Abstract
This study examined the potential of bacteria from river epilithon to mobilize a recombinant catabolic plasmid, pD10, encoding 3-chlorobenzoate degradation and kanamycin resistance. Fifty-four mobilizing plasmids were exogenously isolated by triparental matings between strains of Pseudomonas putida and epilithic bacteria from the River Taff (South Wales, United Kingdom). Frequencies for mobilization ranged from 1.7 x 10(-8) to 4.5 x 10(-3) per recipient at 20 degrees C. The sizes of the mobilizing plasmids isolated ranged from 40 kb to over 200 kb, and 19 of 54 were found to encode mercury resistance. Plasmid-encoded resistance to tetracycline and streptomycin was also found but not resistance to UV light or various heavy metals. Eight plasmids of epilithic bacteria, analyzed by comparing restriction fragmentation patterns, showed significant differences between those isolated from different independent matings. Optimal temperatures for mobilization of pD10 were between 15 and 25 degrees C. Four mercury resistance plasmids were found to be broad host range, transferring mercury resistance and mobilizing pD10 readily to representative species of beta- and gamma-purple bacteria. In general, frequencies of pD10 mobilization by plasmids of epilithic bacteria were 2 to 3 orders of magnitude lower than conjugal transfer frequencies. Thus, there is a high potential for exchange of recombinant genes introduced into the epilithon by mobilization between a variety of bacterial species.
Full text
PDF

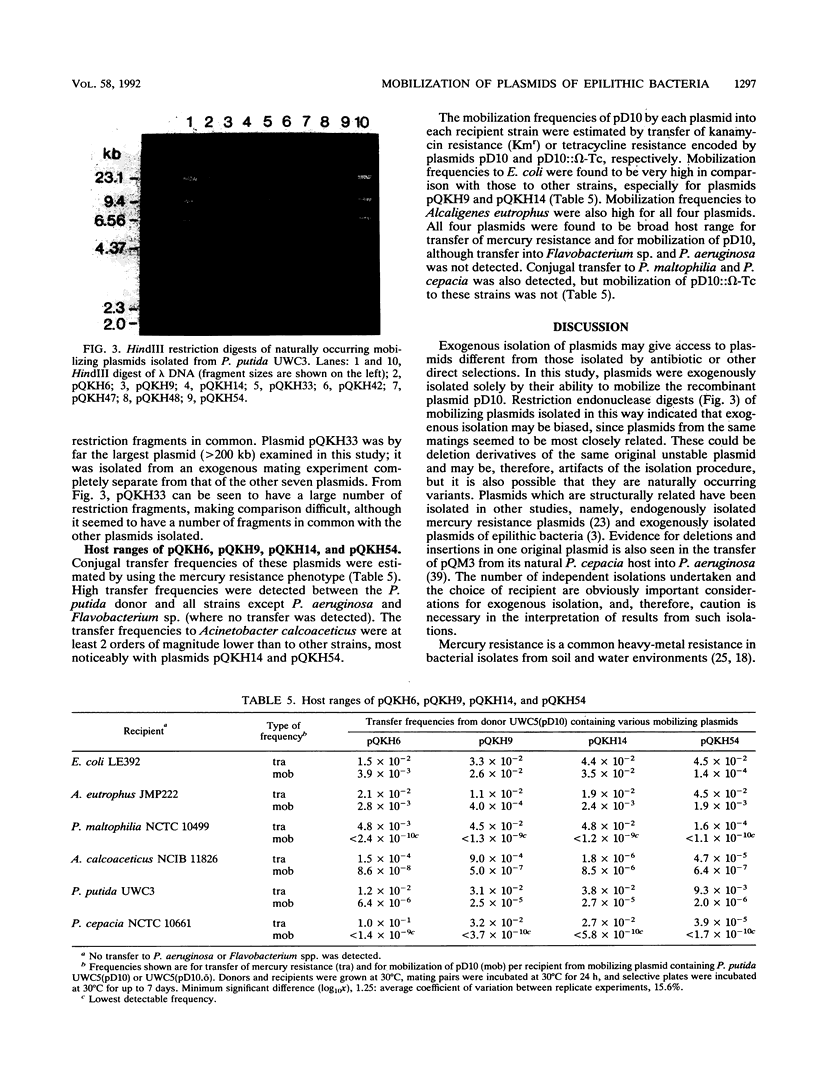



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altherr M. R., Kasweck K. L. In situ studies with membrane diffusion chambers of antibiotic resistance transfer in Escherichia coli. Appl Environ Microbiol. 1982 Oct;44(4):838–843. doi: 10.1128/aem.44.4.838-843.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
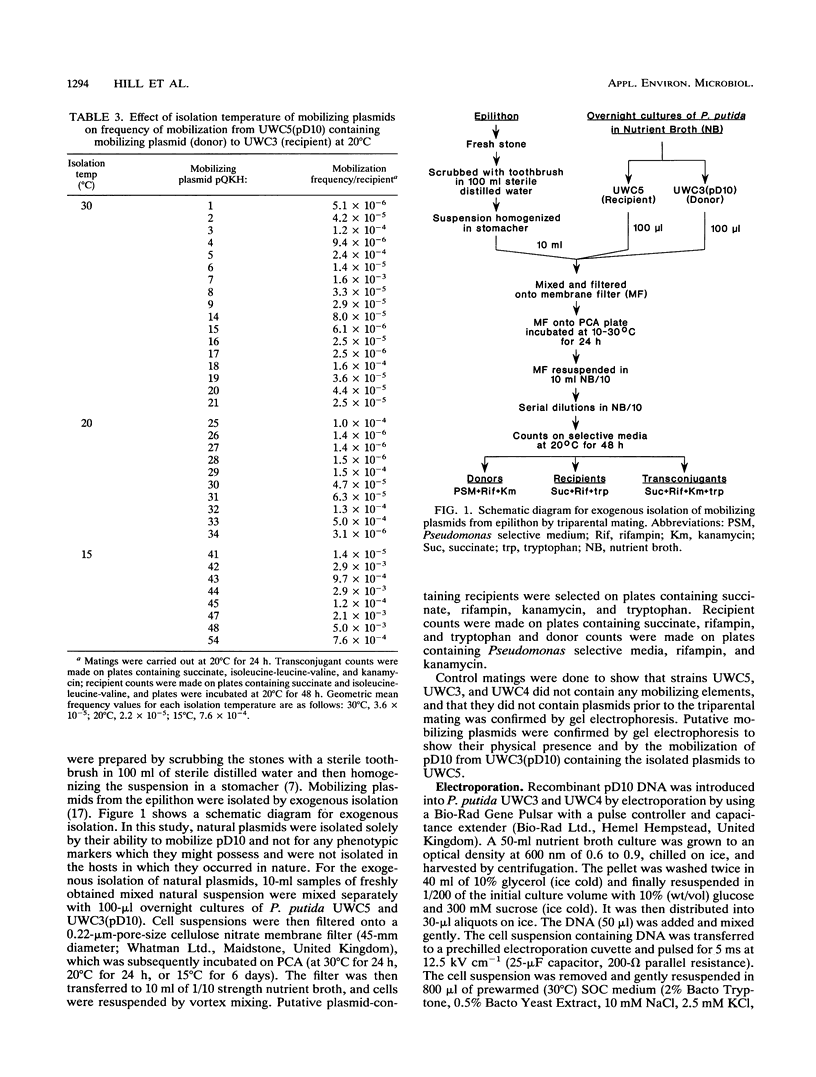
- Bale M. J., Day M. J., Fry J. C. Novel method for studying plasmid transfer in undisturbed river epilithon. Appl Environ Microbiol. 1988 Nov;54(11):2756–2758. doi: 10.1128/aem.54.11.2756-2758.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale M. J., Fry J. C., Day M. J. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J Gen Microbiol. 1987 Nov;133(11):3099–3107. doi: 10.1099/00221287-133-11-3099. [DOI] [PubMed] [Google Scholar]
- Bale M. J., Fry J. C., Day M. J. Transfer and occurrence of large mercury resistance plasmids in river epilithon. Appl Environ Microbiol. 1988 Apr;54(4):972–978. doi: 10.1128/aem.54.4.972-978.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J. Colicin Ib does not cause plasmid-promoted abortive phage infection of Escherichia coli K-12. Mol Gen Genet. 1981;182(3):508–510. doi: 10.1007/BF00293944. [DOI] [PubMed] [Google Scholar]
- Brasch M. A., Meyer R. J. Genetic organization of plasmid R1162 DNA involved in conjugative mobilization. J Bacteriol. 1986 Aug;167(2):703–710. doi: 10.1128/jb.167.2.703-710.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton N. F., Day M. J., Bull A. T. Distribution of bacterial plasmids in clean and polluted sites in a South Wales river. Appl Environ Microbiol. 1982 Nov;44(5):1026–1029. doi: 10.1128/aem.44.5.1026-1029.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Isolation and properties of recombination-deficient mutants of Pseudomonas aeruginosa. Mutat Res. 1974 Apr;23(1):15–23. doi: 10.1016/0027-5107(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Kilbane J. J., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid in soil by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Aug;44(2):514–516. doi: 10.1128/aem.44.2.514-516.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Pemberton J. M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981 Feb;145(2):681–686. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Weightman A. J., Knackmuss H. J., Timmis K. N. Transposon mutagenesis and cloning analysis of the pathways for degradation of 2,4-dichlorophenoxyacetic acid and 3-chlorobenzoate in Alcaligenes eutrophus JMP134(pJP4). J Bacteriol. 1985 Jan;161(1):85–90. doi: 10.1128/jb.161.1.85-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgehill R. U., Finn R. K. Microbial treatment of soil to remove pentachlorophenol. Appl Environ Microbiol. 1983 Mar;45(3):1122–1125. doi: 10.1128/aem.45.3.1122-1125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay R., Frey J., Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52(2-3):147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Gauthier M. J., Cauvin F., Breittmayer J. P. Influence of salts and temperature on the transfer of mercury resistance from a marine pseudomonad to Escherichia coli. Appl Environ Microbiol. 1985 Jul;50(1):38–40. doi: 10.1128/aem.50.1.38-40.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealt M. A., Chai M. D., Alpert K. B., Boyer J. C. Transfer of plasmids pBR322 and pBR325 in wastewater from laboratory strains of Escherichia coli to bacteria indigenous to the waste disposal system. Appl Environ Microbiol. 1985 Apr;49(4):836–841. doi: 10.1128/aem.49.4.836-841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeenes D. J., Williams P. A. Excision and integration of degradative pathway genes from TOL plasmid pWW0. J Bacteriol. 1982 Apr;150(1):188–194. doi: 10.1128/jb.150.1.188-194.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil T. A., Gealt M. A. Temperature, pH, and cations affect the ability of Escherichia coli to mobilize plasmids in L broth and synthetic wastewater. Can J Microbiol. 1987 Aug;33(8):733–737. doi: 10.1139/m87-127. [DOI] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Mancini P., Fertels S., Nave D., Gealt M. A. Mobilization of plasmid pHSV106 from Escherichia coli HB101 in a laboratory-scale waste treatment facility. Appl Environ Microbiol. 1987 Apr;53(4):665–671. doi: 10.1128/aem.53.4.665-671.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure N. C., Weightman A. J., Fry J. C. Survival of Pseudomonas putida UWC1 containing cloned catabolic genes in a model activated-sludge unit. Appl Environ Microbiol. 1989 Oct;55(10):2627–2634. doi: 10.1128/aem.55.10.2627-2634.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEuen S. F., Miller M. G. Metabolism and pharmacokinetics of 1,3-dinitrobenzene in the rat and the hamster. Drug Metab Dispos. 1991 May-Jun;19(3):661–666. [PubMed] [Google Scholar]
- McPherson P., Gealt M. A. Isolation of indigenous wastewater bacterial strains capable of mobilizing plasmid pBR325. Appl Environ Microbiol. 1986 May;51(5):904–909. doi: 10.1128/aem.51.5.904-909.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle P. A., Day M. J., Fry J. C. Occurrence, transfer and mobilization in epilithic strains of Acinetobacter of mercury-resistance plasmids capable of transformation. J Gen Microbiol. 1988 Nov;134(11):2933–2941. doi: 10.1099/00221287-134-11-2933. [DOI] [PubMed] [Google Scholar]
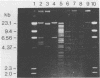
- Rochelle P. A., Fry J. C., Day M. J., Bale M. J. An accurate method for estimating sizes of small and large plasmids and DNA fragments by gel electrophoresis. J Gen Microbiol. 1986 Jan;132(1):53–59. doi: 10.1099/00221287-132-1-53. [DOI] [PubMed] [Google Scholar]
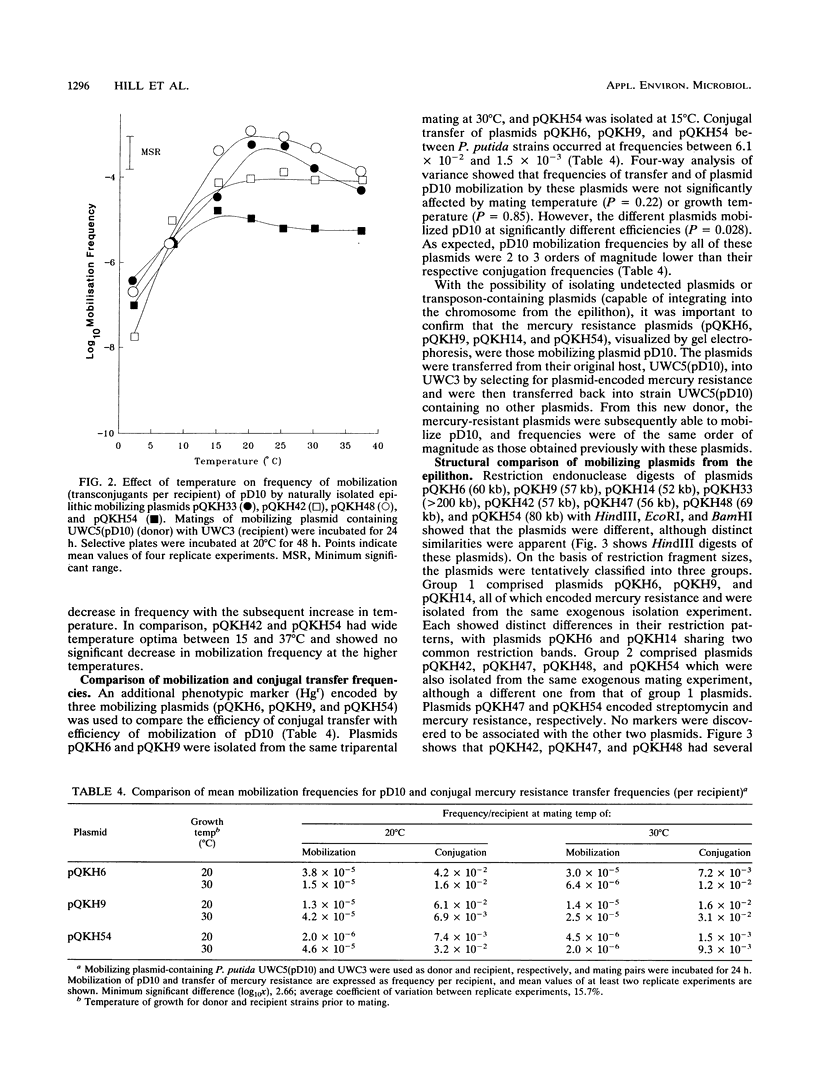
- Rochelle P. A., Fry J. C., Day M. J. Factors affecting conjugal transfer of plasmids encoding mercury resistance from pure cultures and mixed natural suspensions of epilithic bacteria. J Gen Microbiol. 1989 Feb;135(Pt 2):409–424. doi: 10.1099/00221287-135-2-409. [DOI] [PubMed] [Google Scholar]
- Schmidhauser T. J., Helinski D. R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985 Oct;164(1):446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. DNA amplification to enhance detection of genetically engineered bacteria in environmental samples. Appl Environ Microbiol. 1988 Sep;54(9):2185–2191. doi: 10.1128/aem.54.9.2185-2191.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- Willetts N., Crowther C. Mobilization of the non-conjugative IncQ plasmid RSF1010. Genet Res. 1981 Jun;37(3):311–316. doi: 10.1017/s0016672300020310. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]