Abstract
MAPK signaling pathways function as critical regulators of cellular differentiation, proliferation, stress responsiveness, and apoptosis. One branch of the MAPK signaling pathway that culminates in ERK1/2 activation is hypothesized to regulate the growth and adaptation of the heart to both physiologic and pathologic stimuli, given its known activation in response to virtually every stress- and agonist-induced hypertrophic stimulus examined to date. Here we investigated the requirement of ERK1/2 signaling in mediating the cardiac hypertrophic growth response in Erk1−/− and Erk2+/− mice, as well as in transgenic mice with inducible expression of an ERK1/2-inactivating phosphatase in the heart, dual-specificity phosphatase 6. Although inducible expression of dual-specificity phosphatase 6 in the heart eliminated ERK1/2 phosphorylation at baseline and after stimulation without affecting any other MAPK, it did not diminish the hypertrophic response to pressure overload stimulation, neuroendocrine agonist infusion, or exercise. Similarly, Erk1−/− and Erk2+/− mice showed no reduction in pathologic or physiologic stimulus-induced cardiac growth in vivo. However, blockade or deletion of cardiac ERK1/2 did predispose the heart to decompensation and failure after long-term pressure overload in conjunction with an increase in myocyte TUNEL. Thus, ERK1/2 signaling is not required for mediating physiologic or pathologic cardiac hypertrophy in vivo, although it does play a protective role in response to pathologic stimuli.
Keywords: cardiomyopathy, MAPK, signaling
In response to pathophysiologic stress, the adult heart undergoes hypertrophic enlargement characterized by an increase in the cross-sectional area of individual myocytes and whole-organ enlargement. Although cardiac hypertrophy can initially be a compensatory response that temporarily augments/maintains cardiac output, prolonged pathologic hypertrophy can be deleterious and eventually leads to heart failure, loss of myocytes by apoptosis/necrosis, and sudden death associated with arrhythmia (1, 2). The myocardium can also hypertrophy in response to non-disease-causing stimuli that adapt functional performance to changing hemodynamic load, such as after sustained exercise or during pregnancy (3, 4). Both physiologic and pathologic hypertrophy are induced by endocrine, paracrine, and autocrine regulatory circuits that directly signal myocyte growth through membrane-bound G protein-coupled receptors and receptor tyrosine kinases, which in turn activate intermediate signal transduction pathways within the cytoplasm (5). Signaling pathways, such as MAPK, PKC, and calcineurin–nuclear factor of activated T cells, and regulated shuttling of class II histone deacetylases are all critical for transducing myocyte growth (5).
MAPK signaling pathways consist of a sequence of successively acting kinases that ultimately result in the phosphorylation and activation of terminal kinases such as p38, JNK, and ERK (6). The MAPK signaling cascade is initiated in cardiac myocytes by G protein-coupled receptors [angiotensin II (Ang II), endothelin-1, and adrenergic receptors], receptor tyrosine kinases (insulin-like growth factor and fibroblast growth factor receptors), and cardiotrophin-1 (gp130 receptor) and by diverse stress and stretch stimuli (7). Once activated, p38, JNKs, and ERKs each phosphorylate a wide array of intracellular targets that includes transcription factors resulting in the reprogramming of cardiac gene expression. The major upstream activators of ERK1/2 are two MAPK kinases, MEK1 and MEK2, which directly phosphorylate a dual site in the activation loop of ERK1/2 kinases, but not in ERK3/4 or ERK5 (8).
MAPKs are inactivated and recycled by dephosphorylation within their activation loops through specific subclasses of dual-specificity phosphatases (DUSPs) (9). DUSP6, also known as MAPK phosphatase 3, is transcriptionally induced after a stress or mitogen response where it specifically dephosphorylates and inactivates ERK1/2 (9). Here we observed that inducible overexpression of DUSP6 in the heart potently blocked ERK1/2 activity, which predisposed the heart to failure through increased apoptosis after long-term pressure overload, although loss of ERK1/2 activity had no effect on the cardiac growth response. Similarly, Erk1−/− and Erk2+/− mice showed no reduction in pathologic or physiologic cardiac hypertrophy in vivo after multiple stimuli and showed greater propensity toward decompensation after pressure overload.
Results
Assessment of Stress-Induced Hypertrophy in Erk1 and Erk2 Gene-Targeted Mice.
MEK1–ERK1/2 inhibition with pharmacologic agents or a dominant negative MEK1-encoding adenovirus attenuates hypertrophy of neonatal cardiomyocytes in culture after agonist or stress stimulation (10). In vivo, continuous expression of constitutively active MEK1 in the hearts of transgenic mice promoted concentric cardiac hypertrophy, collectively suggesting that MEK1–ERK1/2 signaling was a necessary and sufficient signal-transducing pathway for cardiac hypertrophy (11). However, a definitive loss-of-function approach for ERK1/2 signaling in the adult heart has yet to be performed.
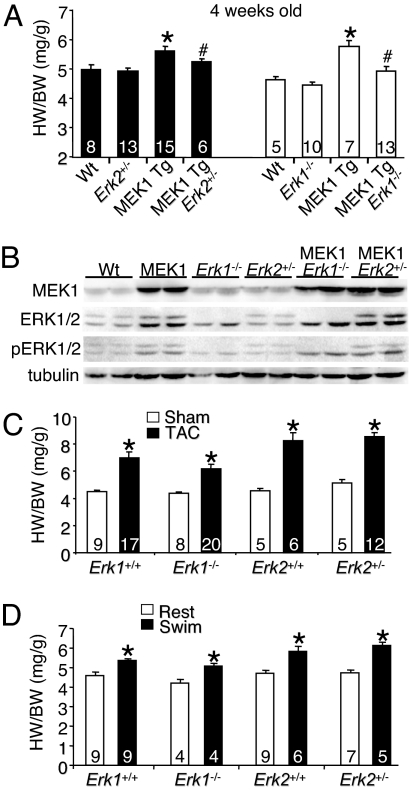
We have previously shown that both Erk1−/− and Erk2+/− mice have a noticeable reduction in the total content of ERK1/2 protein in the heart, a reduction in stress-induced ERK1/2 activity, and greater injury in the heart after ischemia–reperfusion (12). Here we show that both Erk1−/− mice and Erk2+/− mice have less cardiac hypertrophy at 4 weeks of age in the presence of the constitutively active MEK1 transgene (P < 0.05) (Fig. 1A). Activated MEK1 increased both ERK1 and ERK2 protein content, as described previously (11), as well as increased phosphorylation of ERK1/2 protein at baseline and in Erk1−/− and Erk2+/− hearts (Fig. 1B). These results indicate that even a partial reduction in total ERK1/2 protein levels renders MEK1 less effective in driving hypertrophy through this direct pathway. However, Erk1−/− and Erk2+/− mice failed to show a significant reduction in cardiac hypertrophy after a more global stimulation, such as pressure overload induced by transverse aortic constriction (TAC) (Fig. 1C). Moreover, Erk1−/− and Erk2+/− mice also showed no reduction in exercise-induced cardiac hypertrophy after swimming compared with controls (Fig. 1D). These results suggest either that ERK1/2 are not critical for mediating cardiac hypertrophy to pathophysiologic stress or that the remaining ERK1/2 activity in each gene-targeted mouse model was sufficient to mediate the signaling events required to drive the hypertrophic response.
Fig. 1.
Erk1/2 gene-targeted mice undergo normal cardiac hypertrophy. (A) Heart weight/body weight (HW/BW) in the indicated groups of mice, assayed at 4 weeks of age. *, P < 0.05 vs. WT; #, P < 0.05 vs. MEK1 transgenic (Tg). (B) Western blot analysis of MEK1 protein, ERK1/2 protein, phospho-ERK1/2, and α-tubulin (control) from the hearts of the indicated mice (n = 2 blots). (C) Heart weight/body weight in the indicated groups of mice 2 weeks after TAC or a sham procedure. *, P < 0.05 vs. sham of each pairing. (D) Heart weight/body weight in the indicated groups of mice after 20 days of swimming exercise or rest. *, P < 0.05 vs. the rest of each pairing.
Inducible DUSP6 Transgenic Mice Have Inhibited ERK1/2 Activity in the Heart.
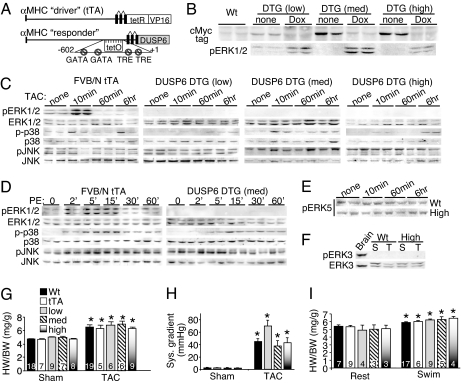
Because Erk2−/− mice are not viable and Erk1−/− Erk2+/− mice (3/4 alleles) are mostly lethal, we needed an alternate approach to more effectively extinguish total ERK1/2 activity within the heart. Accordingly, here we generated inducible transgenic mice that express the ERK1/2-specific DUSP6 protein in the heart. DUSP6 is known to only bind and dephosphorylate ERK1/2 through a highly specific interaction domain (13–17). A modified α-myosin heavy chain (α-MHC) promoter was used as the responder transgene to promote DUSP6 expression (18) when crossed with transgenic mice containing the α-MHC promoter-driven tetracycline transactivator (tTA) protein in the absence of doxycycline (Dox) (18). (Fig. 2A). Multiple lines of DUSP6-containing mice were initially generated, from which we selected low, medium, and high lines based on Western blotting for the Myc tag (engineered in DUSP6) and the ability of DUSP6 expression to be tightly regulated by Dox when double transgenic (DTG) with the tTA line (Fig. 2B). Remarkably, induction of DUSP6 in the hearts of low-expressing DUSP6 DTG mice severely reduced basal ERK1/2 phosphorylation, whereas medium- and high-DUSP6 DTG mice showed essentially no ERK1/2 phosphorylation (Fig. 2B).
Fig. 2.
Characterization of cardiac-specific DUSP6-inducible transgenic mice. (A) Schematic of the bitransgenic inducible expression system used to regulate DUSP6 in the mouse heart. (B) Western blot for Myc-tagged DUSP6 protein in the hearts of the indicated mice on Dox (shutoff) or without Dox (induced). Corresponding basal ERK1/2 phosphorylation is also shown. (C) Western blot assessment of MAPK phosphorylation from hearts at baseline or after TAC for the indicated times in the different groups of adult mice without Dox (induced). At least six separate samples were analyzed in total per condition (although only two are shown). (D) Western blot assessment of cardiac MAPK phosphorylation at baseline or after acute systemic PE injection for the indicated times in mice without Dox (induced). The entire time course was repeated in separate mice with identical results. (E) Western blot of phosphorylated ERK5 (activated) in the hearts of WT or high-expressing DUSP6 DTG mice after 10 min, 60 min, or 6 h of TAC stimulation. (F) ERK3 phosphorylation was not detected in hearts of sham (S) or TAC (T) stimulated mice that were WT or high-expressing DUSP6 DTG, although ERK3 protein was detected. Brain extract was run as a control. (G) Heart weight/body weight in the indicated groups of mice 2 weeks after TAC or a sham procedure (no Dox, induced). *, P < 0.05 vs. sham of each pairing. (H) Systolic pressure gradient across the aortic constriction. *, P < 0.05 vs. sham. (I) Heart weight/body weight in the indicated groups of mice after 20 days of swimming or rest in the induced stated (no Dox). *, P < 0.05 vs. the rest of each pairing. The key in G applies to H and I as well.
All three lines showed essentially no cardiac ERK1/2 phosphorylation after TAC for 10 min, 60 min, or 6 h, whereas control hearts containing the tTA transgene showed robust ERK1/2 activation at 10 min and some level of continuous activation at later time points (Fig. 2C). Overexpression of DUSP6 did not affect basal or induced activity of JNK1/2 or p38 in the heart (n = 6) (Fig. 2C). Quantitation of ERK1/2, p38, and JNK phosphorylation was also performed from these samples across three independent blots with at least four to six separate hearts [supporting information (SI) Fig. 6 A–C]. A more careful series of ERK1/2 activation assays was performed by acute injection of phenylephrine (PE) into the mouse, which induced a robust cardiac ERK1/2 response within 2 min in WT mice that was sustained thereafter (Fig. 2D). However, DUSP6 DTG mice showed no ERK1/2 phosphorylation at any time point, whereas JNK and p38 phosphorylation were induced normally (Fig. 2D). DUSP6-inducible expression in the heart also had no effect on ERK5 phosphorylation or kinase activity, nor was inducible phosphorylation of ERK5 altered in cultured cardiomyocytes infected with a DUSP6 adenovirus (Fig. 2E and data not shown). In other control experiments, DUSP6 overexpression in hearts of DTG mice had no effect on ERK3/4 protein levels or phosphorylation (Fig. 2F and data not shown), although phosphorylated ERK3 protein was never detected in the heart or in cultured cardiomyocytes under any stimulated conditions (Fig. 2F and data not shown). That ERK3/4 were unaffected by DUSP6 in transgenic hearts or in cultured cardiomyocytes was anticipated because they lack the TEY consensus sequence in the activation loop that is required for DUSP6 binding (19).
Given that low-, medium-, and high-DUSP6 DTG mice essentially lacked cardiac ERK1/2 activity, we reasoned that they would render a more definitive answer to the question of ERK1/2 necessity in promoting cardiac hypertrophy in vivo. Consistent with the results from Erk1−/− and Erk2+/− mice, low-, medium-, and high-DUSP6 DTG mice showed the same hypertrophic response as WT and tTA single transgenic mice after 2 and 4 weeks of TAC (Fig. 2G and data not shown). Importantly, there was no significant difference in the pressure gradients across the aortic constrictions (Fig. 2H). All three lines showed no ERK1/2 phosphorylation in the heart after 14 days of TAC (SI Fig. 7 A–C). All three lines of DUSP6 DTG mice also showed an identical physiologic hypertrophy response as WT and single tTA transgenic mice after swimming exercise for 20 days (Fig. 2I). Induction of hypertrophic marker genes (i.e., atrial natriuretic factor) after TAC was also of similar magnitude between control and DUSP6 transgenic mice (data not shown). These results suggest that ERK1/2 activity is not necessary for mounting a productive cardiac hypertrophic response in vivo after a pathologic and physiologic stimulus.
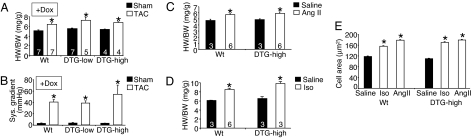
We also maintained low- and high-DUSP6 DTG mice on Dox so that the DUSP6 protein was never expressed in the heart to control for any nonspecific effects that might secondarily influence the hypertrophic response associated with the bitransgenic system itself. No DUSP6 protein expression was observed in the hearts of DTG mice on Dox, nor were any MAPKs altered in phosphorylation (SI Fig. 8 A and B). Both low- and high-DUSP6 DTG mice on Dox showed an identical profile of cardiac hypertrophy as WT mice analyzed at the same relative pressure gradients after TAC stimulation (Fig. 3 A and B).
Fig. 3.
Characterization of cardiac-specific DUSP6-inducible transgenic mice. (A) Heart weight/body weight in the indicated groups of mice on Dox (transgene off) 2 weeks after TAC or a sham procedure. *, P < 0.05 vs. sham of each pairing. (B) Corresponding systolic pressure gradients of the mice in A. (C) Heart weight/body weight in the indicated groups of mice 2 weeks after Ang II infusion or saline (no Dox, induced). *, P < 0.05 vs. saline. (D) Heart weight/body weight in the indicated groups of mice 2 weeks after Iso infusion or saline (no Dox, induced). *, P < 0.05 vs. saline. (E) Histological analysis of myocyte cross-sectional areas from ventricles of the indicated mice and indicated treatments (at least 500 myocytes were counted from three separate hearts in each group). *, P < 0.05 vs. saline.
Finally, we also investigated the ability of DUSP6 DTG mice (high line) to hypertrophy in response to more selective agonists that are known to activate ERK1/2, such as Ang II and isoproterenol (Iso). Inducible expression of DUSP6 in the heart (no Dox) did not reduce the cardiac hypertrophic response after Ang II or Iso infusion compared with WT mice after 14 days (Fig. 3 C and D), further supporting the hypothesis that ERK1/2 are not required for productive cardiac growth in vivo, even after a more selective agonist-based stimulation protocol. Both WT and the high-expressing DUSP6 transgenic mice also showed a similar increase in myocyte cross-sectional areas in the left ventricle after Ang II and Iso simulation (Fig. 3E).
Inhibition of ERK1/2 Renders the Heart More Susceptible to Failure After Stimulation.
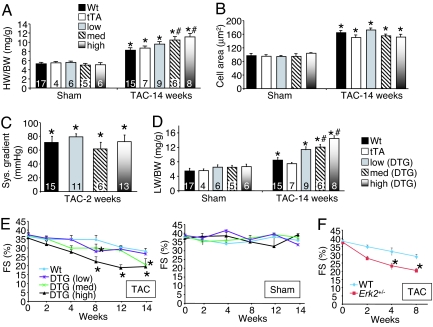
Although chronic inhibition of ERK1/2 was without a pathologic effect in mice as they aged past 1 year of life (based on echocardiography and histology), we did observe a profile of decompensation after long-term pressure overload. Specifically, medium- and high-DUSP6 DTG mice actually showed greater cardiac hypertrophy after 14 weeks of pressure overload, which was associated with greater dilation and inflammation (Fig. 4A). However, at the cellular level myocyte hypertrophy was equally increased across each of the groups (Fig. 4B). Importantly, pressure gradients across the aortic constrictions were not different among the groups at 2 weeks of age (Fig. 4C).
Fig. 4.
Analysis of heart failure in DUSP6-inducible transgenic mice. (A) Heart weight/body weight in the indicated groups of mice 14 weeks after TAC or a sham procedure (no Dox, induced). *, P < 0.05 vs. sham of each pairing; #, P < 0.05 vs. WT or tTA TAC. (B) Histological analysis of myocyte cross-sectional areas from ventricles of the indicated mice and indicated treatments (at least 500 myocytes were counted from three separate hearts in each group). *, P < 0.05 vs. sham. (C) Corresponding systolic pressure gradients at 2 weeks of TAC across the aortic constriction for the mice shown in A. (D) Corresponding lung weight/body weight (LW/BW) for the mice in A. (E) Echocardiography assessment of fractional shortening (FS) in DUSP6 DTG mice after TAC (Left) or subjected to a sham procedure (Right) for the indicated times. *, P < 0.05 vs. WT. (F) Echocardiography assessment of fractional shortening (FS) in WT and Erk2+/− mice after TAC for the indicated time points. *, P < 0.05 vs. WT.
After 14 weeks of TAC stimulation, medium- and high-expressing DTG mice showed greater pulmonary congestion compared with the control groups, suggesting heart failure (P < 0.05) (Fig. 4D). Mice were carefully followed for heart failure signs by echocardiography over 14 weeks, which showed significantly more decompensation (≈2-fold) in high- and medium-expressing DUSP6 DTG mice vs. WT after TAC, whereas the low-expressing DTG mice showed a more mild decrease in function equivalent to the control groups (Fig. 4E). Sham-operated mice from each group showed no signs of cardiac decompensation over a similar time course (Fig. 4E). Erk2+/− mice also showed significant reductions in fractional shortening by 4 and 8 weeks of age (n = 7) after TAC compared with WT mice (Fig. 4F). Erk1−/− mice showed a trend toward reduced function after 2 weeks of TAC (n = 6), although these mice showed prominent lethality by 4 weeks of TAC, precluding a long-term analysis of functional decompensation (data not shown). Erk2+/− mice also showed a significantly greater increase in lung weight normalized to body weight after 8 weeks of TAC (P < 0.05) (data not shown).
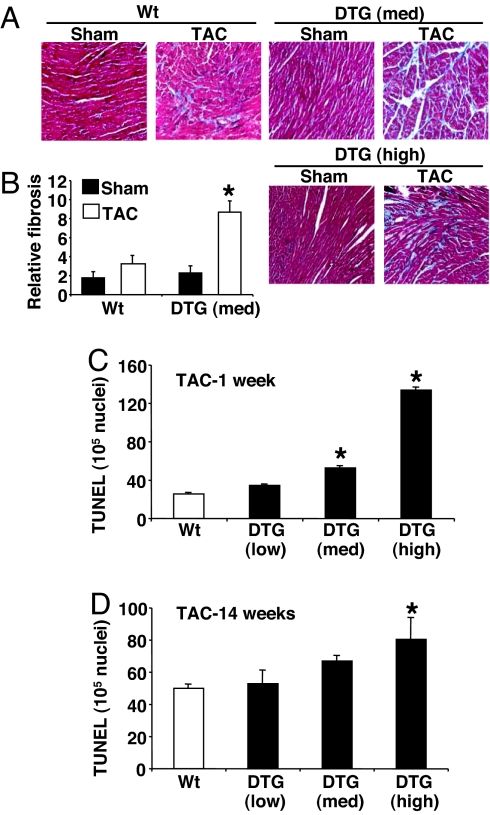
Histological analysis of hearts from medium- and high-expressing DUSP6 DTG mice showed more abundant pathology and a >2-fold increase in fibrosis after 14 weeks of TAC, with areas suggestive of myocyte dropout (Fig. 5 A and B). Indeed, DUSP6 DTG mice showed twice as much fibrosis in the heart after 14 days of Iso infusion compared with control mice (data not shown). Mechanistically, we hypothesized that chronic inhibition of ERK1/2 during long-term pressure overload in high- and medium-DUSP6 DTG mice might predispose myocytes to death. As predicted, acute pressure overload for 1 week increased the TUNEL rates in both high- and medium-DUSP6 DTG hearts, which remained elevated even after 14 weeks of TAC in high-DUSP6 DTG mice (Fig. 5 C and D). These results suggest that ERK1/2 signaling is necessary to protect myocytes from apoptosis after pathologic insults such as pressure overload.
Fig. 5.
Histological assessment of fibrosis and TUNEL in DUSP6 DTG mice after TAC. (A) Representative Masson's trichrome-stained histological sections from the hearts of the indicated mice after 14 weeks of TAC or sham operation. (B) Quantitation of relative fibrosis of the hearts of medium-expressing DUSP6 DTG mice (n = 3 hearts each). (C) Measurement of TUNEL in cardiac histological sections from the indicated mice after 1 week of TAC stimulation (n = 3 hearts each, with at least 200,000 nuclei surveyed per heart). (D) Measurement of TUNEL in cardiac histological sections from the indicated mice after 14 weeks of TAC stimulation (n = 3 hearts each, with at least 200,000 nuclei surveyed per heart). *, P < 0.05 vs. WT TAC.
Discussion
ERK1/2 become activated in cardiac myocytes in response to virtually every type of stress stimulation examined to date (10). For example, neuroendocrine effectors, G protein-coupled receptor agonists, receptor tyrosine kinase agonists, cytokines, reactive oxygen species, and stretch all induce ERK1/2 activation and, in most cases, a hypertrophic response, suggesting the possibility that ERK1/2 directly program growth itself. Mechanistically, ERK1/2 antisense oligonucleotides reduced PE-induced cardiomyocyte hypertrophy (20). Similarly, the MEK1 inhibitor PD98059 reduced sarcomeric organization induced by hypertrophic agonists (21), and a dominant negative MEK1-encoding adenovirus reduced agonist-mediated hypertrophy in cultured cardiomyocytes (22). With respect to a gain-of-function approach, expression of activated MEK1 in cultured neonatal cardiomyocytes by adenoviral gene transfer was shown to induce a prominent growth response (11). In contrast, other studies have shown that inhibition of MEK1–ERK1/2 does not antagonize hypertrophic morphology or cytoskeletal organization in response to agonist treatment in culture (23, 24). Thus, although the use of cultured neonatal cardiomyocytes has provided important clues, little consensus exists as to the causal relationship between ERK1/2 signaling and the cardiac hypertrophic response.
To investigate the ability of MEK1–ERK1/2 signaling to induce cardiac hypertrophy in vivo, we previously generated transgenic mice expressing activated MEK1 under the transcriptional control of the α-MHC promoter (11). MEK1 transgenic mice showed highly specific and constitutive activation of ERK1/2 in the heart, without affects on other stress kinases, resulting in a phenotype of stable concentric hypertrophy (11). More recently, cardiac-specific dominant negative Raf-1 transgenic mice were generated and shown to have attenuated cardiac hypertrophy after pressure overload (25). However, Raf-1 is a complicated kinase that directly complexes with many other signaling proteins, such as Ras, apoptosis signal-regulating kinase 1, Rok-α, cdc25, casein kinase 2, retinoblastoma protein, and MEKK1 (26, 27), any of which could also affect the cardiac hypertrophic response independent of ERK1/2. Thus, the current literature strongly supports the hypothesis that MEK1–ERK1/2 signaling induces cardiac hypertrophy, although the requirement of MEK1–ERK1/2 as mediators of the cardiac growth response has not been evaluated.
Erk1−/− and Erk2+/− mice showed no reduction in growth after pressure overload or exercise stimulation compared with control mice of the same strain. We have previously shown that ERK2 constitutes 70% of the total ERK1/2 protein content of the heart, so that Erk2 heterozygous mice are roughly equivalent in effect to the complete targeting of Erk1, provided the proteins are functionally redundant (12). Indeed, Erk1−/− and Erk2+/− mice show nearly equivalent reductions in total ERK kinase activity at baseline and after agonist stimulation (12). Unfortunately, Erk2−/− mice are not viable and die during early embryonic development because of placental insufficiency (28), and Erk1−/− Erk2+/− (3/4 alleles) mice were essentially nonviable (only a few rare survivors), so a more thorough reduction in total ERK1/2 activity was not possible using gene-targeted mice. Despite the concern of sufficient residual ERK1/2 activity, Erk1−/− and Erk2+/− mice did show significantly less cardiac growth when crossed into the constitutively active MEK1 transgene (Fig. 1A), and Erk2+/− mice were previously shown to have a phenotype of greater myocyte death after ischemia–reperfusion injury to the heart in vivo (12). These results indicate that even a partial reduction in ERK1/2 protein content is sufficient to affect the heart. Thus, the failure of Erk1−/− and Erk2+/− mice to show even a partial reduction in pathologic or physiologic hypertrophy implies that ERK1/2 signaling may not be a necessary event in mediating growth of the adult heart.
Given the concern of residual ERK1/2 activity in both Erk1−/− and Erk2+/− mice, an additional genetic strategy involving inducible overexpression of DUSP6 (MAPK phosphatase 3) was instituted. Inducible expression of DUSP6 in the hearts of three independent transgenic lines all but completely eliminated ERK1/2 activity (>95% in the high line) at baseline and after pressure overload, PE, Iso, or Ang II stimulation, either acutely or chronically. However, inducible DUSP6 transgenic mice productively hypertrophied to both physiologic and pathologic stimuli, further suggesting that ERK1/2 are not necessary mediators of this process. Indeed, long-term pressure overload in medium- and high-expressing DUSP6 DTG mice even showed slightly greater cardiac hypertrophy compared with single transgenic controls and strain-matched WT controls, although this increase was likely due to greater fibrosis, inflammation, and failure, because myocyte cross-sectional areas were not significantly greater. Finally, calcineurin and Akt activity was equally up-regulated in hearts of DUSP6 transgenic and control mice subjected to TAC (data not shown).
DUSP6 is a highly specific member of the DUSP family, where it essentially only regulates ERK1/2. Indeed, even high-expressing DUSP6 DTG mice showed no alterations in p38 or JNK phosphorylation in the heart, nor was ERK5 phosphorylation affected. ERK3/4 are unlikely to be affected because ERK4 protein was not detected in the heart, and ERK3 was not phosphorylated by common agonists in the heart or cultured cardiomyocytes (data not shown). Given these considerations, the simplest interpretation of the data presented here is that ERK1/2 signaling is not required for cardiac hypertrophy after a common physiologic stimulus (exercise) or a number of different pathologic stimuli (pressure overload and neuroendocrine agonists), although we still cannot rule out the possibility that unknown kinases are influenced by DUSP6.
Although ERK1/2 signaling was not required for mediating a productive hypertrophic response in adult mice, the long-term absence of this pathway predisposed the myocardium to decompensation associated with greater TUNEL rates, which is consistent with greater ischemia–reperfusion injury and apoptosis in Erk2+/− mice (12). The mechanisms whereby ERK1/2 might directly protect cardiac myocytes from cell death have yet to be definitively identified in the literature. We did survey expression levels and phosphorylation status of select Bcl-2 family members in the hearts of DUSP6 transgenic mice, showing a significant increase in Bcl-2 and Bcl-xl protein levels after TAC (SI Fig. 9). However, we interpret this up-regulation of Bcl-2 and Bcl-xl in DUSP6 transgenic hearts (high line) to be a compensatory response due to the loss of other survival signals that are more proximal to ERK1/2. Thus, the direct mechanisms whereby ERK1/2 protect the heart from cell death and failure remain elusive.
Methods
Animal Models and Procedures.
Erk1 null mice were obtained from Gilles Pagès (Centre Antoine Lacassagne, Nice, France) (29), and Erk2 heterozygotes were described previously (28). A cDNA encoding mouse DUSP6 was cloned into the modified murine α-MHC promoter expression vector to permit Dox-regulated expression in combination with a cardiac-specific tTA-expressing transgene (18). MEK1 transgenic mice were described previously (11). Cardiac pressure overload by TAC in mice was described previously (30). Swimming for 20 days as a model of exercise-induced hypertrophy was also described previously (30). Echocardiography for assessment of fractional shortening and pressure gradients was described previously (31). Iso and Ang II were infused in saline at 60 mg/kg per day and 2 mg/kg per day, respectively, for 14 days in Alzet minipumps (Alzet, Cupertino, CA) placed under the skin in mice. PE was injected s.c. in 50 μl of saline at a dosage of 15 mg/kg. Dox was administered in the food with a special diet formulated by Purina (St. Louis, MO) (625 mg/kg in pellets). In all experiments that required DUSP6 protein induction, Dox was removed from the food at weaning, resulting in induced expression within 4 additional weeks, although most experiments were conducted 8 weeks after to ensure full induction of the DUSP6 cDNA.
Western Blotting.
Generation of protein samples from tissue, along with Western blotting and chemifluorescent detection, were described previously (11, 32). All antibodies were obtained from Cell Signaling Biotechnology (Beverly, MA), except ERK3 and phospho-ERK3, which were obtained from Abgent (San Diego, CA).
Histology and TUNEL.
Hearts were collected at the indicated times, fixed in 10% formalin-containing PBS, and embedded in paraffin. Serial 5-μm heart sections were cut and stained with Masson's trichrome, from which MetaMorph analysis was performed to quantify the area of fibrosis (31). Assessment of TUNEL and cross-sectional areas from paraffin sections was performed as previously described (31).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health, an Established Investigator Award from the American Heart Association (to J.D.M.), and an international collaborative research grant in cardiovascular disease from Fondation Leducq.
Abbreviations
- α-MHC
α-myosin heavy chain
- Ang II
angiotensin II
- Dox
doxycycline
- DTG
double transgenic
- DUSP
dual-specificity phosphatase
- Iso
isoproterenol
- MEK
MAPK kinase
- PE
phenylephrine
- TAC
transverse aortic constriction
- tTA
tetracycline transactivator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610906104/DC1.
References
- 1.Ho KK, Levy D, Kannel WB, Pinsky JL. J Am Coll Cardiol. 1993;22:6–13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Dickhuth HH, Lehmann M, Auch-Schwelk W, Meinertz T, Keul J. J Cardiovasc Pharmacol. 1987;10:S71–S78. [PubMed] [Google Scholar]
- 4.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 5.Heineke J, Molkentin JD. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 6.Garrington TP, Johnson GL. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 7.Sugden PH, Clerk A. Circ Res. 1998;24:345–352. doi: 10.1161/01.res.83.4.345. [DOI] [PubMed] [Google Scholar]
- 8.Nishimoto S, Nishida E. EMBO Rep. 2006;7:782–786. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farooq A, Zhou MM. Cell Signal. 2004;16:769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Bueno OF, Molkentin JD. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 11.Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, Hewett TE, Jones SP, Lefer DJ, Peng CF, et al. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lips DJ, Bueno OF, Wilkins BJ, Purcell NH, Kaiser RA, Lorenz JN, Voisin L, Saba-El-Leil MK, Meloche S, Pouyssegur J, et al. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 13.Fjeld CC, Rice AE, Kim Y, Gee KR, Denu JM. J Biol Chem. 2000;275:6749–6757. doi: 10.1074/jbc.275.10.6749. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Zhou B, Zheng CF, Zhang ZY. J Biol Chem. 2003;278:29901–29912. doi: 10.1074/jbc.M303909200. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Zhang ZY. J Biol Chem. 2001;276:32382–32391. doi: 10.1074/jbc.M103369200. [DOI] [PubMed] [Google Scholar]
- 16.Zhou B, Wu L, Shen K, Zhang J, Lawrence DS, Zhang ZY. J Biol Chem. 2001;276:6506–6515. doi: 10.1074/jbc.M009753200. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Sun J-P, Zhou B, Zhang Z-Y. Proc Natl Acad Sci USA. 2006;103:5326–5331. doi: 10.1073/pnas.0510506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 19.Cheng M, Boulton TG, Cobb MH. J Biol Chem. 1996;271:8951–8958. doi: 10.1074/jbc.271.15.8951. [DOI] [PubMed] [Google Scholar]
- 20.Glennon PE, Kaddoura S, Sale EM, Sale GJ, Fuller SJ, Sugden PH. Circ Res. 1996;78:954–961. doi: 10.1161/01.res.78.6.954. [DOI] [PubMed] [Google Scholar]
- 21.Clerk A, Michael A, Sugden PH. J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueyama T, Kawashima S, Sakoda T, Rikitake Y, Ishida T, Kawai M, Yamashita T, Ishido S, Hotta H, Yokoyama M. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 23.Thorburn J, Frost JA, Thorburn A. J Cell Biol. 1994;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorburn J, McMahon M, Thorburn A. J Biol Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- 25.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 26.Baccarini M. FEBS Lett. 2005;579:3271–3277. doi: 10.1016/j.febslet.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Hindley A, Kolch W. J Cell Sci. 2002;115:1575–1581. doi: 10.1242/jcs.115.8.1575. [DOI] [PubMed] [Google Scholar]
- 28.Saba-El-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S. EMBO Rep. 2003;4:964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagès G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 31.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 32.Taigen T, De Windt LJ, Lim HW, Molkentin JD. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.