Abstract
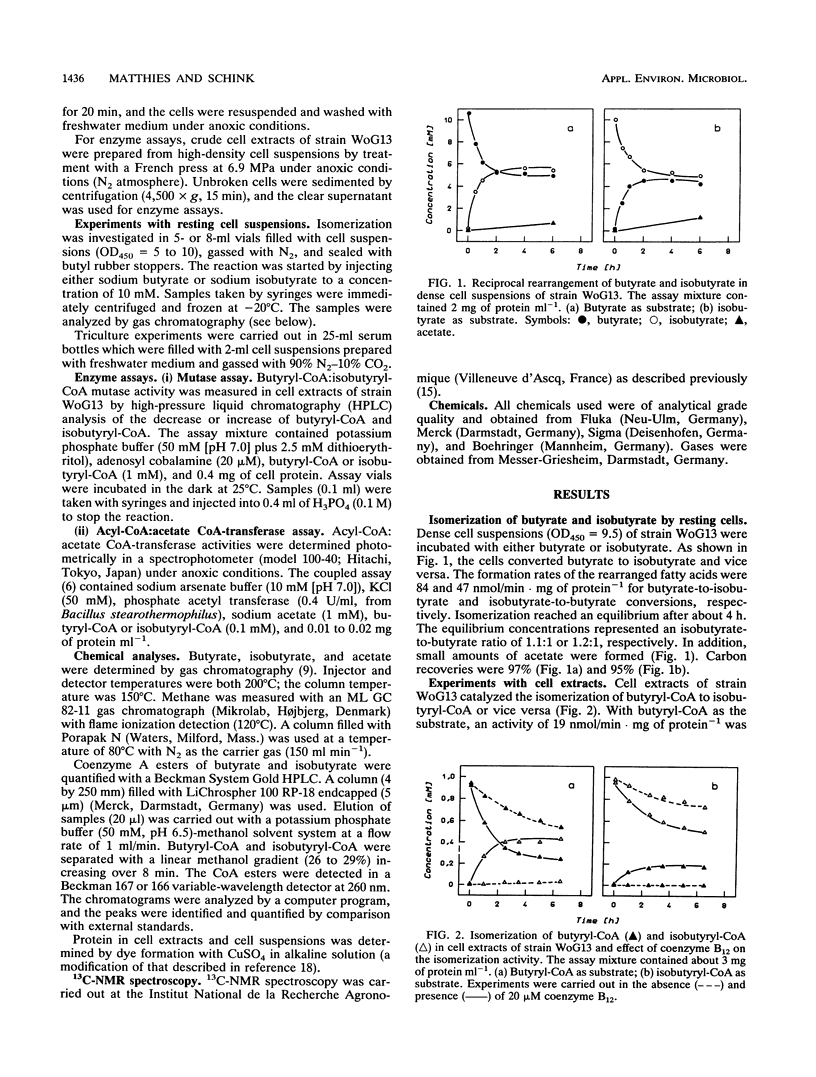
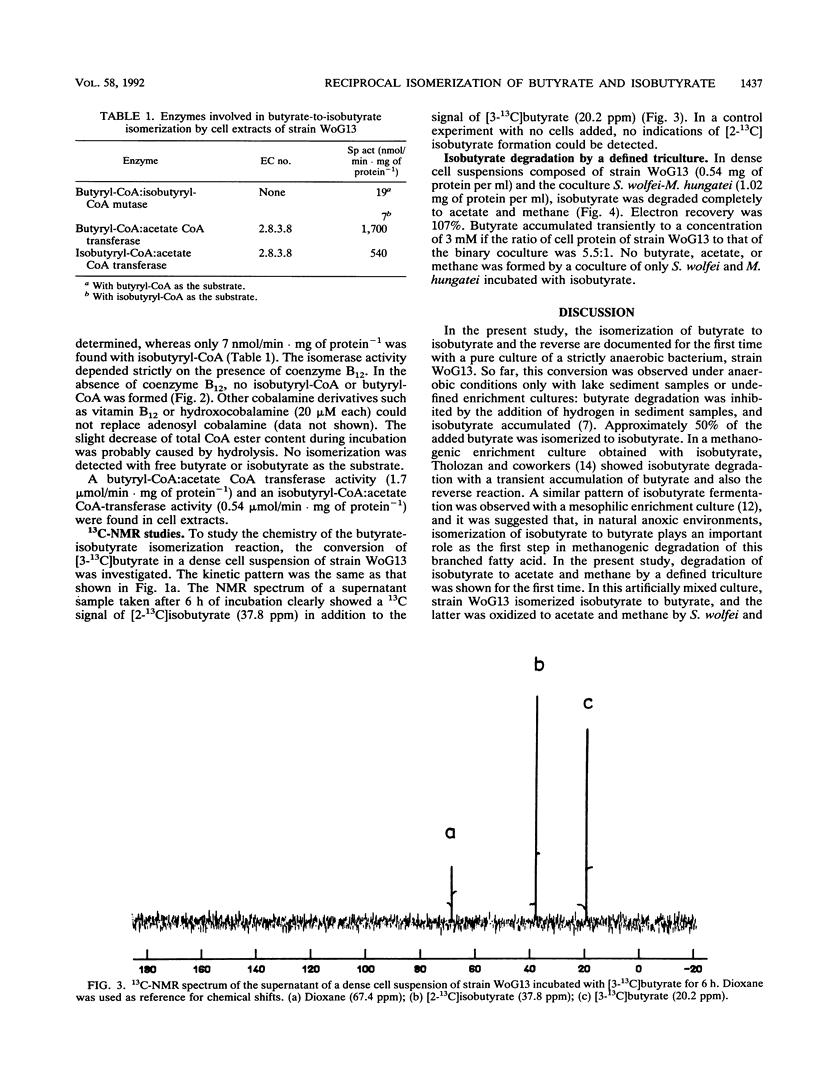
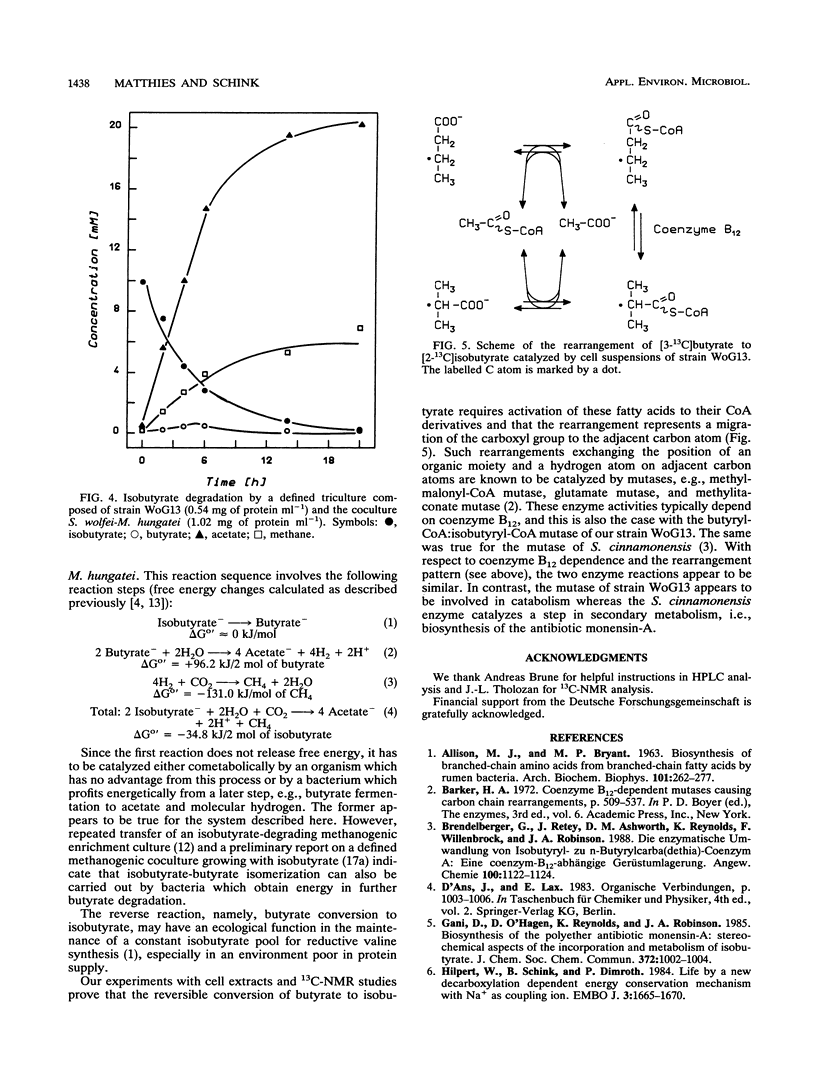
Isomerization of butyrate and isobutyrate was investigated with the recently isolated strictly anaerobic bacterium strain WoG13 which ferments glutarate to butyrate, isobutyrate, CO2, and small amounts of acetate. Dense cell suspensions converted butyrate to isobutyrate and isobutyrate to butyrate. 13C-nuclear magnetic resonance experiments proved that this isomerization was accomplished by migration of the carboxyl group to the adjacent carbon atom. In cell extracts, both butyrate and isobutyrate were activated to their coenzyme A (CoA) esters by acyl-CoA:acetate CoA-transferases. The reciprocal rearrangement of butyryl-CoA and isobutyryl-CoA was catalyzed by a butyryl-CoA:isobutyryl-CoA mutase which depended strictly on the presence of coenzyme B12. Isobutyrate was completely degraded via butyrate to acetate and methane by a defined triculture of strain WoG13, Syntrophomonas wolfei, and Methanospirillum hungatei.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON M. J., BRYANT M. P. Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Arch Biochem Biophys. 1963 May;101:269–277. doi: 10.1016/s0003-9861(63)80012-0. [DOI] [PubMed] [Google Scholar]
- Hilpert W., Schink B., Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na as coupling ion. EMBO J. 1984 Aug;3(8):1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Klug M. J. Intermediary metabolism of organic matter in the sediments of a eutrophic lake. Appl Environ Microbiol. 1982 Mar;43(3):552–560. doi: 10.1128/aem.43.3.552-560.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies C., Schink B. Fermentative degradation of glutarate via decarboxylation by newly isolated strictly anaerobic bacteria. Arch Microbiol. 1992;157(3):290–296. doi: 10.1007/BF00245164. [DOI] [PubMed] [Google Scholar]
- Platen H., Schink B. Anaerobic degradation of acetone and higher ketones via carboxylation by newly isolated denitrifying bacteria. J Gen Microbiol. 1989 Apr;135(4):883–891. doi: 10.1099/00221287-135-4-883. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thede-Reynolds K. R., Motulsky H. J., Feldman R. D. Temperature-dependent binding of hydrophilic beta-adrenergic receptor ligands to intact human lymphocytes. Life Sci. 1986 Oct 13;39(15):1325–1334. doi: 10.1016/0024-3205(86)90330-9. [DOI] [PubMed] [Google Scholar]
- Tholozan J. L., Samain E., Grivet J. P., Moletta R., Dubourguier H. C., Albagnac G. Reductive carboxylation of propionate to butyrate in methanogenic ecosystems. Appl Environ Microbiol. 1988 Feb;54(2):441–445. doi: 10.1128/aem.54.2.441-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate-reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol. 1981 Jul;129(5):395–400. doi: 10.1007/BF00406470. [DOI] [PubMed] [Google Scholar]


