundress-reared or worker-reared (multivariate ANOVA, F4,154 = 6.78, P ≪ 0.0001; Fig. 4). The overall decline in storage protein levels from the larval stage until adult emergence reflects the pattern typical of holometabolous insects (35). Concentrations are high at the end of larval growth and then decline during the pupal stage. However, the difference between foundress-reared and worker-reared offspring resides in only one of the four proteins, Hex 1 (LSD post hoc test, P < 0.0001). That is, the difference due to rearing condition is not a general response to the higher nutrition levels that characterize worker-reared larvae (33); instead, it is a specific response involving only a single hexameric storage protein (LSD post hoc test: Hex 2, P = 0.61; HiGLX, P = 0.08; VHDL, P = 0.14).
Fig. 4.
Quantities (μg/μl of hemolymph) of four storage proteins in hemolymph of P. metricus immatures from cocoon spinning to adulthood. Storage proteins are HEX1, hexamerin 1 (a); HEX2, hexamerin 2 (b); HiGLX (c); VHDL (d). Stages are L, cocooned fifth instar larva (both predefecation and postdefecation prepupae); P1, pupa up to day 11 after cocooning; P2, pupa from day 12 after cocooning; A, adult, including preemergence adults in nest cells and adults collected on the morning after emergence. F-R, foundress reared; W-R, worker-reared; development time was equal for both categories at 19 days. Only the expression level of Hex 1 (a) differed significantly during development of F-R and W-R offspring. This difference in protein levels is supported by recent results showing a corresponding 8-fold difference in mRNA levels between W-R and F-R fifth-instar larvae (J.H.H., A. L. Toth, and T. C. Newman, unpublished data). Both protein (see Fig. 5) and mRNA sequence match to the same 220 bp cDNA fragment from a putative P. metricus hexamerin gene (Contig40833).
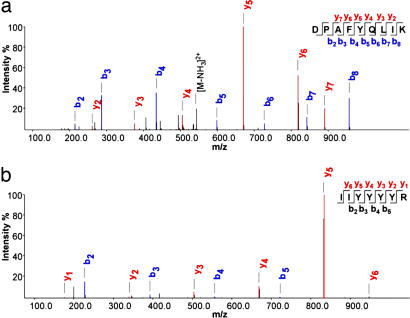
To further validate the key finding of diverging hexamerin levels in P. metricus offspring, we conducted a protein identification analysis combining gel electrophoresis, HPLC, and mass spectrometry. Several high quality spectra were obtained, confirming the specific presence of hexameric storage protein in the bands used for hexamerin quantification (Fig. 5, SI Fig. 6, and SI Table 6). Particularly convincing was the identification of peptides that exactly matched partial P. metricus cDNA sequences for putative hexamerins (Fig. 5b and SI Table 6). Also, it is unlikely that the quantitative protein pattern (Fig. 4) is distorted by sequestration of storage protein in fat body (40), because the signatures identified for hemolymph coincide with partial patterns obtained from whole-tissue preparations (35). Because the identified peptides are encoded by several different Contigs (SI Table 6), it seems likely that we identified more than one hexamerin. This assumption is further supported by the peptide sequences showing homologies to hexamerins of different classes from other organisms (data not shown). In summary, our findings show decisively that the physiology of foundress-reared and worker-reared individual P. metricus differs before emergence, and the divergence is reflected in a diapause protein marker (9, 10).
Fig. 5.
Spectra of selected peptides that correspond to known hexamerins/arylphorins. (a) Peptide with sequence identical to Anthonomus grandis hexamerin. (b) Hexamerin peptide sequence from P. metricus matching a partial cDNA sequence of a putative hexamerin from the same species (Contigs 40833 and 45913). Blue b and red y ions represent fragment ions starting from the N and C termini, respectively, as generated by collision induced dissociation (69, 70). x axis, mass over charge values for the fragment ions; y axis, intensity % describes the relative intensity for the fragment ions related to the strongest peak in the spectrum.
Our results have shown significantly different patterns of both development time and storage protein synthesis and sequestration between foundress-reared (primarily worker-destined) and worker-reared (primarily gyne-destined) females, and these patterns correspond to expectations based on nondiapause and diapause phenotypes. Much evidence suggests that Polistes larvae are biased onto worker vs. gyne life histories under the influence of low nourishment for foundress-reared larvae and higher nourishment for worker-reared larvae (33, 41). Provisioning level to larvae also is the causal basis for morphologically distinct worker and gyne castes in vespine wasps (29), honey bees (42), and some ants (43), but the evolutionary origins of these developmental programs remain largely unknown. We show that hexameric storage protein dynamics diverge throughout late larval and pupal development of foundress-reared and worker-reared Polistes females and that this difference reflects a response in a diapause marker rather than an across-the-board response to higher nourishment. Our data thus provide support for the hypothesis that activation of a facultative, intra-specifically variable diapause program (23, 24) underlies the absence of brood care behaviors at the natal nest and deferment of the onset of reproductive behaviors that are distinguishing behavioral characteristics of the gyne phenotype among the female offspring of a Polistes colony (5, 29). Thereby, the work presented here may exemplify a mechanism with regulatory potential to serve as an origin for developmental programs leading to castes.
Elevated levels of a hexameric storage protein in gyne-destined individuals also can point to a causal basis for the diapause-derived characteristics of this class of offspring. Hexamerins were recently implicated in regulation of caste differentiation in termites, where binding of juvenile hormone to the hexameric molecule results in physiological silencing of hormone action (44). Diapause is characterized by low juvenile hormone levels (45), and application of a juvenile hormone analog can trigger P. metricus gynes to initiate ovarian development (46). Hexamerin 1 protein in Polistes offspring, therefore, might be directly responsible for the phenotype bias of adult females: low levels in workers would permit juvenile hormone action and expression of reproductive behaviors shortly after adult emergence. In the context of the natal nest, these behaviors are channeled into alloparental care such as nursing and foraging (5, 33). High hexamerin 1 levels in gynes can contribute, on the other hand, to a juvenile hormone-deficient phenotype that does not express reproductive behaviors until the next year as a working solitary foundress or dominant or subordinate (working nonovipositing) cofoundress.
Materials and Methods
Field Sites and Procedures.
P. metricus foundresses will individually initiate nests in “nest boxes” provided for them. Wood and hardboard boxes ≈15 cm on a side with open bottoms were placed on posts in oldfields at two sites as in previous studies (47, 48): Tyson Research Center near Eureka, St. Louis County, MO, and Shaw Nature Reserve near Gray Summit, Franklin County, MO. Foundresses initiate nests on the underside of lift-off box lids. Boxes were in place by March 10, 2005; nests were initiated in late March or early April. From May 30 to June 23, 2005, a set of foundress nests was examined daily between 0800 and 1000 hours Central Daylight Time. Each new cocoon was entered on a diagram (“nest map”), whereby the age in days of each developing cocooned wasp was known. A separate set of worker-phase nests was similarly monitored from July 2 to July 29, 2005.
Development Time.
To determine duration from cocoon spinning to adult emergence in the field (SI Table 1), nests not used to collect specimens were monitored daily, and each new cocoon was recorded and dated on a nest map, as was the emergence date of an adult wasp from each cocoon. During the worker phase of the season, because many emerged worker-reared wasps were males and because data were ambiguous if two or more wasps, one of which was a male, emerged on the same day, data from uncollected nests were supplemented with emergence data from nests from which collections (see below) had been made. Daily maximum and minimum temperatures (SI Table 2) were recorded in a standard meteorological station at Tyson Research Center.
To determine “pupation” duration under laboratory conditions (SI Table 3), larvae were gently removed from nest cells in the early morning when newly spun cocoons were discovered. Individual larvae were placed in plastic Petri dishes lined with filter paper, held in the shade at ambient temperature, and transported to a laboratory rearing room with fluorescent lights on a 15:9 light:dark cycle that encompassed a 13:11 light:dark cycle by four incandescent 150-W lights. Specimens were shaded from direct light but exposed to the photoperiod. Heat of the lights raised daytime temperatures, which fell to building ambient at night. Beginning on day 12 of rearing, daily maximum and minimum temperatures were recorded (SI Table 2); rearing conditions for the first 11 days were as for the recorded days.
Each specimen was digitally photographed on the collection day and each following day at ≈1300 hours Central Daylight Time. Two of 22 foundress-reared larvae did not molt from larva to pupa, and 2 did not pigment normally or molt from pupa to adult. Five of 58 worker-reared larvae died before reaching the pupal molt, and 16 did not molt from larva to pupa. Of those that did molt, 24 were males. Of the females, 1 did not pigment normally or molt from pupa to adult.
Sex Determination.
Foundress-reared “early males” (49) have never been seen in 30 years of research with these populations of P. metricus (J.H.H., personal observation). In this study, all foundress-reared offspring collected or observed as pupae or adults were females. All foundress-reared larvae thus were confidently assumed to be females. Worker-reared offspring include males. Male pupae and adults are easily identified. For larvae, we used a molecular method to determine sex.
Hymenoptera are haplodiploid, therefore larvae heterozygous at any locus can be considered diploid and female. Accordingly, we genotyped larvae at six microsatellite loci originally isolated from Polistes bellicosus, Pbe128, Pbe203, Pbe205, Pbe269, Pbe424, and Pbe440 (50). Heterozygosities and allele sizes are given in SI Table 4.
We extracted DNA via a standard salt precipitation protocol (51, 52). We ground 10–50 mg of tissue in 500 μl of grinding buffer (0.1 M NaCl/0.1 M Tris·HCl, pH 9.1/0.05 M EDTA/0.05% SDS), 10 μl of RNase A (10 mg/ml), and 10 μl of proteinase K (20 mg/ml). The homogenate was incubated at 65°C for 30 minutes. Then 86 μl of warm 8 M KOAc was added and the mix incubated at 4°C for 30 min, then centrifuged at 20,800 × g for 5 min, and the supernatant was transferred to clean tubes. The supernatant was cleaned by shaking with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) for 5 min, followed by two cleanings with chloroform:isoamyl alcohol (24:1). After each cleaning, samples were centrifuged at 14,000 rpm for 5 min and the aqueous phase transferred to clean tubes. Finally, we added 2.5 vol of cold 100% EtOH, chilled samples overnight at −20°C, pelleted the DNA, and resuspended it in 50 μl of H2O.
We amplified microsatellite loci via PCR with a PerkinElmer (Norwalk, CT) 2400 thermocycler and 10-μl reactions (1× buffer with 1.5 mM MgCl2/0.1 μM dNTPs/0.5 pmol of each primer/1.0 pmol of M13f-29 primer/0.5 unit FisherBrand Taq polymerase). An M13f-29 sequence was added to the 5′ end of the forward primer for each locus. M13f-29 primers labeled at the 5′ end with either IRDye 700 or IRDye 800 (Li-Cor Bioscience, Lincoln, NE) were then added to the reactions and incorporated into the products during PCR. DNA samples were diluted for use in PCR between 1:5 and 1:20, based on extraction quality as determined by electrophoresis on 1% agarose gels. Three to 5 μl of diluted DNA were used in each reaction. Reactions were initially denatured at 94°C for 5 min, followed by 36 cycles at 94°C for 20 s, the annealing temperature (50° to 52°C depending on the locus) for 20 s, and 72°C for 30 s, and a final extension at 72°C for 3 min.
We visualized PCR products on 5.5% polyacrylamide gels, using a Li-Cor 4300 DNA analyzer (Li-Cor Biosciences, Lincoln, NE). Before loading, PCR products were diluted with 2 μl of 6× loading dye, denatured 3 min at 94°C, and quenched on ice. We ran 1 μl of each sample and a 50- to 350-bp sizing standard (Li-Cor Bioscience, Lincoln, NE). Alleles were scored by eye.
Sex in these wasps is probably determined by heterozygosity at a single locus (53), which can result in diploid males homozygous at that locus. However, diploid male production would have been apparent, as half of the female-destined eggs, on average, would have developed as males. No colonies having that characteristic were observed. Because we used six microsatellite loci, with heterozygosity at a single locus the basis for female determination, our method of sexing larvae is likely to be highly accurate.
Storage Proteins.
Storage protein samples were collected from known-age specimens (SI Table 5). For each, the cocoon was carefully cut away, and the specimen was placed in a tube on ice and transported to the laboratory. One to 3 h after collection, each live specimen's cuticle was cut longitudinally along a lower side of the gaster of a pupa or along the corresponding body wall for larvae. Under 6× magnification, with careful attention to not breaching the gut, ≈10–50 μl of hemolymph was gently aspirated via a 10-μl micropipette, transferred to a tube on ice, then centrifuged 10 min at 8,000 × g at 4°C. After centrifugation, a 1-μl sample of clear interphase (between fat and cell layers) was carefully withdrawn and placed into a 200-μl tube containing 10 μl of Tris-buffered saline with Roche (Indianapolis, IN) Protease Inhibitor Mixture. Samples were stored at −80°C until analysis.
Samples were separated by 7.5% SDS/PAGE, using standard methods (54). Duplicate runs and titration series were run to optimize sample separation. A β-galactosidase standard (Sigma–Aldrich) was included to allow densitometric quantification (55). Storage protein bands corresponding to Hexamerin 1 and 2, HiGLX, and VHDL were identified as described in ref. 35. Densitometrical analysis was performed by Quantity One imaging software (Bio-Rad, Hercules, CA) after staining the gels with Commassie Brilliant Blue (Sigma–Aldrich). Gel-to-gel variation in staining intensity was controlled by background correction and the β-galactosidase standard, which was loaded in equal dilution series on all gels (55). Protein titer was calculated as the detected protein amount in micrograms relative to the original hemolymph sample volume in microliters.
Peptide and Protein Identification via LC-MSn.
Hemolymph proteins were mixed with a protein denaturation buffer (50 mM Tris pH 8.5/2% SDS/5% 2-mercaptoethanol/0.15 M NaCl), vortexed, and boiled (5 min, 95°C). Next, they were concentrated and purified by methanol-chloroform precipitation (56), reconstituted in 1× Laemmli buffer (54), and separated by SDS/PAGE. Protein bands were stained with Commassie brilliant blue, cut from the gels, and digested with trypsin as described in the manual (trypsin proteomics grade; Roche). Peptides were desalted as described before (57) and stored at −20°C.
For LC-MSn analysis, peptides were dissolved in 2% TFA, 5% acetonitrile, separated by reversed-phase HPLC (100-μm monolithic column; Merck, Darmstadt, Germany; 60-min gradient from 2% to 40% ACN containing 0.1% formic acid), and directly eluted into a linear quadrupole ion trap mass spectrometer at 350 nl/min (ThermoElectron, San Diego, CA). An MS scan was followed by five MS/MS scans on the most intense peaks. Recorded spectra were matched against an Apis mellifera database containing additional hexamerin, trypsin, and keratin sequences, using the Open Mass Spectrometry Search Algorithm search engine (58). Generally, only peptides with an e value ≤ 0.01 are reported for the database search. The de novo sequencing program NovoHMM (59) was used to identify additional hexamerin peptides and to validate shown hexamerin peptides obtained from the database search. Sequences obtained by NovoHMM were manually inspected and matched against the same A. mellifera database, using BLAST for short, nearly exact matches (60).
Data Analysis.
Student's t tests were used to test cocoon duration difference between foundress-reared and worker-reared individuals. Mean daily temperatures were analyzed by nested ANOVA for differences between rearing location (field and laboratory) nested within rearing condition (foundress-reared and worker-reared). Fisher's LSD test was used for posthoc comparisons. Differences in lengths of pupal stages were analyzed by one-way ANOVA with developmental stage as categorical factor and time as dependent variable. Between the same developmental stage of foundress-reared and worker-reared individuals, Fisher's LSD test was used to test for level of significance. Putative differences between storage protein levels were examined by multivariate ANOVA with rearing condition and developmental stage as categorical factors and Hex 1, Hex 2, HiGLX, and VHDL as dependent variables. Fisher's LSD test was used for posthoc comparisons. Analyses were performed with Statistica software, Version 6.0.
Supplementary Material
Acknowledgments
We thank D. Larson and J. M. Chase (Tyson Research Center) and J. C. Trager and J. Behrer (Shaw Nature Reserve) for accommodating and facilitating our field work; T. C. Newman, A. L. Toth, G. E. Robinson, and N. Mutti for Polistes sequence information; and M. J. West-Eberhard, K. G. Ross, J. M. Herbers, K. Hartfelder, C. M. Brent, T. Flatt, and three anonymous referees for manuscript review. This work was supported by a University of Missouri–St. Louis Research Award and Research Leave (J.H.H.); the University of Illinois Sociogenomics Initiative (J.H.H.: G. E. Robinson, P.I.); Norwegian Research Council Grants 171958 and 175413, U.S. National Science Foundation Grant 0615502, U.S. National Institute on Aging Grant PO1 AG22500, the PEW Foundation (to G.V.A.); and the Humboldt Foundation (F.W.).
Abbreviation
- LSD
least significant difference.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705660104/DC1.
References
- 1.Wilson EO. The Insect Societies. Cambridge, MA: Belknap Press of Harvard Univ Press; 1971. [Google Scholar]
- 2.Robinson GE, Grozinger CM, Whitfield CW. Nat Rev Gen. 2005;6:257–270. doi: 10.1038/nrg1575. [DOI] [PubMed] [Google Scholar]
- 3.Evans JD, Wheeler DE. BioEssays. 2001;23:62–68. doi: 10.1002/1521-1878(200101)23:1<62::AID-BIES1008>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 4.Amdam GV, Csondes A, Fondrk MK, Page RE. Nature. 2006;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt JH, Amdam GV. Science. 2005;308:264–267. doi: 10.1126/science.1109724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatar M, Chien SA, Priest NK. Amer Nat. 2001;158:248–258. doi: 10.1086/321320. [DOI] [PubMed] [Google Scholar]
- 7.Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Insect Molecular Science. Gilbert LI, Iatrou K, Gill SS, editors. Vol 3. Amsterdam: Elsevier BV; 2005. pp. 615–650. [Google Scholar]
- 8.Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. New York: Oxford Univ Press; 1986. [Google Scholar]
- 9.de Kort CAD, Koopmanschap AB. J Insect Physiol. 1994;40:527–535. [Google Scholar]
- 10.Lewis DK, Spurgeon D, Sappington TW, Keeley LL. J Insect Physiol. 2002;48:887–901. doi: 10.1016/s0022-1910(02)00158-0. [DOI] [PubMed] [Google Scholar]
- 11.Zayed A, Packer L. Insectes Soc. 2002;49:282–288. [Google Scholar]
- 12.Brady SG, Sipes S, Pearson A, Danforth BN. Proc R Soc London Ser B. 2006;273:1643–1649. doi: 10.1098/rspb.2006.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brockmann HJ. J Kansas Entomol Soc. 2004;77:503–527. [Google Scholar]
- 14.Schmidt PS, Paaby AB, Heschel MS. Evolution (Lawrence, Kans) 2005;59:2616–2625. [PubMed] [Google Scholar]
- 15.Schmidt PS, Matzkin L, Ippolito M, Eanes WF. Evolution (Lawrence, Kans) 2005;59:1721–1732. [PubMed] [Google Scholar]
- 16.Schmidt PS, Conde DR. Evolution (Lawrence, Kans) 2006;60:1602–1611. [PubMed] [Google Scholar]
- 17.Wedell N, Nylin S, Janz N. Oikos. 1997;78:569–575. [Google Scholar]
- 18.Nijhout HF. Insect Hormones. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 19.Vargo EL. In: Pheromone Communication in Social Insects. Vander Meer RK, Breed MD, Winston ML, Espelie KE, editors. Boulder, CO: Westview; 1998. pp. 293–313. [Google Scholar]
- 20.Hunt JH, Brodie RJ, Carithers TP, Goldstein PZ, Janzen DH. Biotropica. 1999;31:192–196. [Google Scholar]
- 21.Pratte M, Strambi J, Gervet J, Strambi A. Insectes Soc. 1982;29:383–401. [Google Scholar]
- 22.Giray T, Giovanetti M, West-Eberhard MJ. Proc Natl Acad Sci USA. 2005;102:3330–3335. doi: 10.1073/pnas.0409560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deleurance É-P. Coll Int CNRS. 1952;34:141–155. [Google Scholar]
- 24.Deleurance É-P. C R Acad Sci Paris. 1949;229:303–304. [Google Scholar]
- 25.Reeve HK. In: The Social Biology of Wasps. Ross KG, Matthews RW, editors. Ithaca, NY: Comstock, Cornell Univ Press; 1991. pp. 99–148. [Google Scholar]
- 26.Reeve HK, Peters JM, Nonacs P, Starks PT. Proc Natl Acad Sci USA. 1998;95:13737–13742. doi: 10.1073/pnas.95.23.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dapporto L, Palagi E, Turillazzi S. Ann Zool Fenn. 2005;42:135–139. [Google Scholar]
- 28.Karsai I, Hunt JH. Env Entomol. 2002;31:99–106. [Google Scholar]
- 29.Hunt JH. The Evolution of Social Wasps. New York: Oxford Univ Press; 2007. [Google Scholar]
- 30.Pardi L. A Soc Toscana Sci Nat. 1948;55:1–15. [Google Scholar]
- 31.Gervet J, Pratte M, Semenoff S, Gabouriaut D. Insectes Soc. 1986;33:375–387. [Google Scholar]
- 32.Strassmann JE, Lee RE, Rojas RR, Baust JG. Insectes Soc. 1984;31:291–301. [Google Scholar]
- 33.Hunt JH. In: The Social Biology of Wasps. Ross KG, Matthews RW, editors. Ithaca, NY: Comstock, Cornell Univ Press; 1991. pp. 426–450. [Google Scholar]
- 34.Eickwort K. Insectes Soc. 1969;16:67–72. [Google Scholar]
- 35.Hunt JH, Buck NA, Wheeler DE. J Insect Physiol. 2003;49:785–794. doi: 10.1016/s0022-1910(03)00115-x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson EO, Hölldobler B. Proc Natl Acad Sci USA. 2005;102:13367–13371. doi: 10.1073/pnas.0505858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Platt JR. Science. 1964;146:347–353. doi: 10.1126/science.146.3642.347. [DOI] [PubMed] [Google Scholar]
- 38.Hunt JH. Ann Zool Fenn. 2006;43:407–422. [Google Scholar]
- 39.Chapman RF. The Insects: Structure and Function. Cambridge, MA: Cambridge Univ Press; 1998. [Google Scholar]
- 40.Telfer WH, Kunkel JG. Annu Rev Entomol. 1991;36:205–228. doi: 10.1146/annurev.en.36.010191.001225. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell S. Annu Rev Entomol. 1998;43:323–346. doi: 10.1146/annurev.ento.43.1.323. [DOI] [PubMed] [Google Scholar]
- 42.Winston ML. The Biology of the Honey Bee. Cambridge, MA: Harvard Univ Press; 1987. [Google Scholar]
- 43.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Belknap Press of Harvard Univ Press; 1990. [Google Scholar]
- 44.Zhou X, Oi FM, Scharf ME. Proc Natl Acad Sci USA. 2006;103:4499–4504. doi: 10.1073/pnas.0508866103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders DS, Richard DS, Applebaum SW, Ma M, Gilbert LI. Gen Comp Endocrinol. 1990;79:174–184. doi: 10.1016/0016-6480(90)90102-r. [DOI] [PubMed] [Google Scholar]
- 46.Bohm MK. J Insect Physiol. 1972;18:1875–1883. [Google Scholar]
- 47.Rossi AM, Hunt JH. Ecol Entomol. 1988;13:437–442. [Google Scholar]
- 48.Hunt JH, Dove MA. Ecol Entomol. 2002;27:467–474. [Google Scholar]
- 49.Strassmann JE. Behav Ecol Sociobiol. 1981;8:55–64. [Google Scholar]
- 50.Strassmann JE, Barefield K, Solís CR, Hughes CR, Queller DC. Mol Ecol. 1997;6:97–100. doi: 10.1046/j.1365-294x.1997.00158.x. [DOI] [PubMed] [Google Scholar]
- 51.Miller SA, Dykes DD, Polesky HT. Nucl Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strassmann JE, Solís CR, Peters JM, Queller DC. In: Molecular Zoology: Advances, Strategies and Protocols. Ferraris JD, Palumbi SR, editors. New York: Wiley–Liss; 1996. pp. 528–549. [Google Scholar]
- 53.Cook JH. Heredity. 1993;71:421–435. [Google Scholar]
- 54.Laemmli UK. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 55.Lin H, Winston ML, Haunerland NH. Can Entomol. 1999;131:695–706. [Google Scholar]
- 56.Wessel D, Flugge UI. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- 57.Rappsilber J, Ishihama Y, Mann M. Anal Chem. 2003;75:663–670. doi: 10.1021/ac026117i. [DOI] [PubMed] [Google Scholar]
- 58.Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, Yang X, Shi W, Bryant SH. J Proteome Res. 2004;3:958–964. doi: 10.1021/pr0499491. [DOI] [PubMed] [Google Scholar]
- 59.Fischer S, Samietz J, Dorn S. J Comp Physiol A. 2003;189:723–730. doi: 10.1007/s00359-003-0452-9. [DOI] [PubMed] [Google Scholar]
- 60.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 61.Cowan DP. In: The Social Biology of Wasps. Ross KG, Matthews RW, editors. Ithaca, NY: Comstock, Cornell Univ Press; 1991. pp. 33–73. [Google Scholar]
- 62.O'Neill KM. Solitary Wasps: Behavior and Natural History. Ithaca, NY: Cornell Univ Press; 2001. [Google Scholar]
- 63.Ishay J. Anim Behav. 1975;23:425–431. [Google Scholar]
- 64.Wheeler DE. Amer Nat. 1986;128:13–34. [Google Scholar]
- 65.Pardi L. Physiol Zool. 1948;21:1–13. doi: 10.1086/physzool.21.1.30151976. [DOI] [PubMed] [Google Scholar]
- 66.West-Eberhard MJ. In: Chemistry and Biology of Social Insects. Eder J, Rembold H, editors. Munich: J. Peperny; 1987. pp. 369–372. [Google Scholar]
- 67.West-Eberhard MJ. In: Molds, Molecules, and Metazoa: Growing Points in Evolutionary Biology. Grant PR, Horn HS, editors. Princeton: Princeton Univ Press; 1992. pp. 59–79. [Google Scholar]
- 68.West-Eberhard MJ. In: Natural History and Evolution of Paper-Wasps. Turillazzi S, West-Eberhard MJ, editors. Oxford: Oxford Univ Press; 1996. pp. 290–317. [Google Scholar]
- 69.Jensen C, Haebel S, Andersen SO, Roepstorff P. Int J Mass Spec Ion Proc. 1997;160:339–356. [Google Scholar]
- 70.Johnson RS, Martin SA, Biemann K, Stults JT, Watson JT. Anal Chem. 1987;59:2621–2625. doi: 10.1021/ac00148a019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.