Abstract
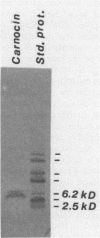
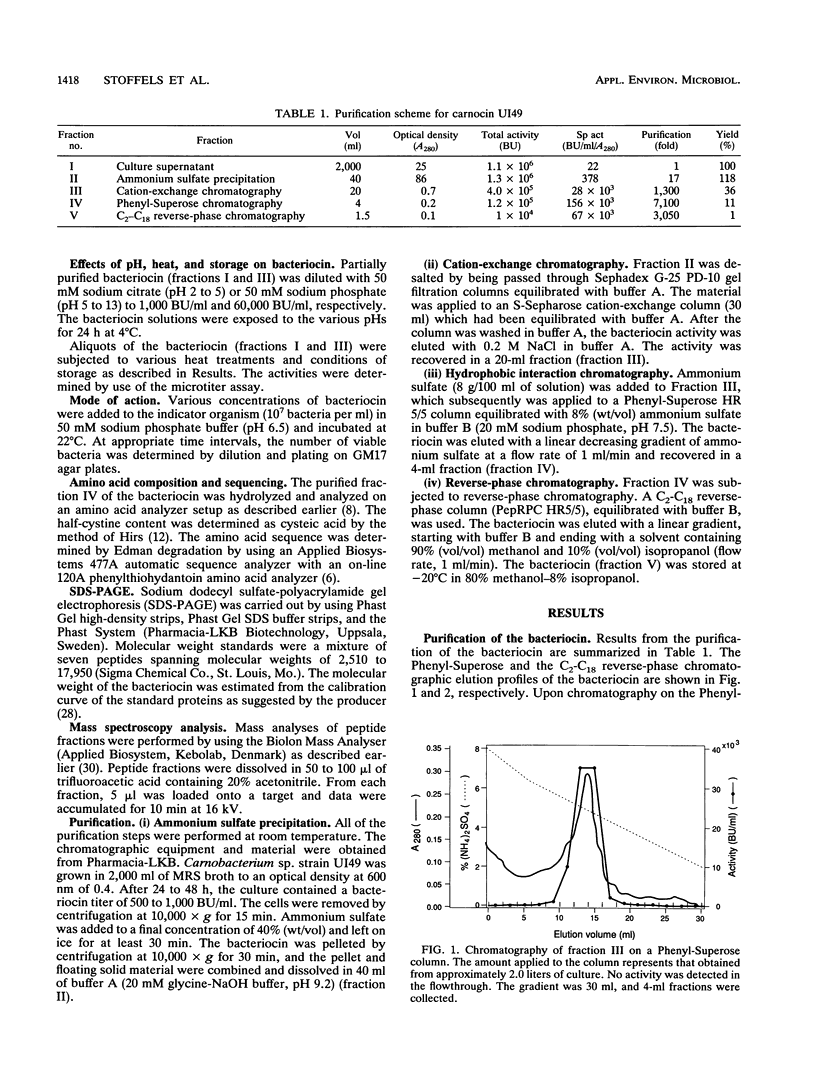
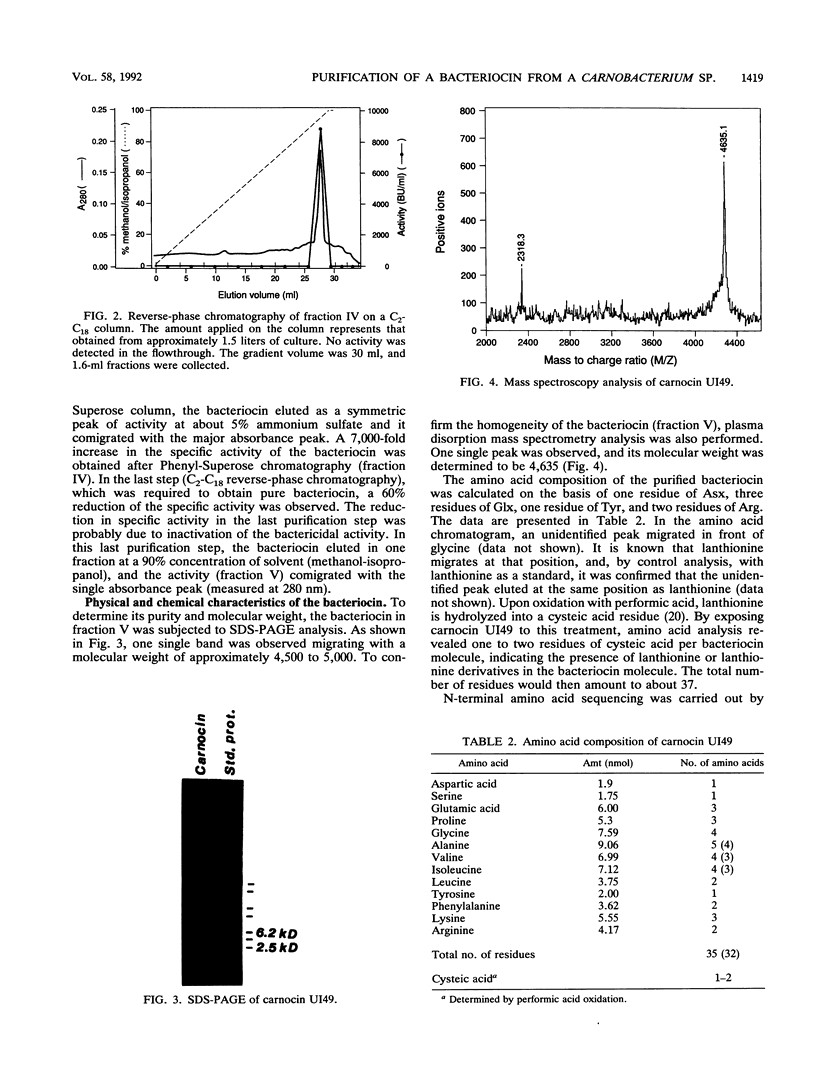
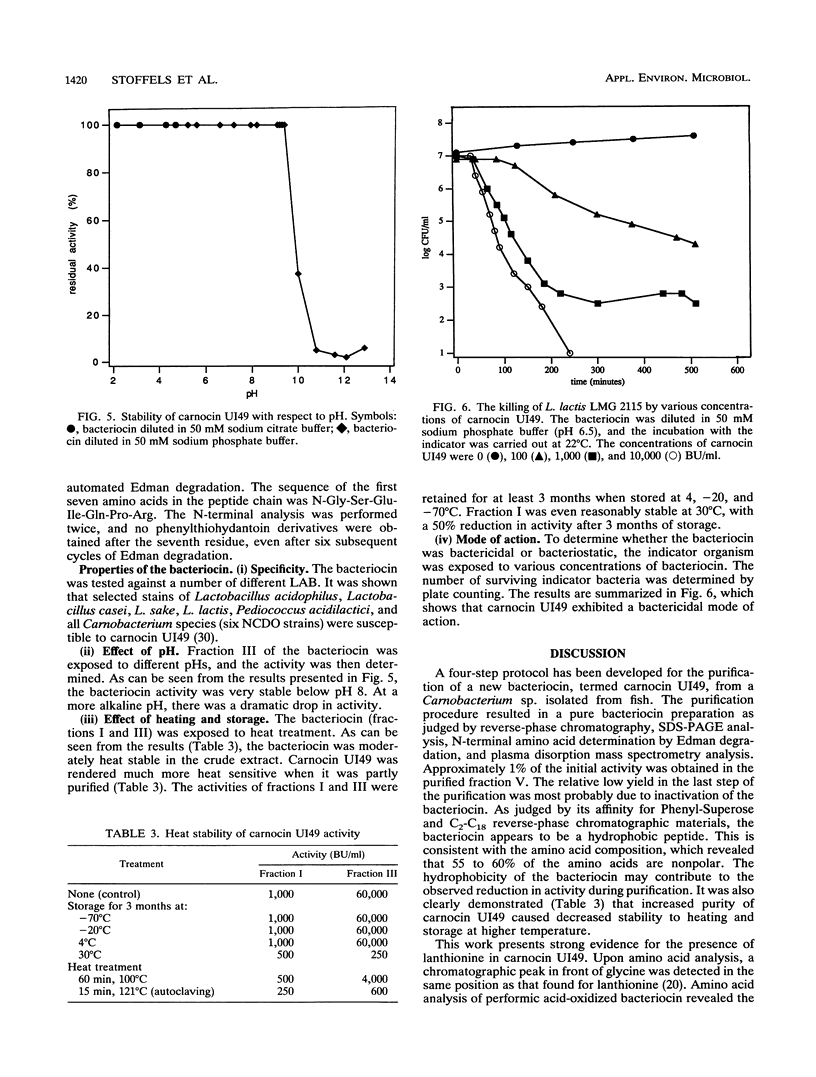
A bacteriocin-producing Carnobacterium sp. was isolated from fish. The bacteriocin, termed carnocin UI49, was purified to homogeneity by a four-step purification procedure, including hydrophobic interaction chromatography and reverse-phase chromatography. Carnocin UI49 has a bactericidal mode of action. It was shown to be heat tolerant and stable between pH 2 and 8. At pH above 8, carnocin UI49 was rapidly inactivated. Amino acid analysis revealed a composition of about 35 to 37 amino acids in addition to an unidentified peak which migrates at the position of lanthionine. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis suggests a molecular weight of about 4,500 to 5,000. Mass spectrometry gave a molecular weight of 4,635, which is about 1,000 larger than that calculated from the amino acid analysis data. Performic acid oxidation of carnocin UI49, followed by amino acid hydrolysis, revealed the presence of cysteic acid. The sequence of the first seven amino acid residues was determined to be N-Gly-Ser-Glu-Ile-Gln-Pro-Arg. After the seventh amino acid, carnocin UI49 was not available for further Edman degradation. The results suggest that carnocin UI49 belongs to the class of bacteriocins termed lantibiotics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn C., Stiles M. E. Plasmid-associated bacteriocin production by a strain of Carnobacterium piscicola from meat. Appl Environ Microbiol. 1990 Aug;56(8):2503–2510. doi: 10.1128/aem.56.8.2503-2510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R. E., Daeschel M. A., Hassan H. M. Antibacterial activity of plantaricin SIK-83, a bacteriocin produced by Lactobacillus plantarum. Biochimie. 1988 Mar;70(3):381–390. doi: 10.1016/0300-9084(88)90211-8. [DOI] [PubMed] [Google Scholar]
- Bhunia A. K., Johnson M. C., Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988 Oct;65(4):261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Sletten K., Johansson B., Westermark P. Evidence that the amyloid fibril protein in senile systemic amyloidosis is derived from normal prealbumin. Biochem Biophys Res Commun. 1988 Jul 29;154(2):648–653. doi: 10.1016/0006-291x(88)90188-x. [DOI] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Fykse E. M., Sletten K., Husby G., Cornwell G. G., 3rd The primary structure of the variable region of an immunoglobin IV light-chain amyloid-fibril protein (AL GIL). Biochem J. 1988 Dec 15;256(3):973–980. doi: 10.1042/bj2560973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis A., Singh J., Teuber M. Potential of lactic streptococci to produce bacteriocin. Appl Environ Microbiol. 1983 Jan;45(1):205–211. doi: 10.1128/aem.45.1.205-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Kiltz H. H. The number and nature of , -unsaturated amino acids in subtilin. Biochem Biophys Res Commun. 1973 Jan 23;50(2):559–565. doi: 10.1016/0006-291x(73)90876-0. [DOI] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Holo H., Nilssen O., Nes I. F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991 Jun;173(12):3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger M. C., Klaenhammer T. R. Cloning, expression, and nucleotide sequence of the Lactobacillus helveticus 481 gene encoding the bacteriocin helveticin J. J Bacteriol. 1990 Nov;172(11):6339–6347. doi: 10.1128/jb.172.11.6339-6347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Lindgren S. E., Dobrogosz W. J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol Rev. 1990 Sep;7(1-2):149–163. doi: 10.1111/j.1574-6968.1990.tb04885.x. [DOI] [PubMed] [Google Scholar]
- Lipton S. H., Bodwell C. E., Coleman A. H., Jr Amino acid analyzer studies of the products of peroxide oxidation of cystine, lanthionine, and homocystine. J Agric Food Chem. 1977 May-Jun;25(3):624–628. doi: 10.1021/jf60211a050. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Cloning, phenotypic expression, and DNA sequence of the gene for lactacin F, an antimicrobial peptide produced by Lactobacillus spp. J Bacteriol. 1991 Mar;173(5):1779–1788. doi: 10.1128/jb.173.5.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørtvedt C. I., Nissen-Meyer J., Sletten K., Nes I. F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991 Jun;57(6):1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger U., Lücke F. K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol. 1989 Aug;55(8):1901–1906. doi: 10.1128/aem.55.8.1901-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Entian K. D., Schneider U., Götz F., Zähner H., Kellner R., Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988 May 19;333(6170):276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- Sørensen H. H., Thomsen J., Bayne S., Højrup P., Roepstorff P. Strategies for determination of disulphide bridges in proteins using plasma desorption mass spectrometry. Biomed Environ Mass Spectrom. 1990 Nov;19(11):713–720. doi: 10.1002/bms.1200191110. [DOI] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Geis A., Kok J., Venema G. Cloning of two bacteriocin genes from a lactococcal bacteriocin plasmid. Appl Environ Microbiol. 1989 May;55(5):1187–1191. doi: 10.1128/aem.55.5.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Jeeninga R. E., Kok J., Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991 Feb;57(2):492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]