Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system postulated to be a cell-mediated autoimmune disease in which interferon γ (IFN-γ) plays an important role. There is increased IFN-γ secretion in MS, and IFN-γ administration induces exacerbations of disease. We found that interleukin 12 (IL-12) was responsible for raised IFN-γ secretion in MS as anti-IL-12 antibodies reversed raised anti-CD3-induced IFN-γ in MS patients to normal levels. Furthermore, we found a marked increase in T cell receptor-mediated IL-12 secretion in progressive MS patients vs. controls (24.8 ± 7.7 pg/ml vs. 1.5 ± 1.0 pg/ml, P = 0.003) and vs. relapsing–remitting patients (3.7 ± 1.4 pg/ml, P < 0.05). Investigation of the cellular basis for raised IL-12 demonstrated that T cells from MS patients induced IL-12 secretion from non-T cells, and that T cells from MS patients could even drive non-T cells from normal subjects to produce increased IL-12. Anti-CD40 ligand antibody completely blocked IL-12 secretion induced by activated T cells, and we found increased CD40 ligand expression by activated CD4+ T cells in MS patients vs. controls. The CD40 ligand-dependent Th1-type immune activation was observed in the progressive but not in the relapsing–remitting form of MS, suggesting a link to disease pathogenesis and progression and providing a basis for immune intervention in the disease.
Keywords: autoimmunity, gp39, interferon-γ
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system postulated to be a T cell-mediated autoimmune disease (1). Interferon γ (IFN-γ), a cytokine that is the hallmark of Th1-type immune responses, plays an important role in disease pathogenesis as increased production of IFN-γ precedes clinical attacks (2, 3), and injection of MS patients with recombinant IFN-γ-induced exacerbations of the disease (4). Furthermore, within the nervous system, the inflammatory process is characterized by increased IFN-γ expression (5). Little is known about the immune basis for raised IFN-γ in MS or how it relates to different stages of the disease. Interleukin 12 (IL-12), a cytokine produced by non-T cells, is the most potent inducer of IFN-γ and Th1-type immune responses (6). We recently observed increased expression of IL-12p40 mRNA associated with inflammation in the central nervous system of MS patients but not in patients suffering from stroke (7). Given the importance of IL-12 in IFN-γ induction, we investigated IFN-γ secretion in MS and its potential link to IL-12.
MATERIALS AND METHODS
Subjects.
MS patients were studied from the outpatient MS clinic of the Brigham and Women’s Hospital. Relapsing–remitting patients (n = 18; average age = 44 ± 1.3 years) had an average expanded disability status (EDSS) of 3.9 ± 0.5, and chronic progressive MS patients (n = 33; average age = 46 ± 1.1 years) had an EDSS of 5.7 ± 0.3. A disability of 6 or greater involves use of a cane or other support. Patients had not received immunosuppressive therapy in the past or steroid treatment in the 6 months before blood drawing. The control group consisted of age and sex matched healthy subjects (n = 29; average age = 43 ± 1.8 years). The number of patients used for each individual experiment is given in the corresponding table or figure legends.
Cell Separation.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood by Ficoll/Hypaque density gradient centrifugation (Pharmacia LKB). Cells were resuspended (106 cells/ml) in complete culture media consisting of RPMI 1640 medium (BioWhittaker) supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 25 mM Hepes buffer, 50 units/ml penicillin, and 50 μg/ml streptomycin (all from BioWhittaker). Separation of T cells from PBMC was performed by using negative depletion of non-T cells with human T cell enrichment column (R & D Systems) according to manufacturer’s instructions. Separation of non-T cells (antigen-presenting cells; APCs) from PBMC was done by using negative depletion of T cells with Dynabeads M-450 Pan T (CD2) (Dynal; Great Neck, NY) according to manufacturer’s instructions. Separation of T cells into CD4 depleted (CD4−) and CD8 depleted (CD8−) T cells was performed using Dynabeads M-450 CD4 and Dynabeads M-450 CD8, respectively (Dynal).
Cell Culture.
PBMC activation with soluble anti-CD3. In preliminary experiments during which culture conditions were established, we found that following 2 days of in vitro culture, prominent IFN-γ secretion was observed with anti-CD3 mAb stimulation but not with IL-2 or IL-12. Thus to study the T cell receptor complex (TcR)-mediated pathway of IFN-γ secretion, we chose culture conditions in which 1 ml of PBMC (1 × 106 cells) was placed in polypropylene culture tubes (Fisher Scientific), cultured for 2 days in medium, washed, and then activated with 1 μg/ml of anti-CD3 mAb (American Type Culture Collection; clone OKT3, mouse IgG2a) and culture supernatants were collected 24 or 48 h later. Activated cells were also used for flow cytometry analysis of IL-12 receptor (IL-12R) positive cells.
T cell activation with immobilized anti-CD3.
T cells (1 × 106 cells/well) and/or APCs (5 × 106 cells/well) were placed in the total volume of 1 ml in the wells of a 24-well flat-bottom plate with immobilized anti-CD3 or with immobilized control mouse IgG2a. Culture supernatants were collected after 24-h incubation. Activated T cells were also studied for CD40 ligand expression by flow cytometry after 20-h incubation and at various other times for kinetic studies.
Cytokine ELISA.
IFN-γ and IL-4 determinations in culture supernatants was performed by ELISA using a cytokine ELISA protocol from PharMingen. For IFN-γ and IL-4, 1 μg/ml of capture mouse anti-human IFN-γ mAb or mouse anti-human IL-4 mAb, and 1 μg/ml of biotinylated mouse anti-human IFN-γ mAb or biotinylated rat anti-human IL-4 mAb were used (all from PharMingen). IL-12p70 and IL-2 were determined by appropriate ELISA kits (R & D Systems and BioSource International, Camarillo, CA, respectively). For standards, recombinant human IFN-γ (GIBCO/BRL), recombinant human IL-2 (Boehringer Mannheim), recombinant human IL-4 (PharMingen), and recombinant human IL-12 (R & D Systems) were used. Sensitivity of IFN-γ, IL-2, IL-4, and IL-12 ELISA were 32, 32, 8, and 2 pg/ml, respectively.
Flow Cytometry.
IL-12R-bearing cells were detected as described by Desai et al. (8). Briefly, 1 × 106 cells in 0.1 ml staining buffer (PBS/2% fetal calf serum/0.1% sodium azide) were sequentially incubated with 40 nM unlabeled IL-12 for 40 min, followed by biotinylated rat anti-human IL-12 (Clone 4D6, IgG1, provided by M. K. Gately (Hoffmann–La Roche) or control biotinylated rat IgG1 (clone MP4-25D2; PharMingen) for 20 min, and finally with Streptavidin–phycoerythrin for 20 min. Cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated mAb specific for CD3, CD16 (both from AMAC, Westbrook, ME), CD4, CD8, or control FITC-conjugated mouse Ig (all from Coulter Immunology) according to the manufacturer’s recommendations). T cells activated with immobilized anti-CD3 were stained with FITC-conjugated mAb specific for CD4 (Coulter Immunology) and phycoerythrin-conjugated anti-CD40 ligand mAb (PharMingen) or control FITC- and phycoerythrin-conjugated mouse Ig (Coulter Immunology) according to the manufacturer’s recommendations. All incubations were carried out at 4°C in staining buffer, and cells were washed twice between incubations. Flow cytometric analysis of 5–10 × 103 cells from each sample was performed on an FACSort flow cytometer (Beckton Dickinson) according to standard procedures.
Statistical Analysis.
Results are presented as mean ± SEM for each group. Statistical significance was calculated using Student’s t test.
RESULTS AND DISCUSSION
To investigate the basis for raised IFN-γ production in MS, we stimulated T cells through the TcR with anti-CD3 mAb. We first studied a group of chronic progressive MS patients and healthy control subjects and found a clear increase in TcR-mediated IFN-γ production in MS. Specifically, anti-CD3-induced IFN-γ production was almost 3-fold higher in progressive MS (n = 25) than in control subjects (n = 21), 2132 ± 207 pg/ml in MS vs.756 ± 121 pg/ml in controls, P < 0.001.
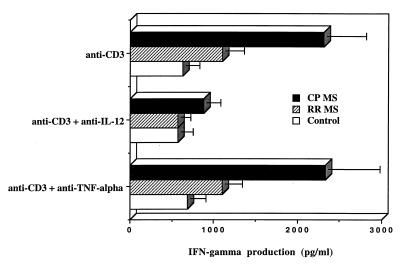
To investigate the possible role of IL-12 in the raised IFN-γ secretion in progressive MS, we added anticytokine neutralizing antibody (Ab) to cultures stimulated with anti-CD3. In addition to progressive MS, we also studied patients with relapsing–remitting disease. Clinically, a classic feature of MS is a course that initially involves relapses followed by remission of disease (9). The relapsing–remitting phase is often followed by a progressive phase that involves progressive neurologic deterioration and is a major source of disability in MS. Some patients have progressive disease from the onset. Why the character of the illness changes from relapsing-remitting to progressive is unknown. As shown in Fig. 1 Top, an increase in anti-CD3-induced IFN-γ production was seen in patients with the chronic progressive form of MS but only minimally in relapsing–remitting subjects. In addition (Fig. 1 Middle), anti IL-12 Ab reversed the elevated anti-CD3-induced IFN-γ secretion in chronic progressive MS to the level of control subjects (P = 0.009). Anti-TNF-α did not affect anti-CD3-induced IFN-γ production in either MS or controls (lower panels). Anti-CD3-induced proliferation, and IL-4 and IL-5 production in chronic progressive MS or controls, were not affected by anti-IL-12 (data not shown).
Figure 1.
Increased TcR-mediated IFN-γ production in MS is linked to defective regulation by endogenous IL-12. PBMC (1 × 106/ml) from controls, relapsing–remitting MS (RR MS), or chronic progressive MS (CP MS) were stimulated with anti-CD3 mAb as described. Neutralizing goat anti-human IL-12 (10 μg/ml, R & D Systems) or isotype control goat anti-human tumor necrosis factor α (TNF-α; 10 μg/ml, R & D Systems) were added to the cultures. After 2 days the levels of IFN-γ production in culture supernatants were measured by ELISA; data are expressed in pg/ml. Data are mean ± SEM from tested control subjects (n = 10), relapsing–remitting MS (n = 9), and chronic progressive MS (n = 11) patients. IFN-γ production in CP MS was significantly higher then in control subjects (P < 0.005) or relapsing–remitting MS (P < 0.05). Neutralizing anti-IL-12 significantly reduced IFN-γ production in chronic progressive MS (P = 0.009) to the level of control subjects. T cell proliferative responses or IL-4 production in MS patients or in control subjects were not affected by anti-IL-12. Isotype control mouse IgG2a did not induce any detectable level of IFN-γ or IL-4.
The IL-12-dependent increase in anti-CD3-induced IFN-γ in chronic progressive MS we observed could be secondary either to increased IL-12R expression on activated T cells in MS or increased biologically active IL-12 production by non-T cells in MS patients. It has been reported that recombinant IL-12 (rIL-12) can provide a costimulatory effect for activated human T cells that express detectable levels of IL-12R, particularly after mitogen stimulation (8, 10). To investigate these possibilities, we first studied IL-12R expression on anti-CD3 activated T cells. However, we found no difference between the number of IL-12R positive activated T cells in eight chronic progressive MS patients (22.6 ± 3.3%) vs. seven control subjects (21.4 ± 4.0%).
We thus investigated whether the raised IFN-γ secretion was secondary to increased IL-12 production in MS patients. To investigate this possibility, we added anti-CD3 to PBMC and measured secretion of IL-12, IL-4, and IL-2 in culture supernatants. As shown in Table 1, there was a marked increase in IL-12 production in chronic progressive MS subjects, which was not observed either in control subjects or relapsing–remitting MS subjects. No changes were observed in IL-4 or IL-2 secretion. To determine whether the amount of increased IL-12 observed in MS patients (24 pg/ml) was sufficient to affect IFN-γ production, we added rIL-12 to cultures of PBMC from control subjects in conjunction with anti-CD3 stimulation. We found that 10 pg/ml of rIL-12 increased anti-CD3-induced INF-γ production 3-fold and this was reversed by neutralizing anti-IL-12 Ab (data not shown).
Table 1.
Increased T cell receptor-mediated IL-12 production in progressive MS
| IL-12, pg/ml | IL-4, pg/ml | IL-2, pg/ml | |
|---|---|---|---|
| Control | 1.5 ± 1.0 (17) | 39.1 ± 7.7 (17) | 719 ± 196 (7) |
| RR MS | 3.7 ± 14 (9) | 37.9 ± 10.4 (9) | ND |
| CP MS | 24.8 ± 7.7 (15)* | 44.5 ± 9.1 (15) | 905 ± 279 (10) |
PBMC were stimulated with anti-CD3 mAb as described, and supernatants were collected 24 h later. The levels of IL-12p70, IL-4, and IL-2 were measured by ELISA and expressed in pg/ml. Data are presented as mean ± SEM (number of subjects tested) for controls, relapsing–remitting MS patients (RR MS) and chronic progressive MS patients (CP MS).
IL-12 secretion in CP MS patients vs control subjects (P = 0.003) and vs. RR MS patients (P < 0.05).
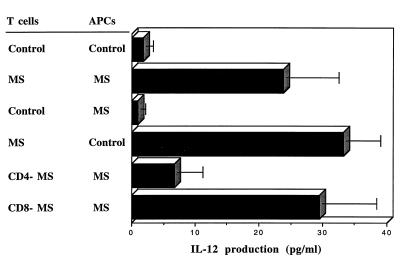
Because anti-CD3 stimulates T cells and IL-12 is produced by non-T cells, we assumed that both cell populations were required for the increased IL-12 secretion in progressive MS patients. To identify whether one or both of the cell populations were abnormal in MS and which cells were responsible for the increased IL-12 in MS, we separated PBMC into T cell or non-T cell populations (APCs) and added them separately to plates coated with immobilized anti-CD3. IL-12 secretion in progressive MS patients was only observed when all three components were present: T-cells, APCs, and anti-CD3 (data not shown). Given this, we then mixed separated populations of T-cells and APCs from MS and normal subjects and measured IL-12 secretion. As shown in Fig. 2, minimal IL-12 was secreted by T cells plus APCs from control subjects whereas large amounts were secreted by MS subjects. However, when mixing experiments were performed, increased IL-12 was only observed when T cells from MS patients were cultured with APCs either from MS patients or controls. No increased IL-12 was observed when APCs from MS patients were cultured with T cells from controls subjects. These results clearly demonstrate that T cells from MS patients are responsible for the increased secretion of IL-12 in progressive MS, which act by inducing the non-T cell population to produce IL-12. To determine which T cell populations were involved, we separated T cells into CD4- and CD8-depleted populations; as shown in Fig. 2, we found that depletion of CD4 cells abrogated increased IL-12 secretion whereas depletion of CD8 cells had no effect.
Figure 2.
Activated T cells but not APCs from progressive MS patients are responsible for increased IL-12 production. PBMC from control subjects and progressive MS patients were separated into APCs (non-T cells), T cells, CD4-depleted T cells (CD4−), or CD8-depleted T cells (CD8−). T cells or their subsets (1 × 106) from the control subject or from a chronic progressive MS patient were then activated with immobilized anti-CD3 mAb in the presence APCs (5 × 105) of either control subject or MS patient. The levels of biologically active IL-12p70 was measured in 24-h culture supernatants by ELISA and are expressed in pg/ml. Data represented by four top bars are mean ± SEM of six independent experiments with different control subjects and MS patients. Data represented by two bottom bars are mean ± SEM of four independent experiments with four different MS patients. APCs from MS patients produced significantly more IL-12 when cultured with activated T cells from MS patients vs. T cells from control subjects (P < 0.025). APCs from control subjects also secreted more IL-12 when cultured with activated T cells from MS patients vs. controls (P < 0.001). APCs from MS patients produced more IL-12 when cultured with autologous CD8-depleted (CD8−) T cells vs. CD4-depleted (CD4−) T cells (P < 0.05). No detectable IL-12 (less then 2 pg/ml) was secreted by APCs alone or T cells alone activated with immobilized anti-CD3, or T cells plus APCs in the presence of immobilized isotype control mouse IgG2a.
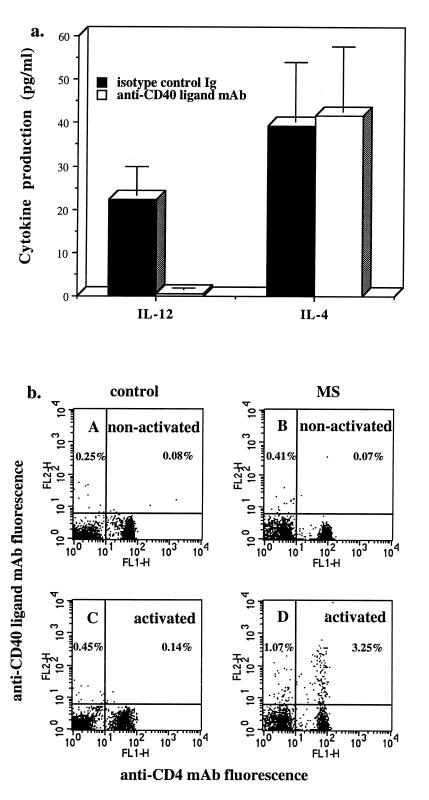
Although we found that the interaction between anti-CD3 activated CD4+ T cells from MS patients with APCs was responsible for raised IL-12 production in CP MS, the nature of the T cell/APC interaction was unknown. To investigate this, we attempted to block IL-12 secretion in chronic progressive MS using mAb directed against cell surface structures known to be involved in T cell interaction with APCs. As shown in Fig. 3a, anti-CD40 ligand mAb completely blocked increased production of IL-12 in patients with progressive MS. No effect of anti-CD40 ligand Ab was seen on IL-4 production in the same culture supernatants. In addition, we found no significant effect on IL-12 production in MS using other Ab or soluble adhesion molecules to block interaction of LFA-1, CD2, or CD28/CTLA-4 (cytolytic T lymphocyte-associated antigen) expressed by T cells with appropriate ligands expressed by APCs (data not shown). These results suggested that anti-CD3 mAb was inducing increased CD40 ligand expression on T cells from MS patients, but not controls. As shown in Fig. 3b, this indeed was the case as a small, but significant increase in CD40 ligand expression occurred in anti-CD3 activated T cells from progressive MS patients but not in controls or relapsing–remitting patients. The expression of CD40 ligand occurred preferentially on activated CD4+ T cells, although a small number of CD40 ligand positive CD8+ T cells was also detected. Because optimal CD40 ligand expression may occur at earlier time points, we investigated the kinetics of CD40 ligand expression in purified T cells from MS patients and controls. As shown in Table 2, CD40 ligand expression first became evident at 24 h and was only observed in progressive MS patients. Although CD40 ligand is required for IL-12 production, other T cell-secreted cytokines may act synergistically with CD40 ligand. We have found that neutralizing anti-IFN-γ antibody reduced, though did not abolish, IL-12 secretion. Additional experiments were performed using phorbol 12-myristate 13-acetate (PMA) plus ionomycin, which is one of the strongest inducers of T cell activation. PMA plus ionomycin dramatically up-regulated CD40 ligand expression on T cells to a similar extent in both control subjects and MS patients with peak expression (56–78%) at 4 h after stimulation.
Figure 3.
(a) Increased TcR-mediated IL-12 production in MS is mediated by activated T cells via CD40 ligand. T cells and APCs from chronic progressive MS patients were activated with immobilized anti-CD3 mAb as described in the legend for Fig. 2. Anti-CD40 ligand mAb (10 μg/ml, clone m92, mouse IgG2a, provided by W. Fanslow, Immunex) or isotype control mouse IgG2a (10 μg/ml; PharMingen) were added from the beginning of the cultures. The levels of biologically active IL-12p70, IL-4, and IFN-γ were measured in 24-h culture supernatants by ELISA in pg/ml. Data are mean ± SEM of seven different experiments with different MS patients. Production of IL-12 was significantly reduced in the presence of anti-CD40 ligand mAb (P = 0.007 vs. isotype control Ig). Production of IFN-γ was 1381 ± 302 pg/ml in cultures with control mouse IgG2a and 634 ± 188 pg/ml in cultures with anti-CD40 ligand mAb. (b) Increased CD40 ligand expression by activated T cells in progressive MS. T cells from a control subject (A and C) or chronic progressive MS subject (B and D) were cultured with immobilized mouse IgG2a (A and B) or immobilized anti-CD3 (C and D) for 20 h. T cells were then stained with FITC-conjugated mAb specific for CD4 (Coulter Immunology) and phycoerythrin-conjugated anti-CD40 ligand mAb (PharMingen), according to the manufacturer’s recommendations. Flow cytometric analysis of 5 × 103 cells from each sample was performed on an FACSort flow cytometer (Beckton Dickinson) according to standard procedures. Data are representative of six separate experiments. For all tested subjects, a significantly higher number of anti-CD3 activated T cells from progressive MS patients expressed CD40 ligand (2.5 ± 0.3%, n = 6) vs. controls (0.6 ± 0.2%, n = 8) or relapsing remitting MS patients (0.6 ± 0.24%, n = 6), P < 0.001, chronic progressive MS vs. control or relapsing–remitting MS. Less than 0.2% of anti-CD3-activated T cells from any tested donor were positively stained with combination of control FITC- and phycoerythrin-conjugated mouse IgG (all from Coulter Immunology).
Table 2.
Kinetics of CD40 ligand expression
| Time after activation, h | Percentage of T cells expressing CD40 ligand
|
||
|---|---|---|---|
| Control | RR MS | CP MS | |
| 0 | 0.05 | 0.10 | 0.26 |
| 6 | 0.08 | 0.17 | 0.64 |
| 12 | 0.10 | 0.11 | 0.80 |
| 24 | 0.14 | 0.17 | 2.58 |
| 36 | 0.07 | 0.14 | 3.14 |
| 48 | 0.94 | 0.43 | 6.45 |
Purified T cells were activated with immobilized anti-CD3 as described in the legend for Fig. 3b. The percentage of T cells that expressed CD40 ligand was determined at various time points after activation. Results shown are representative of three independent experiments. RR MS, relapsing–remitting MS; CP MS, chronic progressive MS.
There are two series of investigations describing anti-CD3-induced CD40 ligand expression by human peripheral blood T cells. In the first, T cells were isolated by positive separation with sheep red blood cells (11–13). This separation led to CD2/LFA3 (lymphocyte function-associated antigen 3) interaction and CD2 engagement on the surface of T cells during separation. It has been reported recently that CD2/LFA3 interaction augments the expression of CD40 ligand on activated human CD4+ T cells (14). Presumably this explains why the level of CD40 ligand expression by T cells was higher in these studies than in our studies. In contrast, when T cells from normal individuals were activated without previous CD2 engagement (15), stimulation of peripheral blood T cells through CD3 by mAb immobilized on plates induced only a minimal expression of the CD40 ligand, that was analogous to our system. Of note is that the phenotype of T cells directly after isolation from normal individuals vs. CP MS did not show generalized activation as measured by increased IL-2R, HLA-DR (human leukocyte antigen, DR region), or IL-12R expression (data not shown).
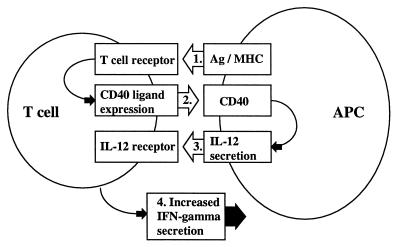
Our results provide an explanation for the altered regulation of IFN-γ in progressive MS patients and suggest that a self-perpetuating series of immune interactions occurs that results in a Th1-type immune response (see Fig. 4). The initiating immunologic event must involve repeated stimulation of the T cells through the Ag/major histocompatibility complex (MHC)/TcR complex, because stimulation through the TcR is required for CD40 ligand expression. It is postulated that this occurs in the relapsing–remitting form of the illness when patients have repeated attacks before entering the progressive phase. Although the trigger for these attacks is unknown, epidemiologic studies suggest that attacks are related in part to repeated viral infections and an increased number of attacks early in the disease is a poor prognostic sign (9, 16, 17). Increased expression of CD40 ligand on T cells then triggers the APC through CD40, an interaction that is known to induce IL-12 secretion by APCs (18–20). IL-12 then acts on T cells to induce the secretion of IFN-γ. Once secreted, IFN-γ itself acts on APCs to further up-regulate IL-12 secretion and perpetuate the cycle leading to a chronic state of Th1-type immune activation. It remains to be determined the degree to which increased CD40 ligand expression in MS is related to an inherent defect in MS T cells or is secondary to the state of T cell differentiation following chronic in vivo activation.
Figure 4.
Mechanism of increased IFN-γ secretion in progressive MS.
The association of IL-12 and CD40 ligand expression with raised IFN-γ secretion in MS is consistent with what is known about the effect of IL-12 and CD40 ligand on Th1-type autoimmune diseases in animals (21, 22). Thus, administration of IL-12 induces the rapid onset of insulin-dependent diabetes mellitus (23) in the NOD mouse. In the experimental autoimmune encephalomyelitis (EAE) model, animals treated with IL-12 in vivo have a more severe and prolonged form of EAE whereas anti-IL-12 reduces the incidence and severity of adoptively transferred EAE (24). Administration of IL-12 enhances, in an IFN-γ-dependent fashion, collagen-induced arthritis (25). Biologically active IL-12 secretion has not been studied in human autoimmune diseases in which it would be expected that enhanced production would be associated not only with disease progression but with increased IFN-γ production. This indeed was found in our MS patient population. IL-12 has also been studied in other human disease states including HIV, where IL-12 production was decreased and exogenous rIL-12 was reported to restore HIV specific cell-mediated immunity in vitro (26, 27).
CD40 ligand is a 33-kDa type II glycoprotein which is transiently expressed on the surface of T cells following activation (28). In the murine system, CD40 ligand is involved in T cell-dependent induction of nitric oxide, TNF-α (29), and IL-12 (18) secretion by macrophages and activated differentiated Th1 cells express a 20-fold greater amount of CD40 ligand than activated nondifferentiated T cells (30). In murine collagen-induced arthritis, disease is blocked by Ab to CD40 ligand (22). In addition, it has recently been shown by Gerritse et al. (31) that CD40–CD40 ligand interactions play an important role in EAE as treatment of animals with anti-CD40 ligand mAb completely prevented development of the disease. Furthermore, these investigators reported an increased number of CD4+ T cells expressing CD40 ligand in MS patient brain sections (31). Our results demonstrate that functional immune abnormalities in the blood of MS patients are linked to CD40–CD40 ligand interactions and are related to disease progression. Thus, increased IL-12 production in progressive MS is mediated by activated T cells via CD40 ligand, a mechanism that has not been previously described in human disease states. Of note is that Stuber et al. (32) have recently observed CD40 ligand-mediated increased IL-12 and Th1-type responses in the mouse model of 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Also, Grewal et al. (33) have recently reported impairment of antigen-specific T cell priming in mice lacking CD40 ligand expression and postulated that antagonists of CD40–CD40 ligand interaction may be of benefit in the treatment of autoimmune diseases such as multiple sclerosis.
In summary our findings establish the presence of immune activation associated with raised IFN-γ in MS which is linked to IL-12 production by non-T cells and to CD40 ligand expression by CD4+ T cells. These immune abnormalities are seen most prominently in patients with the progressive form of the disease, suggesting an important link to disease pathogenesis and progression. Furthermore, these findings not only provide an immunological basis to understand disease mechanisms in MS but identify two molecules that may serve as targets for treatment of the disease.
Acknowledgments
We thank Vijay Kuchroo and Byron Waksman for helpful discussions and critical reading of the manuscript. K.E.B. is a Susan Fuhrbacher Conroy Fellow in Multiple Sclerosis. This research was supported by National Institutes of Health Grants NS23132 and NS2424, a grant from the National Multiple Sclerosis Society, the Foundation for Neurologic Diseases, and the Nancy Davis Center Without Walls.
Footnotes
Abbreviations: MS, multiple sclerosis; IFN-γ, interferon γ; PBMC, peripheral blood mononuclear cells; EAE, experimental autoimmune encephalomyelitis; APC, antigen presenting cell; Ab, antibody; FITC, fluorescein isothiocyanate; IL, interleukin; IL-12R, IL-12 receptor; rIL-12, recombinant IL-12; TNF-α, tumor necrosis factor α; TcR, T cell receptor.
References
- 1.Martin R, McFarland H F, McFarlin D E. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Acta Neurol Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- 3.Lu C-Z, Jensen M A, Arnason B G W. J Neuroimmunol. 1993;46:123–128. doi: 10.1016/0165-5728(93)90241-p. [DOI] [PubMed] [Google Scholar]
- 4.Panitch H S, Hirsch R L, Haley A S, Johnson K P. Lancet. 1987;i:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 5.Woodroofe M N, Cuzner M L. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 7.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe M N, Cuzner M L, Hafler D A. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai B B, Quinn P M, Wolitzky A G, Mongini P K A, Chizzonite R, Gately M K. J Immunol. 1992;148:3125–3132. [PubMed] [Google Scholar]
- 9.Matthews W B, Acheson E D, Batchelor J R, Weller R O. In: McAlpine’s Multiple Sclerosis. Matthews W B, editor. Edinburg: Churchill Livingstone; 1985. pp. 49–72. [Google Scholar]
- 10.Chizzonite R, Truitt T, Desai B B, Nunes P, Podlaski F J, Stern A S, Gately M K. J Immunol. 1992;148:3117–3124. [PubMed] [Google Scholar]
- 11.Patel H R, Oshiba A, Jeppson J D, Gelfand E W. J Immunol. 1996;156:1781–1787. [PubMed] [Google Scholar]
- 12.Splawski J B, Nishioka J, Nishioka Y, Lipsky P E. J Immunol. 1996;156:119–127. [PubMed] [Google Scholar]
- 13.Spriggs M K, Armitage R J, Strockbine L, Clifford K N, Macduff B M, Sato T A, Maliszewski C R, Fanslow W C. J Exp Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmann K, Hughes C C W, Fanslow W C, Pober J C. Eur J Immunol. 1996;26:610–617. doi: 10.1002/eji.1830260316. [DOI] [PubMed] [Google Scholar]
- 15.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Eur J Immunol. 1992;22:2573–2578. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 16.Panitch H S. Ann Neurol. 1994;36:S25–S28. doi: 10.1002/ana.410360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sibley W A, Bamford C R, Clark K. Lancet. 1985;i:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy M K, Picha K S, Fanslow W C, Grabstein K H, Alderson M R, Clifford K N, Chin W A, Mohler K M. Eur J Immunol. 1996;26:370–378. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 19.Shu U, Kinawa M, Wu C Y, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 20.Kato T, Hakamada R, Yamane H, Nariuchi H. J Immunol. 1996;156:3932–3938. [PubMed] [Google Scholar]
- 21.Trembleau S, German T, Gately M K, Adorini L. Immunol Today. 1995;16:383–386. doi: 10.1016/0167-5699(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 22.Durie F H, Fava R A, Foy T M, Aruffo A, Ledbetter J A, Noelle R J. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 23.Trembleau S, Penna G, Bosi E, Mortara A, Gately M K, Adorini L. J Exp Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard J P, Waldburger K E, Goldman S G. J Exp Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Germann T, Szeliga J, Hess H, Storkel S, Podlaski F J, Gately M K, Schmitt E, Rude E. Proc Natl Acad Sci USA. 1995;92:4823–4827. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clerici M, Lucey D R, Berzofsky J A, Pinto L A, Wynn T A, Blatt S P, Dolan M J, Hendrix C W, Wolf S F, Shearer G M. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 27.Chehimi J, Starr S E, Frank I, D’Andrea A, Ma X, MacGregor R R, Sennelier J, Trinchieri G. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanslow W C, Srinivasan S, Paxton R, Gibson M G, Spriggs M K, Armitage R J. Semin Immunol. 1994;6:267–278. doi: 10.1006/smim.1994.1035. [DOI] [PubMed] [Google Scholar]
- 29.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 30.Roy M, Waldschmidt T, Aruffo A, Ledbetter J A, Noelle R J. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 31.Gerritse K, Laman J D, Noelle R J, Aruffo A, Ledbetter J A, Boersma W J A, Claassen E. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stuber E, Strober W, Neurath M. J Exp Med. 1996;184:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grewal I S, Xu J, Flavell R A. Nature (London) 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]