Abstract
Specific and sensitive detection of indigenous and introduced degradative organisms is an essential prerequisite to their use in remediation of toxic waste and soil systems. Procedures were employed for the use of polymerase chain reaction and gene probes for sensitive detection of the 2,4-dichlorophenoxyacetic-acid-degrading bacterium, Alcaligenes eutrophus JMP134(pJP4). Two 20-mer oligonucleotide primers were identified for amplification of a 205-bp region of the tfdB gene of pJP4, and optimum conditions for amplification were determined. Both the polymerase chain reaction amplification process and hybridization with the 5'-end-labelled probe were found to be specific to organisms containing plasmid pJP4 or its derivative pRO103. Detection limits were determined for the template supplied either as bacterial cells or purified plasmid DNA. The detection was sensitive up to an initial inoculum of 3,000 CFU or 156 pg of total plasmid DNA. However, when the amplified product was transferred to a nylon membrane and hybridized with the 5'-end-labelled probe, the detection sensitivity increased to 300 CFU or 15.6 pg of plasmid DNA. This sensitive detection method is more specific than use of traditional indicator media (M. A. Loos, Can. J. Microbiol. 21:104-107, 1975). An oligonucleotide (20 bases) complementary to a sequence internal to the 205-bp region was synthesized and utilized as a probe to confirm the specificity of the detection.
Full text
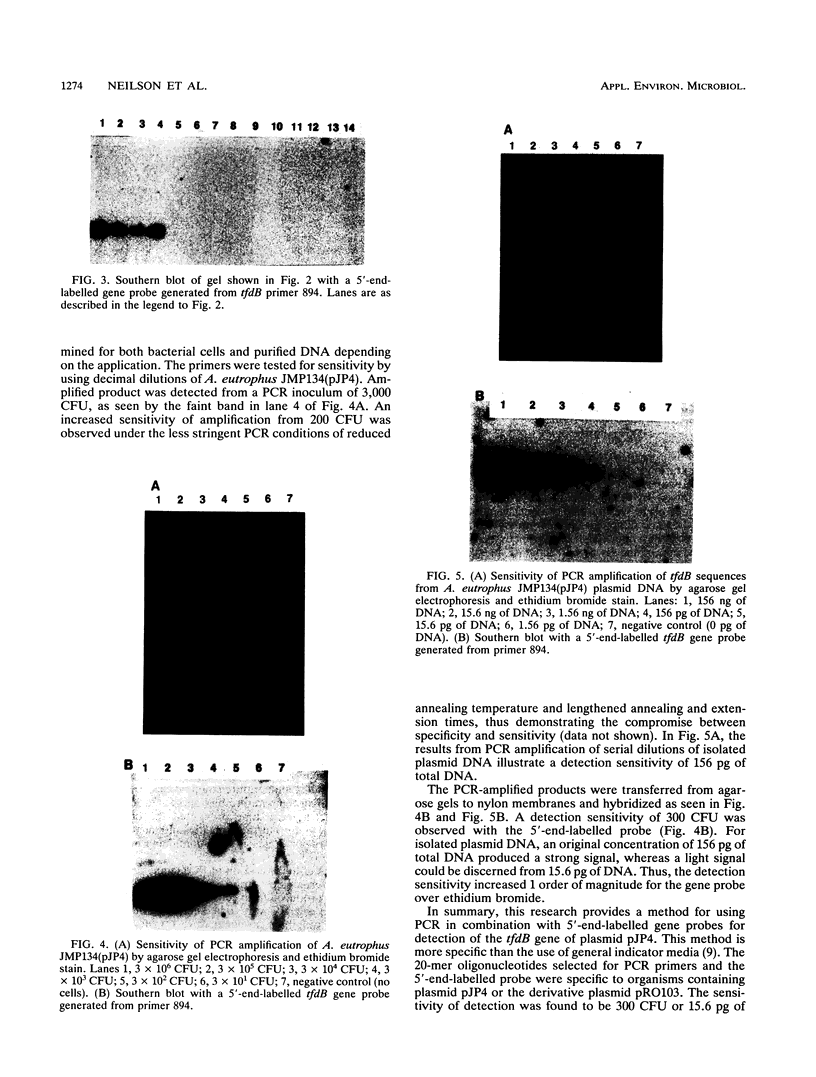
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Don R. H., Pemberton J. M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981 Feb;145(2):681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Plasmids in the degradation of chlorinated aromatic compounds. Basic Life Sci. 1985;30:667–686. doi: 10.1007/978-1-4613-2447-8_47. [DOI] [PubMed] [Google Scholar]
- Harker A. R., Olsen R. H., Seidler R. J. Phenoxyacetic acid degradation by the 2,4-dichlorophenoxyacetic acid (TFD) pathway of plasmid pJP4: mapping and characterization of the TFD regulatory gene, tfdR. J Bacteriol. 1989 Jan;171(1):314–320. doi: 10.1128/jb.171.1.314-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J. G., Colwell R. R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990 Sep;54(3):305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M. A. Indicator media for microorganisms degrading chlorinated pesticides. Can J Microbiol. 1975 Jan;21(1):104–107. doi: 10.1139/m75-016. [DOI] [PubMed] [Google Scholar]
- Molin G., Nilsson I. Degradation of phenol by Pseudomonas putida ATCC 11172 in continuous culture at different ratios of biofilm surface to culture volume. Appl Environ Microbiol. 1985 Oct;50(4):946–950. doi: 10.1128/aem.50.4.946-950.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins E. J., Gordon M. P., Caceres O., Lurquin P. F. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J Bacteriol. 1990 May;172(5):2351–2359. doi: 10.1128/jb.172.5.2351-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S. D., Josephson K. L., Bailey R. L., Gerba C. P., Pepper I. L. Rapid method for processing soil samples for polymerase chain reaction amplification of specific gene sequences. Appl Environ Microbiol. 1991 Aug;57(8):2283–2286. doi: 10.1128/aem.57.8.2283-2286.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler G. S., Shields M. S., Tedford E. T., Breen A., Hooper S. W., Sirotkin K. M., Davis J. W. Application of DNA-DNA colony hybridization to the detection of catabolic genotypes in environmental samples. Appl Environ Microbiol. 1985 May;49(5):1295–1303. doi: 10.1128/aem.49.5.1295-1303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K. A., Doyle J. D., King R. J., Seidler R. J., Stotzky G., Olsen R. H. Effects of 2,4-dichlorophenol, a metabolite of a genetically engineered bacterium, and 2,4-dichlorophenoxyacetate on some microorganism-mediated ecological processes in soil. Appl Environ Microbiol. 1991 Feb;57(2):412–418. doi: 10.1128/aem.57.2.412-418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
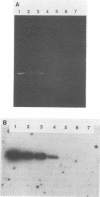
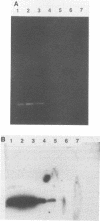
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. R., van Neerven A. R., de Vries E. J., de Vos W. M., Zehnder A. J. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J Bacteriol. 1991 Jan;173(1):6–15. doi: 10.1128/jb.173.1.6-15.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]