Abstract
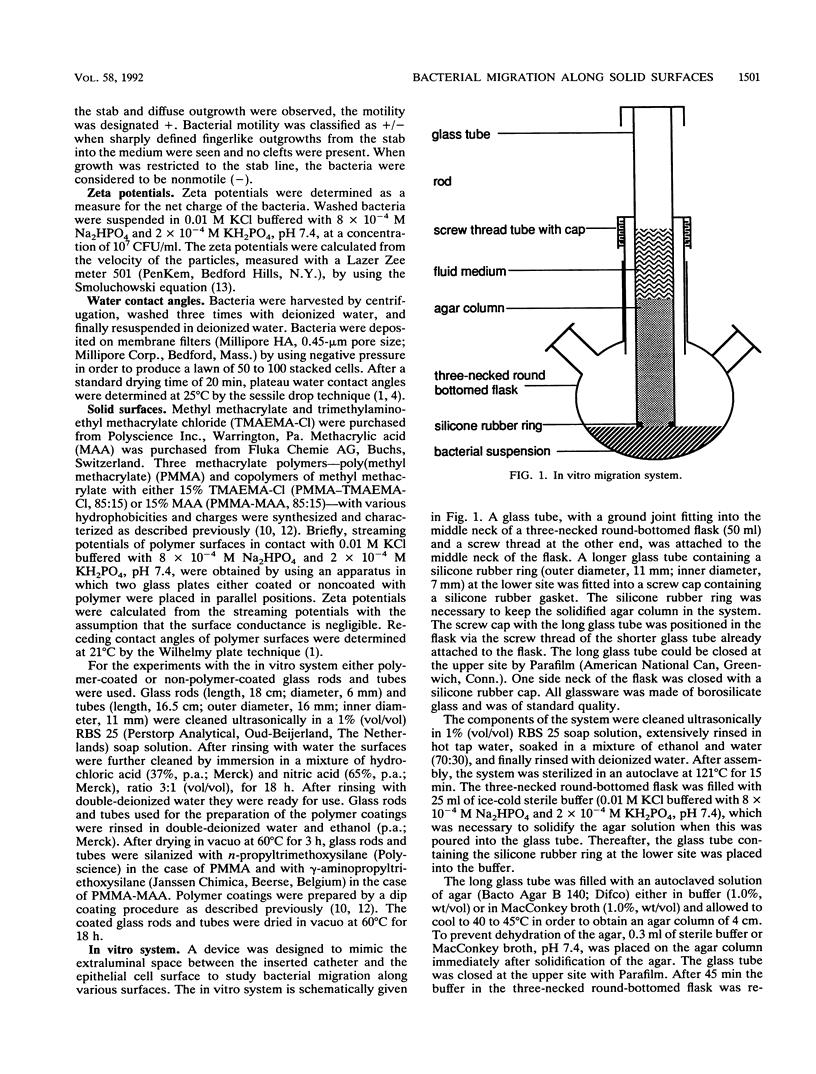
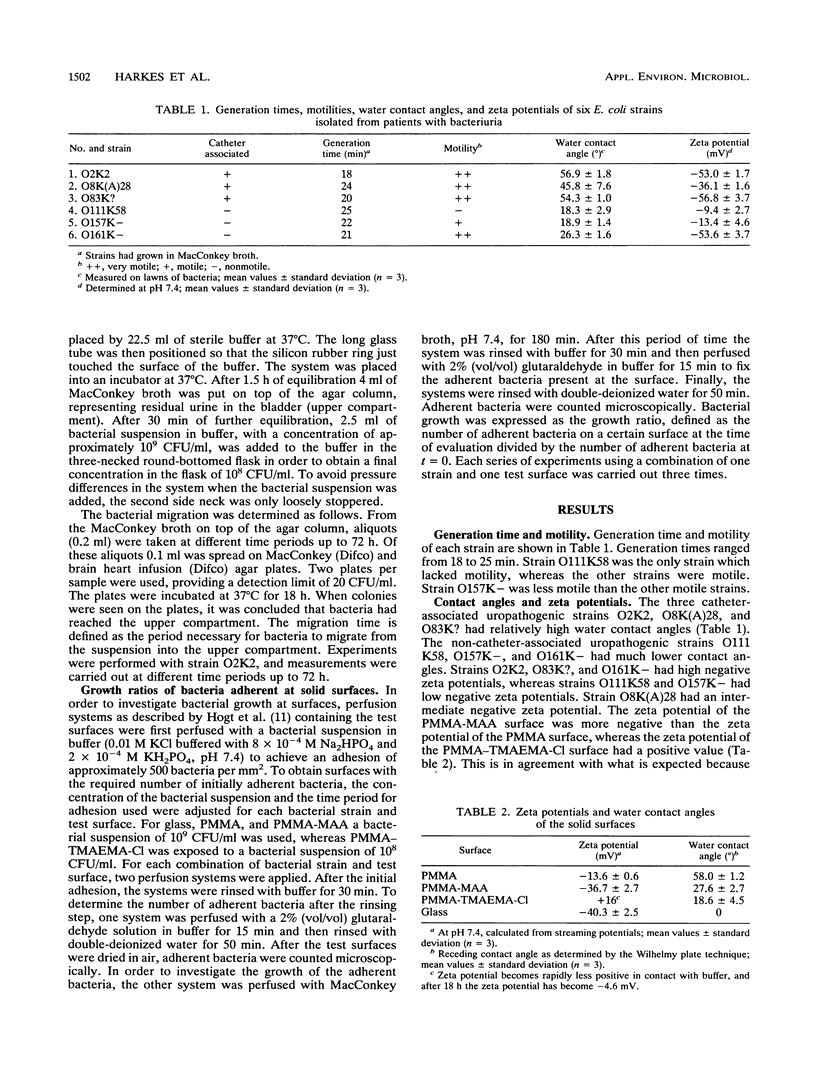
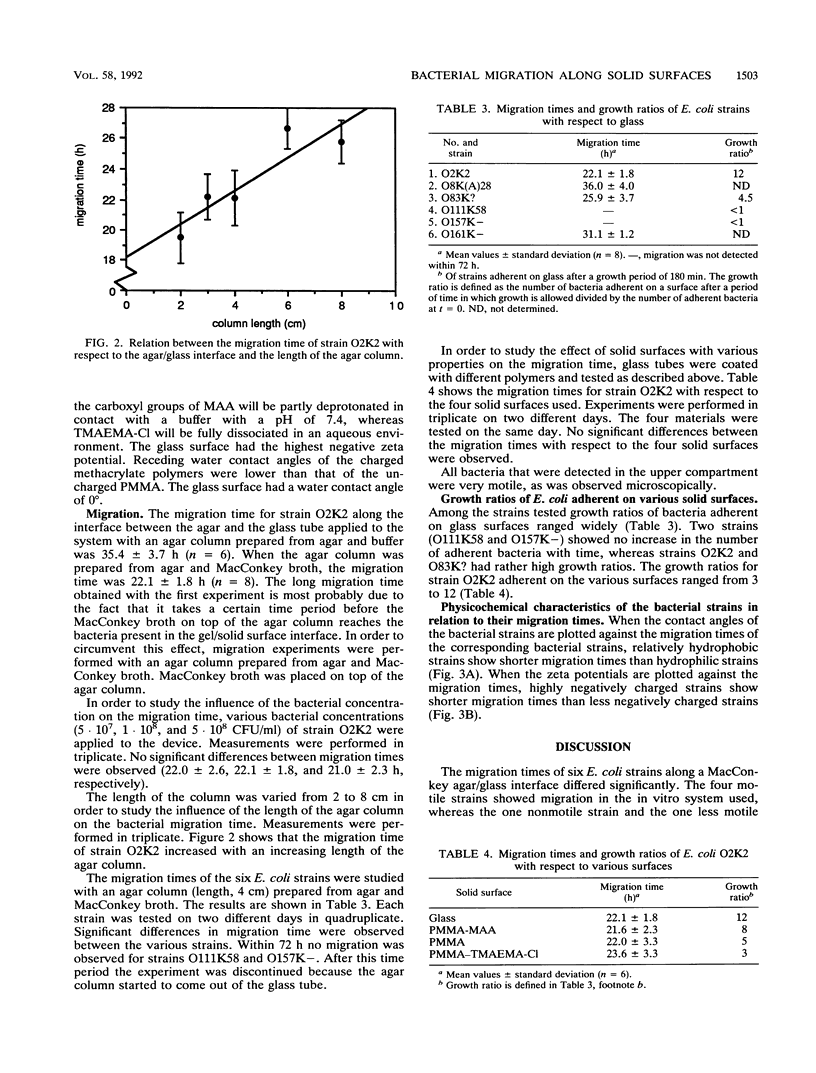
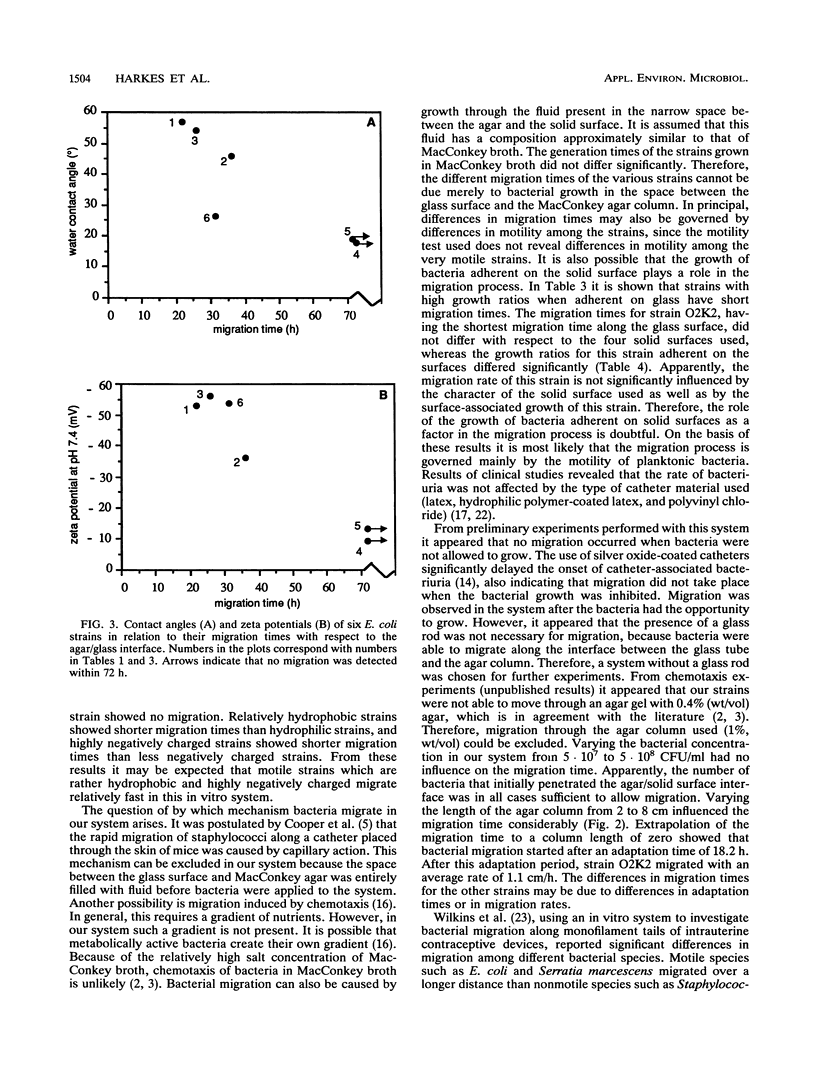
An in vitro system was developed to study the migration of uropathogenic Escherichia coli strains. In this system an aqueous agar gel is placed against a solid surface, allowing the bacteria to migrate along the gel/solid surface interface. Bacterial strains as well as solid surfaces were characterized by means of water contact angle and zeta potential measurements. When glass was used as the solid surface, significantly different migration times for the strains investigated were observed. Relationships among the observed migration times of six strains, their contact angles, and their zeta potentials were found. Relatively hydrophobic strains exhibited migration times shorter than those of hydrophilic strains. For highly negatively charged strains shorter migration times were found than were found for less negatively charged strains. When the fastest-migrating strain with respect to glass was allowed to migrate along solid surfaces differing in hydrophobicity and charge, no differences in migration times were found. Our findings indicate that strategies to prevent catheter-associated bacteriuria should be based on inhibition of bacterial growth rather than on modifying the physicochemical character of the catheter surface.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busscher H. J., Weerkamp A. H., van der Mei H. C., van Pelt A. W., de Jong H. P., Arends J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol. 1984 Nov;48(5):980–983. doi: 10.1128/aem.48.5.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. L., Schiller A. L., Hopkins C. C. Possible role of capillary action in pathogenesis of experimental catheter-associated dermal tunnel infections. J Clin Microbiol. 1988 Jan;26(1):8–12. doi: 10.1128/jcm.26.1.8-12.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A. J., Hukins D. W., Sutton T. M. Infection of catheterised patients: bacterial colonisation of encrusted Foley catheters shown by scanning electron microscopy. Urol Res. 1989;17(6):349–352. doi: 10.1007/BF00510523. [DOI] [PubMed] [Google Scholar]
- Harkes G., Feijen J., Dankert J. Adhesion of Escherichia coli on to a series of poly(methacrylates) differing in charge and hydrophobicity. Biomaterials. 1991 Nov;12(9):853–860. doi: 10.1016/0142-9612(91)90074-k. [DOI] [PubMed] [Google Scholar]
- Hogt A. H., Dankert J., Feijen J. Adhesion of coagulase-negative staphylococci to methacrylate polymers and copolymers. J Biomed Mater Res. 1986 Apr;20(4):533–545. doi: 10.1002/jbm.820200409. [DOI] [PubMed] [Google Scholar]
- Johnson J. R., Roberts P. L., Olsen R. J., Moyer K. A., Stamm W. E. Prevention of catheter-associated urinary tract infection with a silver oxide-coated urinary catheter: clinical and microbiologic correlates. J Infect Dis. 1990 Nov;162(5):1145–1150. doi: 10.1093/infdis/162.5.1145. [DOI] [PubMed] [Google Scholar]
- Monson T., Kunin C. M. Evaluation of a polymer-coated indwelling catheter in prevention of infection. J Urol. 1974 Feb;111(2):220–222. doi: 10.1016/s0022-5347(17)59932-2. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Gristina A. G., Costerton J. W. Electron microscopic study of an infected Foley catheter. Can J Surg. 1985 Jan;28(1):50-1, 54. [PubMed] [Google Scholar]
- Ohkawa M., Sugata T., Sawaki M., Nakashima T., Fuse H., Hisazumi H. Bacterial and crystal adherence to the surfaces of indwelling urethral catheters. J Urol. 1990 Apr;143(4):717–721. doi: 10.1016/s0022-5347(17)40071-1. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd M. J., Gow J. G., Pennington J. H., Shelton J., Scott M. R. Comparison of hydrophilic polymer-coated latex, uncoated latex and PVC indwelling balloon catheters in the prevention of urinary infection. Br J Urol. 1976 Aug;48(4):285–291. doi: 10.1111/j.1464-410x.1976.tb03023.x. [DOI] [PubMed] [Google Scholar]
- Wilkins K. M., Hanlon G. W., Martin G. P., Marriott C. The migration of bacteria through gels in the presence of IUCD monofilament tails. Contraception. 1989 Feb;39(2):205–216. doi: 10.1016/s0010-7824(89)80009-5. [DOI] [PubMed] [Google Scholar]


