Abstract
Bruton’s tyrosine kinase (Btk) is essential for B cell activation, but downstream targets of Btk have not been defined. We now describe a protein, BAP-135, that is associated with Btk in B cells and is a substrate for phosphorylation by Btk. BAP-135, which exhibits no detectable homology to known proteins, contains six occurrences of a hitherto undescribed amino acid repeat and two motifs, similar to the Src autophosphorylation site, that represent potential targets for tyrosine phosphorylation. The pleckstrin homology domain of Btk comprises the principal site of BAP-135 binding. Btk-dependent phosphorylation of BAP-135 is abolished by mutations that impair activation of Btk by Src-related kinases. Btk and BAP-135 exist in a complex before B cell antigen receptor (BCR) engagement; in response to BCR crosslinking, BAP-135 is transiently phosphorylated on tyrosine. Taken together, these observations suggest that BAP-135 may reside downstream of Btk in a signaling pathway originating at the BCR.
Engagement of the B cell antigen receptor (BCR) initiates a cascade of tyrosine phosphorylation, resulting in the activation of Ras- and phospholipase-dependent signal transduction pathways and culminating in proliferative and differentiative responses. Antigenic simulation of B cells induces immediate activation of the Src-related tyrosine kinases Lyn, Fyn, and Blk, followed within minutes by activation of Bruton’s tyrosine kinase (Btk) and the tyrosine kinase Syk (1). Mutations in Btk are associated with X-linked agammaglobulinemia in man (2, 3) and with impaired B cell proliferation in response to antigen receptor engagement in the mouse (4, 5). While such genetic evidence indicates that Btk is essential for normal immune responsiveness, the function of this kinase is unknown.
Btk and its homologues Itk (6, 7), Tec (8), and Bmx (9) are distinguished by the presence of a canonical pleckstrin homology (PH) domain at the amino terminus, followed by cysteine-rich and proline-rich regions, which together have been termed the Tec homology (TH) domain (10). The proline-rich portion of the TH domain mediates interactions with Src homology-3 (SH3) domains in vitro and may stabilize regulatory interactions with Src-type kinases in vivo (11, 12). The cysteine-rich region, which lies adjacent to the canonical PH domain, may represent a functional extension of the latter (10). While interactions between the PH domain of Btk and protein kinase C (13) or G protein βγ subunits (14) have been reported, evidence for formation of stable complexes with these proteins in vivo has been lacking.
As one approach to identifying signal transduction pathways in which Btk and its homologues function, we sought to identify proteins that interact specifically with Btk in vivo. In this communication, we describe a hitherto unidentified protein, BAP-135, that is associated in vivo with the PH domain of Btk. BAP-135 is a substrate for phosphorylation by Btk; mutations that impair activation of Btk by Src-related kinases abolish Btk-dependent phosphorylation of BAP-135. BAP-135 is transiently phosphorylated on tyrosine in response to B cell receptor crosslinking. Taken together, these observations suggest that BAP-135 lies downstream of Btk in a signaling pathway originating at the BCR.
MATERIALS AND METHODS
Cell Lines.
The human B lymphoid cell line RAMOS was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 50 μg/ml streptomycin, and 50 units/ml penicillin. The 293 cell line was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 50 μg/ml streptomycin, and 50 units/ml penicillin. All cells were grown at 37°C in 5% CO2.
Antibodies.
Polyclonal rabbit anti-Btk antibodies Ab1280 and Ab1300 were described previously (12). Polyclonal rabbit antibody Ab2240 was generated against the synthetic peptide KFEAHPNDLYVEGLPENIPFR, corresponding to amino acid residues 453–473 of BAP-135. Goat F(ab′)2 anti-human IgM (μ chain-specific) and goat F(ab′)2 anti-mouse IgG were obtained from Southern Biotechnology Associates; rabbit F(ab′)2 anti-goat IgG was provided by Jackson ImmunoResearch.
Stimulation of RAMOS Cells.
For stimulation with pervanadate, RAMOS cells were washed once with serum-free RPMI 1640 medium, resuspended in serum-free RPMI 1640 medium, and incubated at 37°C for 30 min. Pervanadate was added to 1 mM and cells were incubated at 37°C for 10 min. For BCR stimulation, RAMOS cells (1.5–2 × 107 cells in 1 ml) were washed and preincubated in serum-free medium as above and subsequently incubated for 10 min on ice with 25 μg of goat F(ab′)2 anti-human IgM (μ chain-specific) or goat F(ab′)2 anti-mouse IgG. Following addition of 25 μg of rabbit F(ab′)2 anti-goat IgG, incubation was continued for 10 min at 37°C. For kinetic analysis of BAP-135 phosphorylation, addition of secondary antibodies was omitted, and cells were incubated at 37°C for the indicated times.
Immunoprecipitation and Immune Complex Kinase Assays.
To examine proteins associated with Btk, RAMOS cells were lysed in a buffer containing 150 mM NaCl, 25 mM Tris·Cl (pH 8.0), 1 mM Na3VO4, 1 mM Na2MoO2, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1% Nonidet P-40, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 5 μg/ml pepstatin (buffer N), and Btk was immunoprecipitated as described (12). After three washes with Buffer N, immune complexes were fractionated by SDS/PAGE and transferred to nitrocellulose. For immunoprecipitation of BAP-135, RAMOS cells were lysed in 150 mM NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate (DOC), 0.1% SDS, 50 mM Tris (pH 8.0), 1 mM Na3VO4, 1 mM Na2MoO4, 1 mM PMSF, 1% NP40, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 5 μg/ml pepstatin. Immune complex kinase assays were performed as described (15).
Protein Purification and Peptide Sequencing.
RAMOS cells (5.3 × 109) were washed once with ice-cold PBS containing 5 mM EDTA and lysed (cell density, 3 × 108 cells per ml) in buffer N at 4°C for 1 hr. Lysates were clarified by centrifugation at 100,000 × g and sequentially precleared with rabbit serum-bound agarose and protein-A Sepharose. Btk was immunoprecipitated by addition of 3 mg of affinity-purified antibody Ab1300 at 4°C for 4 hr; following addition of protein-A Sepharose, incubation was continued at 4°C for 3 hr. After washing in buffer N, immunoprecipitated protein was eluted into SDS sample buffer at 37°C for 15 min, and the eluate was fractionated in multiple lanes of a SDS/6% polyacrylamide gel. Protein was transferred electrophoretically to a 0.2-micron polyvinylidene difluoride (PVDF) membrane; before transfer, the lower part of the gel, containing Ig heavy chain, was removed. Filter-bound protein was stained with amido black, and regions of the membrane containing p135 or p140 were excised. Tryptic digestion of p135 and p140, peptide isolation and microsequence determination were carried out under the direction of David Reim (Wistar Protein Microchemistry Laboratory, Philadelphia).
Cotransfection Assays.
Transient transfection into 293 cells was performed as described (12). Cells were washed at 48 hr after transfection with ice-cold PBS containing 5 mM EDTA and lysed in buffer N. Immunoprecipitation of myc epitope-tagged BAP-135 was carried out with anti-myc antibody 9E10 as described above. For reciprocal coprecipitation experiments, cells were lysed in RIPA buffer supplemented with protease inhibitors as described above, and immunoprecipitation was performed using affinity-purified anti-Btk antibody Ab1280. Before fractionation by SDS/PAGE, immune complexes were washed three times with RIPA buffer, once with 500 mM NaCl/50 mM Tris·Cl, pH 7.4, and twice with water. Each wash was carried out for 5 min at 4°C.
Construction of Glutathione S-Transferase (GST) Fusion Proteins.
DNA fragments encoding the SH3 (residues 219–268) or Src homology-2 (SH2; residues 269–382) domain of Btk were amplified by PCR as described (12), using murine Btk cDNA as a template. Forward and reverse primers incorporated BamHI and EcoRI sites, respectively, at their 5′ ends. The amplified products were cloned between the BamHI and EcoRI restriction sites of the Escherichia coli expression vector pGEX-2T to produce the plasmids pGEX-2T-BtkSH3 and pGEX-2T-BtkSH2. All constructions were verified by nucleotide sequencing. Construction of pGEX-2T-BtkN, pGEX-2T-BtkN1, and pGEX-2T-BtkN2, expression of GST fusion proteins, purification, and binding assays have been described (12).
Immunoblot Assays.
After SDS/PAGE, protein was transferred to nitrocellulose or PVDF membranes; these were incubated with primary antibodies for 2–10 hr at room temperature. Anti-phosphotyrosine antibody 4G10 (United Biotechnology, Lake Placid, NY) was used at 1 μg/ml. Anti-Btk antibody Ab1280 was used at 1:500 dilution. Anti-BAP-135 antibody Ab2240 was used at 1:200 dilution. Anti-myc 9E10 ascites fluid was used at 1:1000 dilution. Immunoreactive proteins were detected by incubation with horseradish peroxidase-coupled goat anti-mouse or goat anti-rabbit IgG for 1 hr at room temperature; immobilized HRP was visualized by an enhanced chemiluminescence assay (Amersham)
RESULTS AND DISCUSSION
Identification of Phosphotyrosine-Containing Proteins Associated with Btk in B Lymphoid Cells.
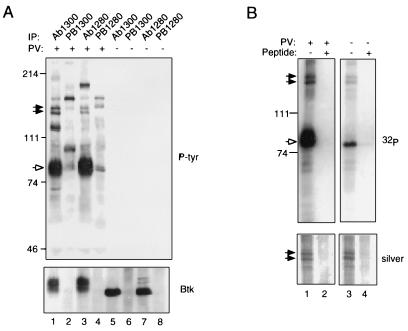
To identify potential substrates associated with Btk in vivo, Btk was immunoprecipitated from the human B lymphoid cell line RAMOS using antibodies directed against two distinct epitopes, and these immunoprecipitates were examined for the presence of common, phosphotyrosine-containing proteins. Two such proteins, with apparent molecular masses of 135 and 140 kDa, were detected in specific immunoprecipitates from pervanadate-treated cells (Fig. 1A, lanes 1 and 3) but not in preimmune controls (Fig. 1A, lanes 2 and 4) or in immunoprecipitates from untreated cells (Fig. 1A, lanes 5–8). In addition to Btk, the p135 and p140 species were phosphorylated in anti-Btk immune complexes in vitro (Fig. 1B Upper, lane 3) and phosphorylation of all three proteins was increased in complexes obtained from pervanadate-treated cells (Fig. 1B Upper, lane 1). Similar amounts of p135 and p140 were detectable by silver staining in Btk immunoprecipitates from stimulated and unstimulated cells (Fig. 1B Lower, lanes 1 and 3), indicating that these proteins are associated with Btk before pervanadate stimulation and that pervanadate treatment results in increased phosphorylation of p135 and p140 in vivo. The results suggest, furthermore, that the pervanadate-induced increase in phosphorylation of p135 and p140 in vitro is the result of increased Btk activity.
Figure 1.
Btk is associated with proteins of 135 and 140 kDa in B lymphoblastoid cells. (A) Coimmunoprecipitation assays. RAMOS cells were treated for 10 min with 1 mM pervanadate (lanes 1–4) or they were left untreated (lanes 5–8), and they were all lysed in the presence of 1% Nonidet P-40 (12). Immunoprecipitation was performed using 10 μl of preimmune sera (lanes 2, 4, 6, and 8) or anti-Btk antisera (lanes 1, 3, 5, and 7) as described (12). Phosphotyrosine-containing proteins present in immune complexes were detected by immunoblotting with anti-phosphotyrosine antibody 4G10 (Upper). Btk was detected using anti-Btk antibody Ab1280 (Lower). Btk is indicated by the open arrow; p135 and p140 by filled arrows. (B) Immune complex kinase assays. Btk was immunoprecipitated from lysates of pervanadate-stimulated (lanes 1 and 2) or unstimulated (lanes 3 and 4) RAMOS cells using affinity-purified antibody Ab1300 in the presence (lanes 2 and 4) or absence (lanes 1 and 2) of competitor peptide. Immune complex kinase assays were carried out as described (15). Protein was fractionated by 7.5% SDS-PAGE and detected by silver staining (Lower); after alkalai treatment to preferentially dephosphorylate phosphoserine and phosphothreonine residues, 32P was detected by autoradiography (Upper). Positions and masses of standards (in kDa) are indicated at left.
Identification of BAP-135 by Purification and Molecular Cloning.
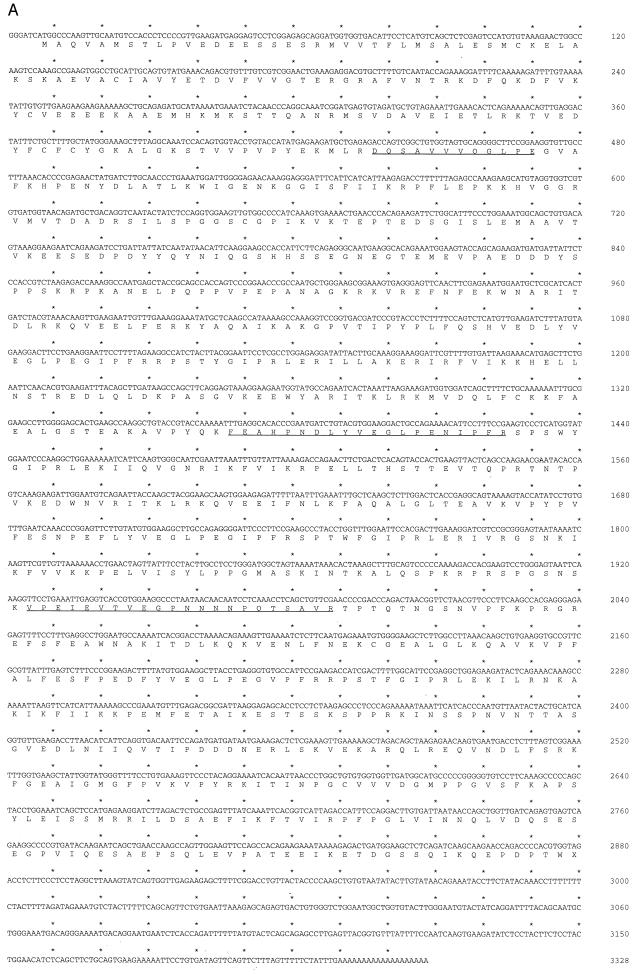
p135 and p140 were isolated from 5 × 109 RAMOS cells by large-scale immunoprecipitation and subjected to tryptic digestion. By reverse-phase chromatography, the tryptic peptide elution profiles for p135 and p140 were nearly identical, indicating that these proteins are highly related (data not shown). Amino acid sequences of three peptides from p135 were determined and these were found to identify overlapping, human expressed sequence tags (ESTs) in the GenBank dbEST data base. Two of these EST clones (identification numbers 147504 and 23797; GenBank accession nos. R81199R81199 and T77416T77416, respectively) were used to construct the intact p135 coding sequence (Fig. 2A). Two p135-derived peptides are encoded in clone 147504; the third peptide is encoded in clone 23797. The nucleotide sequence of the reconstructed cDNA was determined independently and confirmed by comparison to independently derived ESTs in the dbEST data base. The putative initiator methionine codon occurs within a context favorable for translation (16) and is preceded in multiple overlapping ESTs by termination codons in all three phases (data not shown). The resulting open reading frame predicts a protein of 957 aa (molecular mass, 107.9 kDa) that we have termed BAP-135 (for Btk-associated protein of 135 kDa). The discrepancy between the calculated and observed molecular masses may be attributable to a high representation of acidic residues (13.9% Glu or Asp), particularly evident in the amino terminal portion of the protein.
Figure 2.
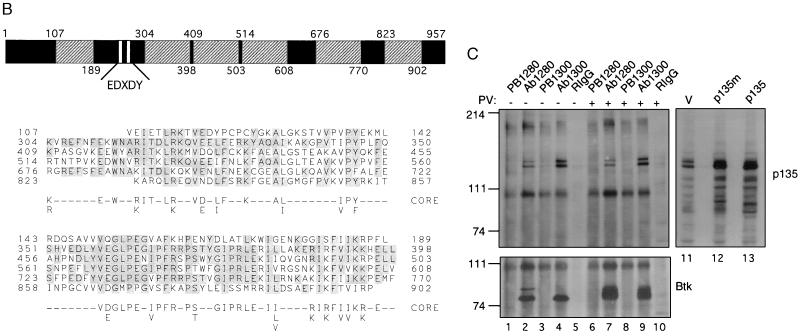
(A) Sequence of BAP-135. The nucleotide sequence is shown on the upper line; the deduced amino acid sequence is shown on the lower line. Sequences of tryptic peptides used to identify BAP-135 cDNA are underlined. (B) Identification of a repetitive amino acid motif in BAP-135. The BAP-135 amino acid sequence is diagrammed (Upper). The 95-residue repeat unit (hatched boxes), sequences homologous to the Src autophosphorylation site (open boxes), and regions of unique sequence (solid boxes) are indicated. Numbers refer to amino acid residues at the boundaries of repeat units. Amino acid sequences of the individual repeats are compared (Lower). Residues are numbered at left and right; amino acid identities, with reference to the second repeat (residues 304–398), are shaded. A consensus sequence (core) was derived by comparison of the four full-length repeats (residues 304–398, 409–503, 514–608, and 676–770). (C) BAP-135 is associated with Btk in B cells. Immunoprecipitation from lysates of pervanadate-stimulated (lanes 6–10) or unstimulated (lanes 1–5) RAMOS cells was performed using anti-Btk antibodies (Ab1280, lanes 2 and 7; Ab1300, lanes 4 and 9), preimmune sera (lanes 1, 3, 6, and 8), or rabbit IgG (lanes 5 and 10). BAP-135 (Upper) was detected by immunoblotting with Ab2240; Btk (Lower) was detected with Ab1280. Epitope-tagged (lane 12) and untagged (lane 13) BAP-135 coding sequences were transferred to the expression vector pCIS-2 and expressed in 293 cells by transient transfection; BAP-135 was detected in total lysates of untransfected (lane 11) and transfected cells by immunoblotting.
The predicted amino acid sequence of BAP-135, which exhibits no detectable homology to known proteins, is remarkable for six occurrences of a hitherto undescribed amino acid repeat (Fig. 2B). The four inner repeats each contain 95 residues and are the most highly related, exhibiting 55–58% identity. The two flanking repeats are somewhat shorter and less highly conserved. A second noteworthy feature is the occurrence of two motifs, at codons 244–248 and 273–277, similar to the Src autophosphorylation site; these represent potential targets for tyrosine phosphorylation. Among human tissues, BAP-135 transcripts were detected in spleen, thymus, prostate, testes, uterus, intestine, and peripheral blood leukocytes (data not shown).
Using an antibody directed against residues 453–473 of BAP-135 (Ab2240), both p135 and p140 were detected in anti-Btk immunoprecipitates from RAMOS cells (Fig. 2C, lanes 2, 4, 7, and 9) but not in control precipitates performed with preimmune sera (Fig. 2C, lanes 1, 3, 6, and 8) or rabbit IgG (Fig. 2C, lanes 5 and 10). Furthermore, the amount of p135 or p140 coprecipitating with Btk did not change upon pervanadate stimulation (Fig. 2C, compare lanes 2 and 4 to lanes 7 and 9). Lastly, expression of intact or c-myc epitope-tagged versions of the BAP-135 open reading frame in 293 cells yielded a protein, detectable with the anti-BAP-135 antibody, that comigrated with endogenous p135 (Fig. 2C, lanes 11–13). These observations confirm: (i) that the BAP-135 cDNA encodes the 135-kDa protein originally detected in Btk immunoprecipitates; (ii) that p135 and p140 are related; and (iii) that in RAMOS cells both proteins are associated with Btk prior to pervanadate stimulation. As the tryptic peptide maps of p135 and p140 are very similar (see above), their amino acid sequences are likely to be largely identical; thus, the ability of p140 to react with antibody raised against p135 is expected. Because the BAP-135 cDNA appears to direct the specific expression of the faster migrating protein upon transient transfection (Fig. 2C, lanes 11–13), the difference between p135 and p140 is not likely to result from differential posttranslational modification. Rather, p135 and p140 may represent products of distinct RNA species.
Interactions Between BAP-135 and Btk Reconstituted in Vitro and in Vivo.
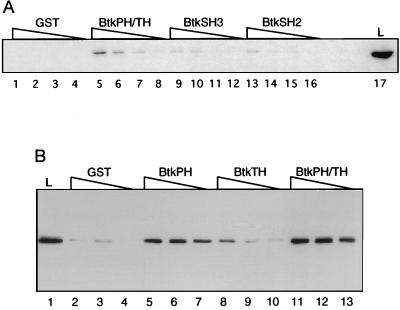
An in vitro binding assay was used to identify regions of Btk responsible for interaction with BAP-135. An epitope-tagged version of BAP-135 (p135m) was preferentially retained by fusion proteins containing residues 1–218 of Btk, comprising the PH and TH domains (Fig. 3A, lanes 5–8) but weakly or not at all by SH3 (Fig. 3A, lanes 9–12), SH2 (Fig. 3A, lanes 13–16), or GST alone (Fig. 3A, lanes 1–4). In a separate experiment, a fusion protein containing the canonical PH domain of Btk, in the absence of of the TH domain, retained the ability to bind BAP-135, albeit with reduced efficiency (Fig. 3B, compare lanes 5–7 and lanes 11–13); the isolated TH domain, in contrast, showed relatively weak binding (Fig. 3B, lanes 8–10). Likewise, p135 and p140, obtained by dissociation of anti-Btk immune complexes from RAMOS cells, were bound preferentially by a fusion protein containing the Btk PH and TH domains (data not shown). These results are consistent with the proposal that in the Btk kinase subfamily the TH domain contains a functional extension of the PH domain (10).
Figure 3.
Specific binding of BAP-135 to the combined PH and TH domains of Btk in vitro. (A) A lysate of 293 cells expressing BAP-135 was diluted 3-fold serially and incubated with agarose beads bearing GST (lanes 1–4), or GST fused to residues 1–218 (PH/TH, lanes 5–8), 219–268 (SH3, lanes 9–12), or 269–382 (SH2, lanes 13–16) of Btk. Retained protein was fractionated by SDS/PAGE and tagged BAP-135 was detected with anti-c-myc antibody 9E10. An amount of total lysate corresponding to the highest dilution used in binding assays was run in lane 17. (B) Binding assays were performed as in A, using GST fusions to residues 1–80 (PH, lanes 5–7), 104–218 (TH, lanes 8–10), or 1–218 (PH/TH, lanes 11–13) of Btk.
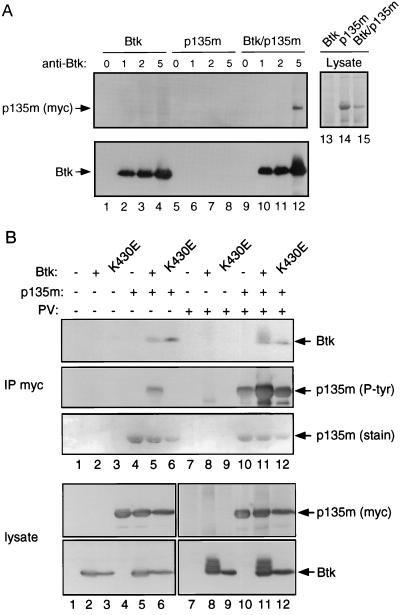
Association of BAP-135 with Btk was reconstituted upon cotransfection. Epitope-tagged BAP-135 was precipitated by an anti-Btk antibody when coexpressed with Btk in 293 cells (Fig. 4A, lanes 9–12) but not in the absence of Btk (Fig. 4A, lanes 5–8). Conversely, Btk could be detected in anti-myc immunoprecipitates only when coexpressed with epitope-tagged BAP-135 (Fig. 4B, compare lanes 5 and 11 with lanes 2 and 8). Association does not require Btk kinase activity, as catalytically inactive Btk also precipitated with BAP-135 (Fig. 4B, lanes 6 and 12). We observed earlier that BAP-135 is phosphorylated in anti-Btk immune complexes (Fig. 1B); consistent with this, BAP-135 was phosphorylated on tyrosine when coexpressed with wild-type Btk (Fig. 4B, lane 5) but not when expressed alone (Fig. 4B, lane 4) or in the presence of catalytically inactive Btk (Fig. 4B, lane 6). In contrast, overexpression of Fyn, while resulting in nonselective phosphorylation of numerous endogenous proteins on tyrosine, had little or no effect on the phosphotyrosine content of BAP-135, as evidenced by anti-phosphotyrosine immunoblotting and phosphoamino acid analysis (data not shown). Treatment of 293 cells with pervanadate resulted in increased tyrosine phosphorylation of BAP-135 regardless of coexpression with Btk (Fig. 4B, lanes 10–12). The extent of tyrosine phosphorylation, however, was substantially greater in the presence of catalytically active Btk (Fig. 4B, lane 11) than in its absence. The increase in tyrosine phosphorylation of BAP-135 seen upon cotransfection with Btk is likely to occur before cell lysis, rather than during immunoprecipitation, as similar results were obtained by anti-phosphotyrosine immunoblotting of total cell lysates prepared by boiling in 1% SDS (Fig. 5, lane 4). Taken together, these observations indicate that BAP-135 is a highly selective substrate in vivo for Btk or for a kinase whose activity depends on Btk.
Figure 4.
Association of Btk with BAP-135 in transfected cells. (A) Cells expressing Btk (lanes 1–4), epitope-tagged BAP-135 (lanes 5–8), or both (lanes 9–12) were lysed in RIPA buffer containing protease inhibitors. Btk was immunoprecipitated in the presence of 0 μg (lanes 1, 5, and 9), 1 μg (lanes 2, 6, and 10), 2 μg (lanes 3, 7, and 11), or 5 μg (lanes 4, 8, and 12) of affinity-purified Ab1280. Precipitates were washed three times with RIPA buffer, once with a buffer containing 0.5 M NaCl and 50 mM Tris (pH 7.4) and twice with water. Proteins were separated by SDS/PAGE; epitope-tagged BAP-135 was detected by immunoblotting with antibody 9E10 (Upper, lanes 1–12) and Btk by Ab1280 (Lower, lanes 1–12). Total cell lysates were fractionated in lanes 13–15 at 1/10 the amount used for immunoprecipitation, and overexpressed BAP-135 was visualized by staining with amido black. (B) 293 cells expressing Btk (lanes 2 and 8), kinase-inactive Btk (lanes 3 and 9), epitope-tagged BAP-135 (lanes 4 and 10), Btk and epitope-tagged BAP-135 (lanes 5 and 11), kinase-inactive Btk and epitope-tagged BAP-135 (lanes 6 and 12), and cells transfected with vector alone (lanes 1 and 7) were lysed in the presence of 1% Nonidet P-40 (12). Before lysis, cells were treated with pervanadate (lanes 7–12) or untreated (lanes 1–6). Immunoprecipitation of epitope-tagged BAP-135 was carried out with antibody 9E10 and proteins were fractionated by SDS/PAGE (IP myc). In the upper three panels, Btk was detected by immunoblotting with Ab1280 (Btk, Top), tyrosine phosphorylated BAP-135 was detected by immunoblotting with antibody 4G10 (p135m [P-tyr], Middle) and BAP-135 protein was detected by amido black staining (p135m [stain], Bottom). In the lower two panels, total cell lysates were fractionated at one-tenth the amount used for immunoprecipitation (lysate); BAP-135 was detected by immunoblotting with 9E10 (p135m [myc], Upper) and Btk was detected with Ab1280 (Btk, Lower).
Figure 5.
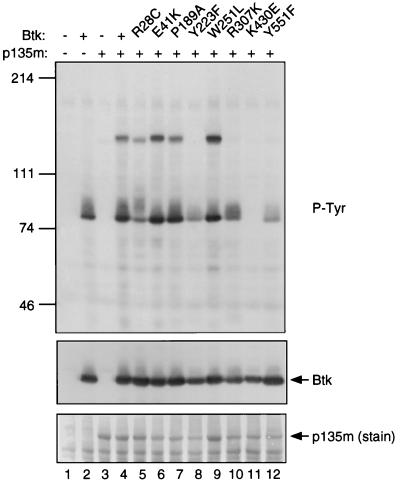
Btk-dependent tyrosine phosphorylation of BAP-135. 293 cells were transfected with vector alone (lane 1), Btk alone (lane 2), epitope-tagged BAP-135 alone (lane 3), epitope-tagged BAP-135 with wild-type Btk (lane 4), or epitope-tagged BAP-135 with the following Btk mutants: R28C (lane 5), E41K (lane 6), P189A (lane 7), Y223F (lane 8), W251L (lane 9), R307K (lane 10), K430E (lane 11), and Y551F (lane 12). Total cell lysates were fractionated by SDS/PAGE, and phosphotyrosine-containing proteins were detected by immunoblotting with antibody 4G10 (P-tyr, Top). The filter was subsequently probed for Btk using Ab1280 (Btk, Middle), and BAP-135 was detected by amido black staining (p135m [stain], Bottom).
Mutations in Btk Affecting Phosphorylation of BAP-135.
Two sites of phosphorylation within Btk, Y551, and Y223, have been implicated as important in regulation of biological activity (17, 18). Phosphorylation of Btk at Y551 by Src-related kinases such as Lyn results in increased enzymatic activity, leading to enhanced autophosphorylation at Y223 (17, 18). While the specific physiologic consequences of Btk autophosphorylation are as yet undefined, mutation of Y223 is synergistic with a second mutation (E41K) in cell transformation assays (17), suggesting that phosphorylation at Y223 alters interactions with factors that regulate Btk’s biological activity. Btk-dependent tyrosine phosphorylation of BAP-135 is profoundly impaired by mutation of either Y551 or Y223 (Fig. 5, lanes 8 and 12). The effect of the Y551F mutation is interpreted to indicate that phosphorylation of BAP-135 requires prior activation of Btk, which is presumably accomplished by a tyrosine kinase expressed endogenously in 293 cells. Phosphorylation of BAP-135 is also impaired by mutation of the phosphotyrosine-binding site in the Btk SH2 domain (R307K; Fig. 5, lane 10), suggesting a role for SH2-mediated interactions in phosphorylation of BAP-135 or in protection of phosphorylated BAP-135 from phosphatase activity. In contrast, point mutations associated with murine X-linked immunodeficiency (R28C), cell transformation (E41K), impairment of SH3 binding to the TH domain (P189A), or SH3 inactivation (W251L) had little or no effect on BAP-135 phosphorylation (Fig. 5, lanes 5–7 and 9).
Phosphorylation of BAP-135 on Tyrosine in Response to BCR Engagement.
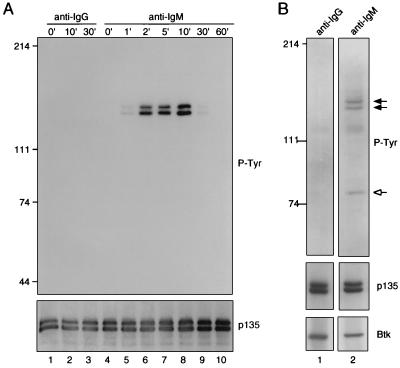
The foregoing experiments indicate that BAP-135 is associated with Btk in vivo and suggest that BAP-135 is phosphorylated on tyrosine as a direct or indirect consequence of Btk activation. Consistent with this relationship and with the activation of Btk by BCR engagement, BAP-135 was sharply and transiently phosphorylated on tyrosine following crosslinking of surface immunoglobulin. Tyrosine phosphorylation of BAP-135 reached a maximum within ≈10 min of BCR stimulation, declining to an undetectable level by 60 min after administration of anti-IgM (Fig. 6A). Similar amounts of BAP-135 were detectable in anti-Btk immune complexes before and after surface immunoglobulin crosslinking (Fig. 6B), indicating (i) that Btk and BAP-135 are physically associated before BCR engagement and (ii) that tyrosine phosphorylation of Btk and BAP-135 in response to BCR stimulation occurs within this preformed complex.
Figure 6.
BAP-135 is phosphorylated on tyrosine in response to surface immunoglobulin crosslinking. (A) Serum-starved RAMOS cells were stimulated with goat anti-human IgM F(ab′)2 (μ heavy chain-specific; lanes 4–10) or goat anti-mouse IgG F(ab′)2 (lanes 1–3) for the indicated times at 37°C. Cells were lysed in RIPA buffer and BAP-135 was immunoprecipitated under conditions described in Fig. 3C. Following fractionation by SDS/PAGE, phosphotyrosine was detected by immunoblotting (Upper); BAP-135 was subsequently detected by probing with Ab2240 (Lower). (B) BCR-induced tyrosine phosphorylation of Btk and BAP-135 occurs within a preformed complex. RAMOS cells were stimulated with goat anti-human IgM F(ab′)2 (lane 2) or goat anti-mouse IgG F(ab′)2 (lane 1) on ice for 10 min, followed by incubation with rabbit anti-goat IgG at 37°C for 10 min. Cells were lysed in the presence of 1% Nonidet P-40 and Btk was immunoprecipitated. Phosphotyrosine-containing proteins (P-tyr, Top), BAP-135 (p135, Middle), and Btk (Btk, Bottom) were detected by sequential immunoblotting.
Aside from receptor or coreceptor components and tyrosine kinases themselves, relatively few targets of BCR-induced phosphorylation have been identified. These include enzymes involved in metabolism of lipid second messengers and potential mediators or regulators of the Ras signaling pathway (19). Through its physical association with Btk, we have identified BAP-135 as an additional target for BCR-induced tyrosine phosphorylation. Moreover, observations presented above suggest that BAP-135 occupies a position immediately downstream of Btk in the BCR-mediated signaling pathway. This hypothesis suggests the existence of immunodeficient patients, lacking mutations in Btk but with a clinical presentation resembling X-linked agammaglobulinemia, who rather have defects in BAP-135. One should note, however, that the tissue distribution of BAP-135 is substantially broader than that of Btk; the phenotypes associated with mutations in BAP-135 and Btk may therefore differ. It should also be noted that the present data do not establish a direct relationship between Btk activity and BAP-135 phosphorylation in vivo, nor do they exclude the possibility that BAP-135 serves as a substrate for tyrosine kinases other than Btk.
PH domains are found in a large and functionally diverse group of proteins (20). Structural comparisons have suggested that PH domains mediate specific interactions with a chemically diverse set of ligands (21). The canonical PH domain of Btk binds specifically to BAP-135 in vitro; while the TH domain alone binds BAP-135 weakly, binding to the combined PH and TH domains is more efficient than binding to the PH domain alone. Together, therefore, the PH and TH domains of Btk comprise the principal target for BAP-135 binding in vitro. Correspondingly, Btk-induced phosphorylation of BAP-135 in transfected cells was abolished by deletion of the Btk PH domain (data not shown). Because Btk autokinase activity was also impaired under these conditions, it is not yet clear whether the effect of PH deletion on phosphorylation of BAP-135 in vivo is direct. Tandem arrangement of PH and TH domains is a characteristic feature of all members of the Btk tyrosine kinase subfamily. While BAP-135 is expressed in a wide variety of cell types, expression of Btk is restricted to cells of the B lymphoid and myeloid lineages. Nonetheless, other members of the Btk tyrosine kinase subfamily, namely Tec and Bmx, exhibit a broader tissue distribution than Btk; it is possible that interaction with BAP-135 is a common feature of Btk-related kinases.
Acknowledgments
We thank D. Reim (Wistar Protein Microchemistry Laboratory) for microsequence analysis of peptides, C. Riley (Howard Hughes Medical Institute Biopolymers Facility, Baltimore) for synthesis of oligonucleotides and peptides, and D. Dordai for expert technical assistance. This work was supported by the Howard Hughes Medical Institute.
Footnotes
Abbreviations: Btk, Bruton’s tyrosine kinase; BCR, B cell antigen receptor; PH, pleckstrin homology; TH, Tec homology; SH2, Src homology-2; SH3, Src homology-3; GST, glutathione S-transferase; EST, expressed sequence tag.
Data deposition: The sequence reported in this paper has been deposited in the GenBank data base (accession no. U77948U77948).
References
- 1.Saouaf S, Mahajan S, Rowley R, Kut S, Fargnoli J, Burkhardt A, Tsukada S, Witte O, Bolen J. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsukada S, Saffran D C, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Mohandas T, Quan S, Belmont J W, Cooper M D, Conley M E, Witte O N. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 3.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, Smith C I E, Bentley D R. Nature (London) 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 5.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copland N G, Jenkins N A, Witte O N. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 6.Siliciano J, Morrow T A, Desiderio S V. Proc Natl Acad Sci USA. 1992;89:11194–11198. doi: 10.1073/pnas.89.23.11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyeck S D, Berg L J. Proc Natl Acad Sci USA. 1993;90:669–673. doi: 10.1073/pnas.90.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mano H, Mano K, Tang B, Koehler M, Yi T, Gilbert D J, Jenkins N A, Copeland N G, Ihle J N. Oncogene. 1993;8:417–424. [PubMed] [Google Scholar]
- 9.Tamagnone L, Lahtinen I, Mustonen T, Virtaneva K, Francis F, Muscatelli F, Alitalo R, Smith C I, Larsson C, Alitalo K. Oncogene. 1994;9:3683–3688. [PubMed] [Google Scholar]
- 10.Vihinen M, Nilsson L, Smith C I E. FEBS Lett. 1994;350:263–265. doi: 10.1016/0014-5793(94)00783-7. [DOI] [PubMed] [Google Scholar]
- 11.Cheng G, Ye Z, Baltimore D. Proc Natl Acad Sci USA. 1994;91:8152–8155. doi: 10.1073/pnas.91.17.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Malek S N, Desiderio S. J Biol Chem. 1995;270:20832–20840. doi: 10.1074/jbc.270.35.20832. [DOI] [PubMed] [Google Scholar]
- 13.Yao L, Kawakami Y, Kawakami T. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsukada S, Simon M I, Witte O N, Katz A. Proc Natl Acad Sci USA. 1994;91:11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langhans-Rajasekaran S A, Wan Y, Huang X Y. Proc Natl Acad Sci USA. 1995;92:8601–8605. doi: 10.1073/pnas.92.19.8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak M. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 17.Park H, Wahl M I, Afar D E H, Turck C W, Rawlings D J, Tam C, Scharenberg A M, Kinet J-P, Witte O N. Immunity. 1996;4:515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 18.Rawlings D J, Scharenberg A M, Park H, Wahl M I, Lin S Q, Kato R M, Fluckiger A C, Witte O N, Kinet J-P. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 19.DeFranco A L. Annu Rev Cell Biol. 1993;9:377–410. doi: 10.1146/annurev.cb.09.110193.002113. [DOI] [PubMed] [Google Scholar]
- 20.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- 21.Lemmon M A, Ferguson K M, Schlessinger J. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]