Abstract
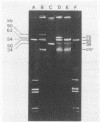
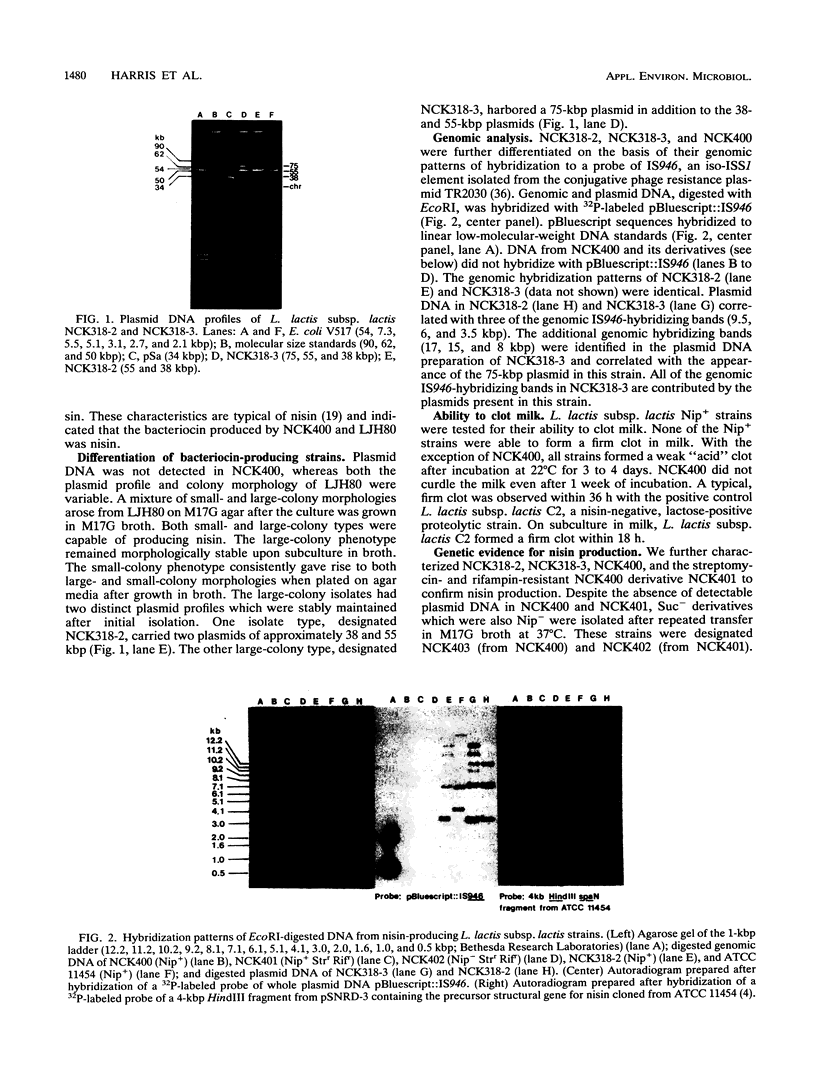
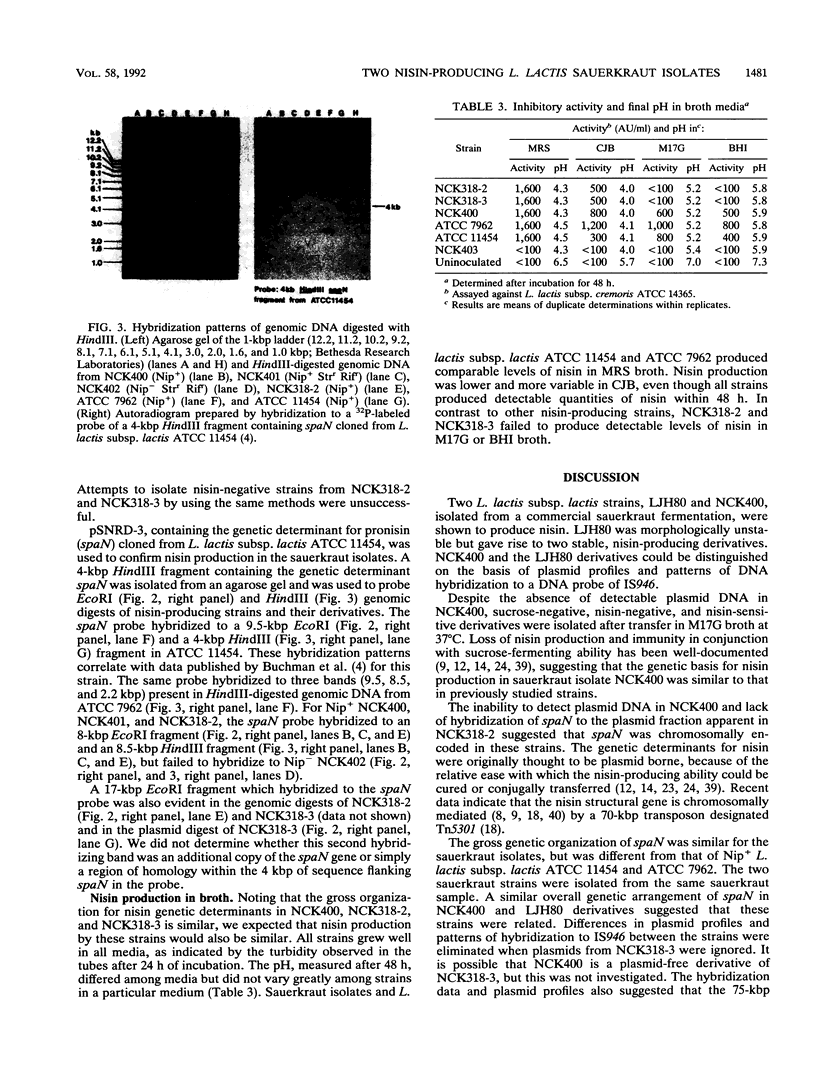
Two Lactococcus lactis subsp. lactis strains, NCK400 and LJH80, isolated from a commercial sauerkraut fermentation were shown to produce nisin. LJH80 was morphologically unstable and gave rise to two stable, nisin-producing (Nip+) derivatives, NCK318-2 and NCK318-3. NCK400 and derivatives of LJH80 exhibited identical morphological and metabolic characteristics, but could be distinguished on the basis of plasmid profiles and genomic hybridization patterns to a DNA probe specific for the iso-ISS1 element, IS946. NCK318-2 and NCK318-3 harbored two and three plasmids, respectively, which hybridized with IS946. Plasmid DNA was not detected in NCK400, and DNA from this strain failed to hybridize with IS946. Despite the absence of detectable plasmid DNA in NCK400, nisin-negative derivatives (NCK402 and NCK403) were isolated after repeated transfer in broth at 37 degrees C. Nisin-negative derivatives concurrently lost the ability to ferment sucrose and became sensitive to nisin. A 4-kbp HindIII fragment containing the structural gene for nisin (spaN), cloned from L. lactis subsp. lactis ATCC 11454, was used to probe genomic DNA of NCK318-2, NCK318-3, NCK400, and NCK402 digested with EcoRI or HindIII. The spaN probe hybridized to an 8.8-kbp EcoRI fragment and a 10-kbp HindIII fragment in the Nip+ sauerkraut isolates, but did not hybridize to the Nip- derivative, NCK402. A different hybridization pattern was observed when the same probe was used against Nip+ L. lactis subsp. lactis ATCC 11454 and ATCC 7962. These phenotypic and genetic data confirmed that unique Nip+ L. lactis subsp. lactis strains were isolated from fermenting sauerkraut.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R. E., Daeschel M. A., Hassan H. M. Antibacterial activity of plantaricin SIK-83, a bacteriocin produced by Lactobacillus plantarum. Biochimie. 1988 Mar;70(3):381–390. doi: 10.1016/0300-9084(88)90211-8. [DOI] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Daeschel M. A., Klaenhammer T. R. Association of a 13.6-Megadalton Plasmid in Pediococcus pentosaceus with Bacteriocin Activity. Appl Environ Microbiol. 1985 Dec;50(6):1538–1541. doi: 10.1128/aem.50.6.1538-1541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Thompson J. Simultaneous loss of N5-(carboxyethyl)ornithine synthase, nisin production, and sucrose-fermenting ability by Lactococcus lactis K1. J Bacteriol. 1990 Jul;172(7):4122–4126. doi: 10.1128/jb.172.7.4122-4126.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming H. P., Etchells J. L., Costilow R. N. Microbial inhibition by an isolate of pediococcus from cucumber brines. Appl Microbiol. 1975 Dec;30(6):1040–1042. doi: 10.1128/am.30.6.1040-1042.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis A., Singh J., Teuber M. Potential of lactic streptococci to produce bacteriocin. Appl Environ Microbiol. 1983 Jan;45(1):205–211. doi: 10.1128/aem.45.1.205-211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Transfer of Sucrose-Fermenting Ability and Nisin Production Phenotype among Lactic Streptococci. Appl Environ Microbiol. 1985 Mar;49(3):627–633. doi: 10.1128/aem.49.3.627-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH A. Growth and nisin production of a strain of Streptococcus lactis. J Gen Microbiol. 1951 Feb;5(1):208–221. doi: 10.1099/00221287-5-1-208. [DOI] [PubMed] [Google Scholar]
- Horn N., Swindell S., Dodd H., Gasson M. Nisin biosynthesis genes are encoded by a novel conjugative transposon. Mol Gen Genet. 1991 Aug;228(1-2):129–135. doi: 10.1007/BF00282457. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar W., Rajchert-Trzpil M., Dobrzański W. T. The effect of proflavin, ethidium bromide and an elevated temperature on the appearance of nisin-negative clones in nisin-producing strains of Streptococcus lactis. J Gen Microbiol. 1974 Aug;83(2):295–302. doi: 10.1099/00221287-83-2-295. [DOI] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989 Feb;55(2):394–400. doi: 10.1128/aem.55.2.394-400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McKay L. L. Functional properties of plasmids in lactic streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):259–274. doi: 10.1007/BF00399502. [DOI] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders J. W., Boerrigter I. J., Rollema H. S., Siezen R. J., de Vos W. M. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur J Biochem. 1991 Nov 1;201(3):581–584. doi: 10.1111/j.1432-1033.1991.tb16317.x. [DOI] [PubMed] [Google Scholar]
- Mundt J. O., Graham W. F., McCarty I. E. Spherical Lactic Acid-producing Bacteria of Southern-grown Raw and Processed Vegetables. Appl Microbiol. 1967 Nov;15(6):1303–1308. doi: 10.1128/am.15.6.1303-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987 Mar;53(3):553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Smiley K. L., Sherman J. M. The Hydrolysis of Arginine by Streptococci. J Bacteriol. 1942 Jun;43(6):651–660. doi: 10.1128/jb.43.6.651-660.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D. A., Klaenhammer T. R. Characterization of insertion sequence IS946, an Iso-ISS1 element, isolated from the conjugative lactococcal plasmid pTR2030. J Bacteriol. 1990 Aug;172(8):4151–4160. doi: 10.1128/jb.172.8.4151-4160.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark P., Sherman J. M. Concerning the Habitat of Streptococcus lactis. J Bacteriol. 1935 Dec;30(6):639–646. doi: 10.1128/jb.30.6.639-646.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. L., McKay L. L. Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl Environ Microbiol. 1986 Jan;51(1):57–64. doi: 10.1128/aem.51.1.57-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M. T., Chung Y. J., Hansen J. N. Characterization of the nisin gene as part of a polycistronic operon in the chromosome of Lactococcus lactis ATCC 11454. Appl Environ Microbiol. 1991 Apr;57(4):1181–1188. doi: 10.1128/aem.57.4.1181-1188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]