Abstract
Bruton tyrosine kinase (Btk) is essential for the development of pre-B cells to mature B cell stages. Btk-deficient mice manifest an X-linked immunodeficiency (xid) defect characterized by a reduction of peripheral IgMlow IgDhigh B cells, a lack of peritoneal CD5+ B cells, low serum levels of IgM and IgG3, and impaired responses to T cell independent type II (TI-II) antigens. We have generated transgenic mice in which expression of the human Btk gene is driven by the murine class II major histocompatibility complex Ea gene locus control region, which provides gene expression from the pre-B cell stage onwards. When these transgenic mice were mated onto a Btk− background, correction of the xid B cell defects was observed: B cells differentiated to mature IgMlowIgDhigh stages, peritoneal CD5+ B cells were present, and serum Ig levels and in vivo responses to TI-II antigens were in the normal ranges. A comparable rescue by transgenic Btk expression was also observed in heterozygous Btk+/− female mice in those B-lineage cells that were Btk-deficient as a result of X chromosome inactivation. These findings indicate that the Btk− phenotype in the mouse can be corrected by expression of human Btk from the pre-B cell stage onwards.
Keywords: B cell development, X-linked agammaglobulinemia
Bruton tyrosine kinase (Btk) is a cytoplasmic protein tyrosine kinase that is essential for B cell development. The Btk gene encodes a 659-amino acid protein that contains a single catalytic domain, the src homology domains SH2 and SH3, and a unique N-terminal region with a pleckstrin homology domain and proline-rich sequences (1, 2). Btk belongs to the Tec subfamily of tyrosine kinases, together with the homologous Tec, Itk, and Bmx genes (3).
Mutations in the Btk gene (4) lead to X-linked agammaglobulinemia (XLA) in humans, which is characterized by an almost complete failure of pre-B cells to differentiate into mature B cells (5–8). In the mouse, Btk defects result in a mild B cell disorder, X-linked immunodeficiency (xid), both in CBA/N mice carrying an Arg-28 pleckstrin homology domain mutation, and in mice with targeted disruptions of Btk in their germ line (9–13). These mice have ≈50% fewer peripheral B cells than normal, with a specific deficiency of mature surface IgMlowIgDhigh cells (14, 15). In the peritoneum CD5+ B cells are absent. Serum levels of IgM and IgG3 are low; responses to T cell independent type II (TI-II) antigens are severely impaired. xid B cells show an aberrant response to B cell receptor cross-linking, interleukin 5 (IL-5), IL-10, CD38, or CD40 (5–8). It has been shown that Btk activity and tyrosine phosphorylation increase upon stimulation of the B cell receptor, and the receptors for IL-5 and IL-6 (16–20).
The defect in XLA and xid is intrinsic to the B cell, as heterozygous females manifest a unilateral X chromosome inactivation in the mature B cell populations (13, 21–23), due to a selective disadvantage of cells that have the defective Btk gene on the active X chromosome. Btk is expressed throughout B cell differentiation, except in plasma cells (8, 13, 24). Although Btk is already present in pro-B cells, the first selective disadvantage of Btk-deficient cells only becomes apparent at the transition from small pre-B cells to immature B cells in the bone marrow (13). Furthermore, Btk is critical during the antigen-driven maturation of selected B cells in the periphery (9, 12, 13).
We generated transgenic mice in which Btk expression is driven by the class II major histocompatibility complex (MHC) Ea gene locus control region (LCR) (25), and mated them onto a Btk− background. We show that the Btk− phenotype can be corrected by transgenic expression of human Btk from the pre-B cell stage onwards.
MATERIALS AND METHODS
Generation of Transgenic Mice.
Using a human Btk (hBtk) cDNA clone phBtk2.55, which was isolated from a pro-B cell cDNA library (24), the 5′ end of the cDNA up to the EcoRI site at cDNA position 1453 (1) was subcloned by EcoRI digestion and religation (phBtk1.45). A 670-bp EcoRI-blunted HindIII fragment (positions 1453–2123) from phBtk2.55 was cloned into EcoRI and EcoRV digested phBtk1.45. From the resulting plasmid a 2.1-kb blunted NotI–SalI fragment, containing the hBtk cDNA with 16 bp of 3′ untranslated region, was ligated to a 6-kb blunted AatII–SalI fragment from pEV3 (Clare Gooding, ICI Pharmaceuticals), containing 2.8 kb of 3′ hβ-globin sequences. Subsequently, a PvuI linker was introduced at a unique MluI site in the polylinker just 3′ of hβ-globin. Using a unique PvuI site at position +33 in hBtk, a 4.9-kb PvuI fragment containing hBtk–hβ-globin was cloned into a unique PvuI site at position +14 in the MHC class II Ea gene on cosmid 32.1 (25). This cosmid (BALB/c H-2d haplotype) contained 23 kb of 5′ Ea sequence, with the 5 DNase I hypersensitive sites of the Ea LCR. A 29-kb MluI fragment (see Fig. 1A) was injected into pronuclei of FVB × FVB fertilized oocytes at a concentration of 2 ng/μl.
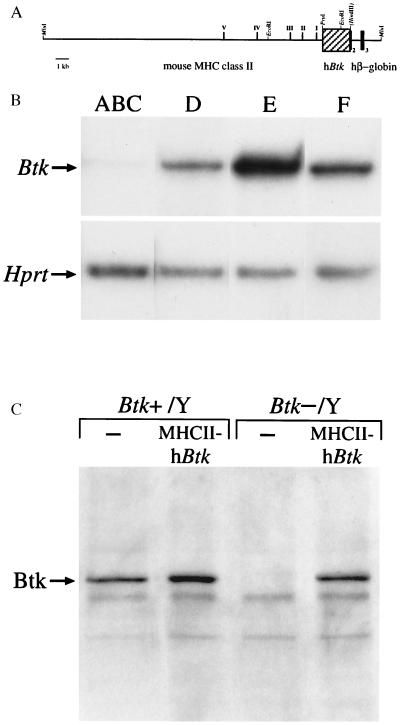
Figure 1.
Structure and expression of the MHCII–hBtk transgene. (A) Map of the transgene construct, showing the locations of five DNase I hypersensitive sites present in the 23-kb MluI–PvuI mouse MHC class II upstream Ea gene fragment, the 2.1-kb PvuI–HindIII hBtk cDNA fragment (hatched box), as well as a 2.8-kb hβ-globin fragment containing part of exon 2, exon 3, and 3′ untranslated region. (B) RT-PCR analyses of MHCII–hBtk transgene expression during B cell development. cDNA samples were prepared from sorted bone marrow populations of MHCII–hBtk transgenic Btk−/Y mice and yields were normalized using ≈250 bp HPRT RT-PCR products. The MHCII–hBtk transgene primers generated single ≈230-bp bands, with the following densities (relative to fraction E): lane ABC, B220+CD43+IgM− pro-B cells (28): 1.8%; lane D, B220+CD43−IgM− pre-B cells: 25%; lane E, B220+IgM+ immature B cells: 100%; and lane F, B220highIgM+ mature B cells: 35%. Data are shown as a representative of three mice examined. (C) Western blot analysis of Btk expression in total cell lysates from spleen of 3-month-old male wild-type mice (Btk+/Y) or Btk-deficient mice (Btk−/Y), either nontransgenic or MHCII–hBtk transgenic. The position of the 77-kDa Btk band is indicated.
Protein Analyses and Reverse Transcription–PCR (RT-PCR).
For RT-PCR analysis, RNA was isolated from sorted cell populations using the Ultraspec RNA isolation system (Biotecx Laboratories, Houston) and cDNA was synthesized using reverse transcriptase (Super RT; HT Biotechnology, Cambridge, U.K.) and an oligo(dT) primer. PCRs were performed in 50 μl PCR buffer (Life Technologies, Paisley, U.K.) with 1.5 mM MgCl2, 100 ng of each primer, and 5 μCi (1 Ci = 37 GBq) of [α-32P]dATP. Amplification was for 27 cycles with denaturation at 94°C for 30 s, annealing at 55°C (Btk transgene) or 60°C (hypoxanthine phosphoribosyltransferase; HPRT) for 30 s, and extension at 72°C for 45 s. PCR products were visualized by electrophoresis through an 6% polyacrylamide/0.5× TBE gel and autoradiography. Quantifications were performed using imagequant (Molecular Dynamics). Primers specific for the hBtk-transgene were in Btk exon 19 (5′-ATGGA TGAAG AATCC TGAGC-3′) and β-globin exon 3 (5′-TGGAC AGCAA GAAAG CGAG-3′). HPRT-specific primers were 5′-CACAG GACTA GAACA CCTGC-3′ and 5′-GCTGG TGAAA AGGAC CTCT-3′.
Western blotting analysis was performed as described (13).
Flow Cytometric Analyses and Cell Sorting.
Preparation of single-cell suspensions, loading cells with fluorescein di-β-d-galactopyranoside, three-color flow cytometric analysis, and mAbs have been described (13). For analysis of Ki-67 expression, cells were lysed in lysis buffer (Becton Dickinson) after staining with surface markers. For cell-sorting experiments, 108 bone marrow cells were incubated with phycoeythrin (PE)-conjugated anti-CD43, fluorescein isothiocyanate-labeled anti-B220, biotin-conjugated IgM (PharMingen) and streptavidin-TriColor (Caltag, South San Francisco, CA) as a second step. Cell sorts were performed on a FACS Vantage cell sorter (Becton Dickinson); the purity of the obtained cell populations was ≈95%, as determined using cellquest (Becton Dickinson) software.
Immunizations, Ig ELISA, and in Vitro Stimulations.
To measure TI-II responses, 4- to 6-month-old mice were injected intraperitoneally with 50 μg 2,4-dinitrophenol (DNP)-Ficoll in PBS and trinitrophenol (TNP)-specific IgG3 was analyzed at day 7 by ELISA. To measure T cell-dependent (TD) responses, mice were immunized with 100 μg TNP keyhole limpet hemocyanin (TNP-KLH) precipitated with alum (26), and TNP-specific IgM was analyzed at day 7. A booster dose of 100 μg TNP-KLH was given at day 14, and TNP-specific IgG1 and IgG2a was analyzed at day 21. In the TNP-specific sandwich ELISA assays, plates were coated with isotype-specific antibodies and serum dilutions were incubated for 3 h. Subsequent steps were peroxidase labeled TNP-KLH and azino-bis-ethylbenz-thiazoline-sulfonic acid. Serum Ig subclasses were determined by isotype-specific ELISA (13, 26).
For in vitro stimulations, total spleen cell populations were cultured with 2 μg/ml goat-anti-mouse IgM (Southern Biotechnology Associates) or bacterial lipopolysaccharide (5 μg/ml) for 3 days, and analyzed for expression of Ki-67 by flow cytometry.
RESULTS
MHCII–hBtk Transgenic Mice.
A transgenic construct (Fig. 1A) was generated in which the hBtk gene is put under the control of the murine class II MHC Ea gene LCR—i.e., a 23-kb genomic DNA fragment that contained the promoter proximal and B cell control regions (27), as well as more upstream DNase I hypersensitive sites required for position-independent, copy number-dependent expression (25). The hBtk 3′ untranslated region was replaced by a 3′ hβ-globin genomic fragment. The transgenic construct was injected into fertilized oocytes and six founder mice were obtained, as identified by genomic Southern blotting analyses using probes specific for 3′ hβ-globin and for hBtk. Five of these showed germ-line transmission of the transgene and in two lines expression of the transgene was observed by S1 analyses. No differences were found between these two lines in any of the performed analyses. The offspring did not exhibit developmental defects or any increased susceptibility to infectious diseases or malignancies, for over 1 year of age.
Consistent with the expression of MHC class II molecules in B lymphocytes and thymic epithelium (27), the highest expression levels of the transgene construct were found in spleen and thymus by S1 protection assays (data not shown). The transgene was also found to be expressed at a low level in brain, liver, and kidney. To confirm that the MHCII–hBtk transgene is transcribed in the B cell lineage only from the pre-B cell stage onwards, RT-PCR experiments were performed on sorted bone marrow cell populations. Transgene transcripts were readily detected in pre-B cells, immature and mature B cells (Fig. 1B). In contrast, expression in the sorted pro-B cells [B220+/IgM−/CD43+ cells, fraction ABC (28)] was very low, with densities of the amplified signals that were <2% of those in fraction E [immature B220+IgM+ B cells (28)]. These faint Btk signals may well originate from contaminating cells that were present (up to 5%) in the pro-B cell preparations.
Male mice with one allele of the MHCII–hBtk transgene were mated to female Btk+/− mice—i.e., mice in which on one allele the Btk gene is inactivated through a targeted in-frame insertion of a lacZ reporter in exon 8 (13). In the offspring the four possible genotypes among males, wild-type (Btk+/Y) and knock-out (Btk−/Y), either nontransgenic or carrying the MHCII–hBtk transgene, were found in the expected ratio of 1:1:1:1. Protein expression of the MHCII–hBtk transgene construct was analyzed by Western blot experiments on spleen total cell lysates (Fig. 1C). Using a polyclonal rabbit antiserum specific for Btk amino acids 69–88 which are 100% conserved between human and mouse, a 77-kDa protein was detected in the spleen of normal male mice (Btk+/Y), but not in the mice with the targeted disruption (Btk−/Y). In the Btk−/Y MHCII–hBtk transgenic mice, Btk protein expression in the spleen was restored, apparently to levels similar to those found in normal mice. When sorted bone marrow cell suspensions were analyzed in these mice, Btk protein was not detected in pro-B cells, but was found to be expressed from the pre-B cell stage (fraction D) onwards (data not shown).
Transgenic MHCII–hBtk Expression Corrects B Cell Numbers and Surface Ig Profiles in Btk− Mice.
The four groups of male littermates described above were investigated to assess lymphocyte surface phenotypes and numbers in spleen, mesenteric lymph node (MLN), bone marrow, thymus, and peritoneal cavity by flow cytometry (Table 1 and Fig. 2). No significant differences were found in the T cell compartment nor in the myeloid lineage (Table 1 and data not shown).
Table 1.
Flow cytometric analysis of the B lymphocyte compartment in MHCII–hBtk transgenic mice
| Tissue | Cell population | Btk+/Y | Btk+/Y MHCII-hBtk | Btk−/Y | Btk−/Y MHCII–hBtk |
|---|---|---|---|---|---|
| Spleen | Nucleated cells, ×106 | 142 ± 45 | 138 ± 43 | 96 ± 30 | 113 ± 34 |
| B220+ cells, % | 59 ± 3 | 54 ± 4 | 45 ± 6* | 52 ± 9† | |
| IgMlowIgDhigh cells, % | 30 ± 4 | 26 ± 4 | 9 ± 5* | 20 ± 7† | |
| CD4+ cells, % | 18 ± 2 | 21 ± 3 | 22 ± 4 | 23 ± 3 | |
| CD8+ cells, % | 9 ± 2 | 8 ± 1 | 11 ± 2 | 11 ± 2 | |
| MLN | B220+ cells, % | 38 ± 10 | 33 ± 6 | 20 ± 5* | 31 ± 14† |
| IgMlowIgDhigh cells, % | 31 ± 8 | 22 ± 7 | 6 ± 1* | 20 ± 10† | |
| CD4+ T cells, % | 36 ± 6 | 40 ± 9 | 48 ± 7 | 40 ± 8 | |
| CD8+ T cells, % | 19 ± 4 | 20 ± 6 | 28 ± 4 | 22 ± 3 | |
| Peritoneum | CD5+ B cells, % | 4 ± 2 | 6 ± 4 | 0.3 ± 0.3* | 4 ± 2† |
| CD5− B cells, % | 13 ± 4 | 11 ± 5 | 5 ± 3* | 14 ± 5† | |
| CD5+ T cells, % | 5 ± 3 | 4 ± 2 | 7 ± 3 | 7 ± 4 | |
| Bone Marrow | B220+ cells, % | 24 ± 1 | 22 ± 1 | 19 ± 1 | 22 ± 1 |
| IgMlowIgDhigh cells, % | 2.5 ± 0.6 | 3.8 ± 1.2 | 0.7 ± 0.2* | 3.0 ± 1.4† |
Mice were 4–6 months old. Numbers of mice analyzed are 10–14 (spleen), 3–6 (MLN), 7–10 (peritoneum), and 4 (bone marrow) for all groups.
P < 0.05, compared with Btk+/Y mice, in the Mann–Whitney U test.
Not significantly different, compared with Btk+/Y mice.
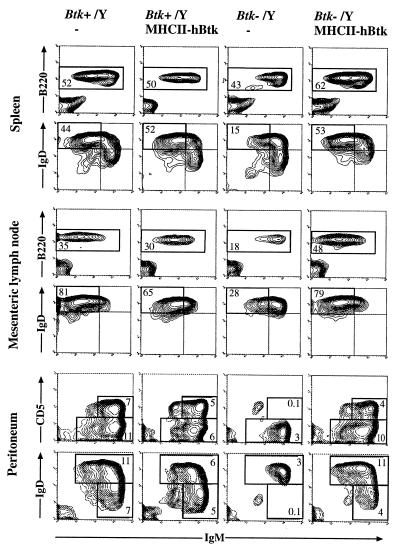
Figure 2.
The effect of the MHCII–hBtk transgene on peripheral B lymphocytes. Three-color flow cytometric analysis of spleen, MLN, and peritoneum from 4-month-old normal and transgenic Btk+/Y males, as well as nontransgenic and transgenic Btk−/Y males. Spleen and MLN cell suspensions were stained with biotinylated anti-IgM and streptavidin-TriColor, anti-IgD-PE, and anti-B220-fluorescein isothiocyanate. (Top) Percentages of cells displayed that are B220+/IgM+ are indicated. (Bottom) Percentages of gated B220+ cells displayed that are IgMlowIgDhigh (fraction I; refs. 14 and 15) are indicated. Peritoneal cells were stained with anti-B220, anti-IgM, and either anti-CD5-PE or anti-IgD-PE; B220+ cells are displayed. The percentages of total cells (including peritoneal macrophages) that are CD5+ (and IgDlow) B cells or conventional (CD5− and IgDhigh) B cells are given. Data are shown as 5% probability contour plots representative of the mice examined; dead cells were gated out, based on forward and side scatter characteristics.
In the B cell lineage no significant differences were detected between normal and MHCII–hBtk transgenic mice. As previously described (13) the Btk−/Y mice had fewer B220+ B cells and, more particularly, a decrease in mature IgMlow/IgDhigh B cells in spleen, MLN, and bone marrow. In the peritoneum, the numbers of conventional B cells were reduced (≈40-50% of normal) and CD5+ B cells were virtually absent. However, correction of this B cell deficiency was observed in the Btk−/Y mice that expressed the MHCII–hBtk transgene. In the spleen and MLN the B cell numbers reached close to normal values and mature IgMlow/IgDhigh B cells were present in normal numbers in the spleen, MLN, and bone marrow (Table 1 and Fig. 2). In the peritoneum the numbers of CD5+ and conventional B cells were in the normal ranges. The percentages of the pro-B, pre-B, and immature B cell subsets in the bone marrow were not significantly different between the four groups (data not shown).
Correction of Btk-Deficient Cells in Btk+/− Heterozygous Females.
As the expression of the disrupted Btk allele could be identified by lacZ activity, in Btk+/− females the competition between B cells that express wild-type Btk and those expressing Btk−/lacZ—due to the phenomenon of X chromosome inactivation—was assessed. Cell samples from spleen and MLN were evaluated for lacZ activity using fluorescein di-β-d-galactopyranoside as a substrate in conjunction with surface expression of IgM and IgD by flow cytometry.
In the absence of any selective disadvantage, lacZ-expressing cells from Btk+/− females would be expected to represent 50% of any B cell developmental population. However, because of the proliferative disabilities of Btk-deficient cells, the fraction of lacZ-expressing cells in the heterozygous females decreased during B cell maturation in the periphery from ≈35% in IgMhighIgDlow (fraction III) to 10% in IgMhigh IgDhigh (fraction II) and to almost undetectable levels in IgMlowIgDhigh (fraction I) B cell stages (ref. 13 and Fig. 3A). Also in Btk+/− females that express the MHCII–hBtk transgene, a selective disadvantage of lacZ-expressing cells was observed, although the development of these cells did not appear to be compromised to such a large extent as in nontransgenic Btk+/− females. Significant fractions (≈10%) of mature IgMlowIgDhigh fraction I cells in spleen and MLN-manifested lacZ activity (Fig. 3A).
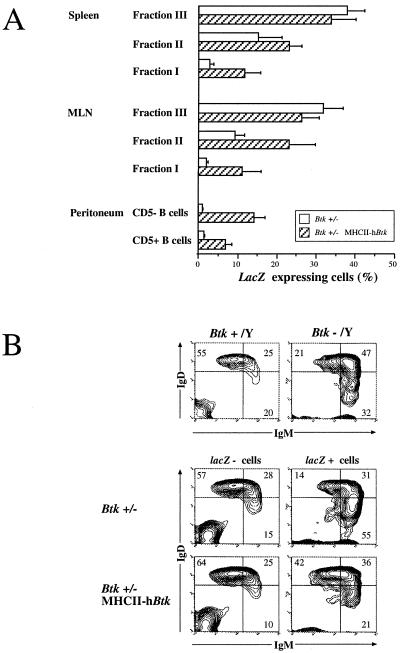
Figure 3.
The effect of the MHCII–hBtk transgene in Btk+/− female mice. Cell suspensions were stained for lacZ activity, anti-IgM (biotinylated)/streptavidin-TriColor and anti-IgD-PE (spleen and MLN) or anti-CD5-PE (peritoneum). (A) The percentages of lacZ-expressing cells as mean values and standard deviations in the three peripheral B cell compartments (fraction III, IgMhighIgDlow; fraction II, IgMhigh IgDhigh; and fraction I, IgMlowIgDhigh; refs. 14 and 15). Values are corrected for individual variations in X chromosome inactivation ratios, using lacZ expression values from peritoneal macrophages (13). Data for spleen and MLN are from 6-week-old mice, and for peritoneum they are from 4-month-old mice. (B) Surface expression of IgM and IgD in gated lacZ− and lacZ+ spleen cells from nontransgenic and MHCII–hBtk transgenic Btk+/− female mice. (Upper) For comparison the profiles in (lacZ−) Btk+ males and in (lacZ+) Btk− males are given. The distribution of IgM+IgD+ cells over the compartments III, II, and I in percentages is indicated. Data are shown as 5% probability contour plots representative of the mice examined.
When peritoneal cells from Btk+/− mice were investigated for LacZ activity and surface expression of IgM and CD5, no lacZ+ cells could be detected in the CD5+ B cells nor in the conventional CD5− B cell population (Fig. 3A). However, 7% of CD5+ B cells and 14% of CD5− B cells expressed lacZ in MHCII–hBtk transgenic Btk+/− females.
Furthermore, we analyzed the surface IgM/IgD profile of the lacZ− and the lacZ+ cells in female spleen and MLN (shown for spleen in Fig. 3B). In nontransgenic heterozygous females, the gated lacZ− mature B cells exhibited an IgM/IgD surface profile that was identical to the profile of B cells in normal mice, whereas the gated lacZ+ cells manifested an IgMhigh phenotype, reminiscent of the B cell population found in Btk−/Y mice (Fig. 3B). In contrast, the gated lacZ+ cells in MHCII–hBtk transgenic heterozygous females, showed an IgM/IgD surface profile that was more similar to the profile in normal mice.
Transgenic MHCII–hBtk Expression Corrects Serum Ig Levels, TI-II Responses, and B Cell Proliferation.
The serum concentrations of IgM, IgG1, IgG2a, and IgG3 were determined by ELISA. The knock-out mice (Btk−/Y) had decreased levels of IgM and IgG3, when compared with normal littermates; this defect was corrected by transgenic Btk expression, as the MHCII–hBtk mice manifested serum Ig levels that were in the normal range (Fig. 4A).
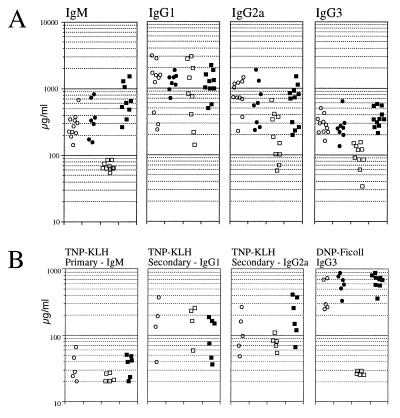
Figure 4.
Correction of serum Ig levels and in vivo antigen responses by expression of the MHCII–hBtk transgene. (A) Serum concentrations of Ig subclasses in unimmunized 4-month-old mice. (B) Serum concentrations of DNP/TNP-specific antibodies were determined after primary and secondary immunizations with the TD antigen TNP-KLH, and after immunization with the TI-II antigen DNP-Ficoll in 4- to 6-month-old mice. In transgenic Btk+/Y males TD responses were not determined. ○, nontransgenic Btk+/Y males; •, MHCII–hBtk transgenic Btk+/Y males; □, nontransgenic Btk−/Y males; ▪, MHCII–hBtk transgenic Btk−/Y males.
To analyze the in vivo responsiveness to TD antigens, mice were immunized with TNP-KLH, and primary IgM and secondary IgG1 and IgG2a responses were measured by ELISA. The primary TNP-specific response was low in Btk−/Y mice, consistent with previous findings in Btk-deficient mice (9, 12) but comparable between normal and transgenic MHCII–hBtk knock-out (Btk−/Y) mice (Fig. 4B). The secondary TNP responses were not significantly different between the groups of mice. Because xid mice are unresponsive to TI-II antigens (9, 12), we also investigated the IgG3 response to the TI-II antigen DNP-Ficoll. The results showed that the Btk−/Y knock-out mice did not respond and that this defect was completely corrected by MHCII–hBtk expression (Fig. 4B).
To determine the proliferative capacity of B cells, total spleen cell suspensions were stimulated with anti-μ and proliferation was quantified by flow cytometric analyses of the nuclear Ki-67 antigen, which is expressed in all proliferating cells during late G1, S, M, and G2 phases of the cell cycle (29). After 3 days of anti-μ stimulation, Ki-67 was expressed in 68 ± 8% of B220+ cells from normal mice and in 35 ± 17% of B220+ cells from Btk-deficient animals. In the MHCII–hBtk transgenic Btk+/Y and Btk−/Y mice, the induction of Ki-67 expression was clearly in the normal range (62 ± 11% and 67 ± 8%, respectively, n = 3 for each group).
When cells were cultured with 5 μg/ml lipopolysaccharide for 3 days, the Ki-67 antigen was expressed in 70–80% of splenic B220+ cells in all four mice groups. However, the fraction of lymphoblasts in the B220+ cell fraction, as determined by forward scatter characteristics, was low in Btk−/Y mice (32 ± 5%, and 45 ± 7% in normal littermates). In the MHCII–hBtk transgenic Btk+/Y and Btk−/Y mice these values were 49 ± 3% and 49 ± 13%, respectively.
DISCUSSION
We have generated transgenic mice in which expression of the hBtk gene is driven by the murine class II MHC Ea gene LCR, which was shown to provide position-independent, copy number-dependent expression and could therefore be used to target genes to B lineage cells (25). MHC class II antigens are constitutively expressed on B cells and can be induced on a variety of other cell types, including macrophages and cells of nonhaematopoietic lineage (27). During B cell development, class II genes are first expressed on a major proportion of the pre-B cells (30). Although mature B cells manifest considerable heterogeneity in expression levels, the levels are generally higher than in pre-B cells (27, 30). In plasma cells the expression is down-regulated. Our RT-PCR and Western blot experiments show expression of the MHCII–hBtk transgene from the pre-B cell stage onwards.
When the transgenic mice were mated onto a Btk− background, correction of the xid B cell defects was observed. In the spleen and MLN of MHCII–hBtk transgenic Btk−/Y males, B cell numbers and their surface IgM/IgD phenotype were normal; in the peritoneal cavity conventional B cells and CD5+ B cells were present in normal numbers. In addition, expression of the transgene restored the serum IgM and IgG3 concentrations, the responses to TI-II antigen, and the proliferative capacity of B cells to anti-μ and lipopolysaccharide. These results directly show that the presence of Btk from the pre-B cell stage onwards is sufficient for normal development of the conventional and CD5+ mature B cell populations. They also agree with previous findings that in Btk-deficient B lineage cells, defects only become apparent at the transition from small pre-B cells to immature B cells in the bone marrow (13, 22, 31). To date, it is unknown which of the signaling functions of Btk are mainly responsible for the immunodeficient phenotypes of XLA and xid. Nevertheless, the outcome of our transgenic rescue experiments are compatible with a role for Btk in signaling both from the Ig-μ H chain—surrogate L chain complex in pre-B cells and from the antigen receptor complex in mature B cells. Such a role for Btk is further supported by recent findings that the B cell defects in Btk-deficient mice resemble those in a mouse mutant that lacks most of the cytoplasmic tail of the B cell receptor-associated signaling molecule Ig-α (32).
Rescue by transgenic MHCII–hBtk expression was also observed in heterozygous Btk+/− female mice in those B-lineage cells that were Btk-deficient as a result of X chromosome inactivation. In MHCII–hBtk transgenic females the mature B cells that expressed the Btk−/lacZ allele did not manifest the aberrant surface IgMhigh profile, typical for the xid phenotype (Fig. 3B). However, as the fractions of lacZ-expressing cells still did not reach 50%, it can be concluded that mature B cells that only have transgenic hBtk still have a slight selective disadvantage over those cells that have both the endogenous murine and the transgenic human gene. This phenomenon may originate from (i) minor insufficiencies in the expression of the transgene, (ii) dominant negative effects of the expression product of the targeted Btk–lacZ allele, or (iii) functional differences between the human and murine Btk protein. Twelve out of 659-amino acid residues are different between murine and hBtk, most of which are conservative substitutions throughout the protein (1, 2). However, three amino acid changes are clustered at positions 207–214 in the proline-rich region, partly located in one of the two 10-amino acids motifs implicated in the interaction of Btk with SH3 domains of Fyn, Lyn, and Hck (33). Hence it remains possible that human and murine Btk show differences in affinity or specificity for SH3 domains, leading to slight selective disadvantages of murine B cells expressing hBtk. Yet, in MHCII–hBtk transgenic Btk+/− heterozygous females the selection against cells expressing the Btk−/lacZ allele was not as pronounced as in the nontransgenic females (Fig. 3A).
The finding that the xid phenotype can be corrected in the mouse by transgenic expression of Btk from the pre-B cell stage onwards has important implications for the design of strategies for gene therapy of XLA. Our findings in the mouse show that the Btk gene does not need to be introduced in the very small populations of hematopoietic stem cells or early pro-B cells to restore B cell development in XLA patients. Instead, Btk gene transfer to any of the precursor B cell stages up to the small pre-B cell would correct the XLA defect. Thus, ≈10% of total cells or ≈50% of the B-lineage cells (28) in the bone marrow would be suitable targets. In addition to this large target cell population, XLA is also a good candidate for gene therapy because Btk expression only needs to be transient [Btk is not required in plasma cells (8, 24)].
The observed correction of the B cell defects by transgenic expression of wild-type hBtk driven by the MHC class II LCR makes it attractive to perform similar studies with mutated Btk transgenes to investigate the function of the various Btk domains.
Acknowledgments
We thank A. Boonstra, L. Braam, M. de Bruijn, J. Guy, A. Langeveld, A. Maas, S. Philipsen, and M. de Weers for advice, assistance, and materials at various stages of the project. R.W.H. was supported by the Royal Academy of Arts and Sciences. These studies were supported in part by the Dutch Prevention Foundation (G.M.D.).
Footnotes
Abbreviations: Btk, Bruton tyrosine kinase; h, human; KLH, keyhole limpet hemocyanin; LCR, locus control region; MHC, major histocompatibility complex; MLN, mesenteric lymph node; TD, T cell dependent; TI-II, T cell independent type II; TNP, trinitrophenol; XLA, X-linked agammaglobulinemia; xid, X-linked immunodeficiency; HPRT, hypoxanthine phosphoribosyltransferase; PE, phycoeythrin; DNP, 2,4-dinitrophenol.
References
- 1.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammerström L, Kinnon C, Levinsky R, Bobrow M, Smith C I E, Bentley D R. Nature (London) 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 2.Tsukada S, Saffran D C, Rawlings D J, Parolini O, Allen R C, Klisak I, Sparkes R S, Kubagawa H, Thuluvancheri M, Quan S, Belmont J W, Cooper M D, Conley M E, Witte O N. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 3.Neet K, Hunter T. Genes Cells. 1996;1:147–169. doi: 10.1046/j.1365-2443.1996.d01-234.x. [DOI] [PubMed] [Google Scholar]
- 4.Vihinen M, Iwata T, Kinnon C, Kwan S-P, Ochs H D, Vorechovsky I, Smith C I E. Nucleic Acids Res. 1996;24:160–165. doi: 10.1093/nar/24.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattsson P T, Vihinen M, Smith C I. BioEssays. 1996;18:825–834. doi: 10.1002/bies.950181009. [DOI] [PubMed] [Google Scholar]
- 6.Conley M E, Parolini O, Rohrer J, Campana D. Immunol Rev. 1994;138:5–21. doi: 10.1111/j.1600-065x.1994.tb00844.x. [DOI] [PubMed] [Google Scholar]
- 7.Rawlings D J, Witte O N. Immunol Rev. 1994;138:105–119. doi: 10.1111/j.1600-065x.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 8.Sideras P, Smith C I E. Adv Immunol. 1995;59:135–223. doi: 10.1016/s0065-2776(08)60631-8. [DOI] [PubMed] [Google Scholar]
- 9.Scher I. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- 10.Rawlings D J, Saffran D C, Tsukada S, Largaespada D A, Grimaldi J C, Cohen L, Mohr R N, Bazan J F, Howard M, Copeland M G, Jenkins N A, Witte O N. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 11.Thomas J D, Sideras P, Smith C I E, Vorechovsky I, Chapman V, Paul W E. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 12.Kahn W F, Alt F W, Gerstein R M, Malynn B A, Larsson I, Rathbun G, Davidson L, Müller S, Kantor A B, Herzenberg L A, Rosen F S, Sideras P. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 13.Hendriks R W, de Bruijn M F T R, Maas A, Dingjan G M, Karis A, Grosveld F. EMBO J. 1996;15:4862–4872. [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy R R, Hayakawa K, Haaijman J, Herzenberg L A. Nature (London) 1982;297:589–591. doi: 10.1038/297589a0. [DOI] [PubMed] [Google Scholar]
- 15.Hardy R R, Hayakawa K, Parks D R, Herzenberg L A. Nature (London) 1983;306:270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- 16.de Weers M, Brouns G S, Hinshelwood S, Kinnon C, Schuurman R K B, Hendriks R W, Borst J. J Biol Chem. 1994;269:23857–23860. [PubMed] [Google Scholar]
- 17.Saouaf S J, Mahajan S, Rowley R B, Kut S A, Fargnoli J, Burkhardt A L, Tsukada S, Witte O N, Bolen J B. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki Y, Isselbacher K J, Pillai S. Proc Natl Acad Sci USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, Tsukada S, Kitamura D, Watanabe T, Witte O, Takatsu K. J Exp Med. 1994;180:2101–2111. doi: 10.1084/jem.180.6.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda T, Takahashi-Tezuka M, Fukada T, Okuyama Y, Fujitani Y, Tsukada S, Mano H, Hirai H, Witte O N, Hirano T. Blood. 1995;85:627–633. [PubMed] [Google Scholar]
- 21.Conley M E, Brown P, Pickard A R, Buckley R H, Miller D S, Raskind W H, Singer J W, Fialkow P J. N Engl J Med. 1986;315:564–570. doi: 10.1056/NEJM198608283150907. [DOI] [PubMed] [Google Scholar]
- 22.Forrester L M, Ansell J D, Micklem H S. J Exp Med. 1987;165:949–958. doi: 10.1084/jem.165.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahm M H, Paslay W J, Davie J M. J Exp Med. 1983;158:920–931. doi: 10.1084/jem.158.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Weers M, Verschuren M C M, Kraakman M E M, Mensink R G J, Schuurman R K B, Van Dongen J J M, Hendriks R W. Eur J Immunol. 1993;23:3109–3114. doi: 10.1002/eji.1830231210. [DOI] [PubMed] [Google Scholar]
- 25.Carson S, Wiles M V. Nucleic Acids Res. 1993;9:2065–2072. doi: 10.1093/nar/21.9.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Ommen R, Vredendaal A E C M, Savelkoul H F J. Scand J Immunol. 1994;40:1–9. doi: 10.1111/j.1365-3083.1994.tb03425.x. [DOI] [PubMed] [Google Scholar]
- 27.Glimcher L H, Kara C J. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 28.Hardy R R, Carmack C E, Shinton S A, Kemp J D, Hayakawa K. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 30.Tarlinton D. Immunol Rev. 1994;137:203–229. doi: 10.1111/j.1600-065x.1994.tb00666.x. [DOI] [PubMed] [Google Scholar]
- 31.Reid G K, Osmond D G. J Immunol. 1985;135:2299–2302. [PubMed] [Google Scholar]
- 32.Torres R M, Flaswinkel H, Reth M, Rajewski K. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 33.Cheng G, Ye Z-S, Baltimore D. Proc Natl Acad Sci USA. 1994;91:8152–8155. doi: 10.1073/pnas.91.17.8152. [DOI] [PMC free article] [PubMed] [Google Scholar]