Abstract
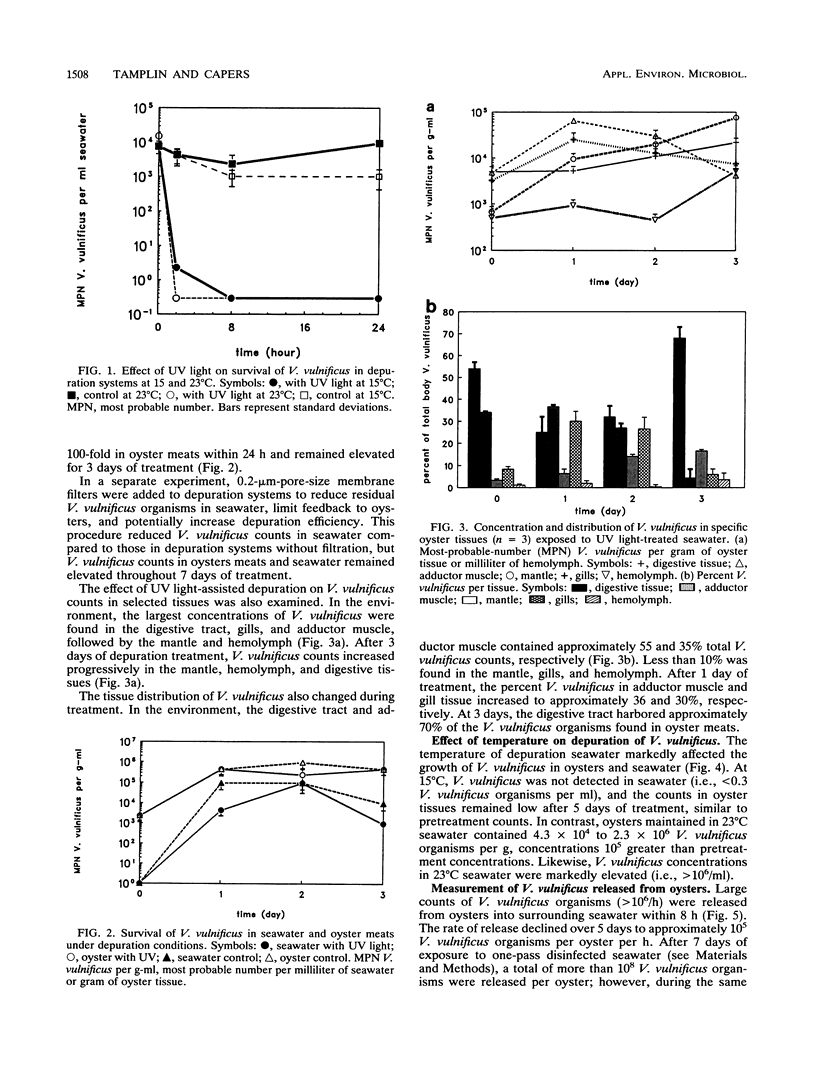
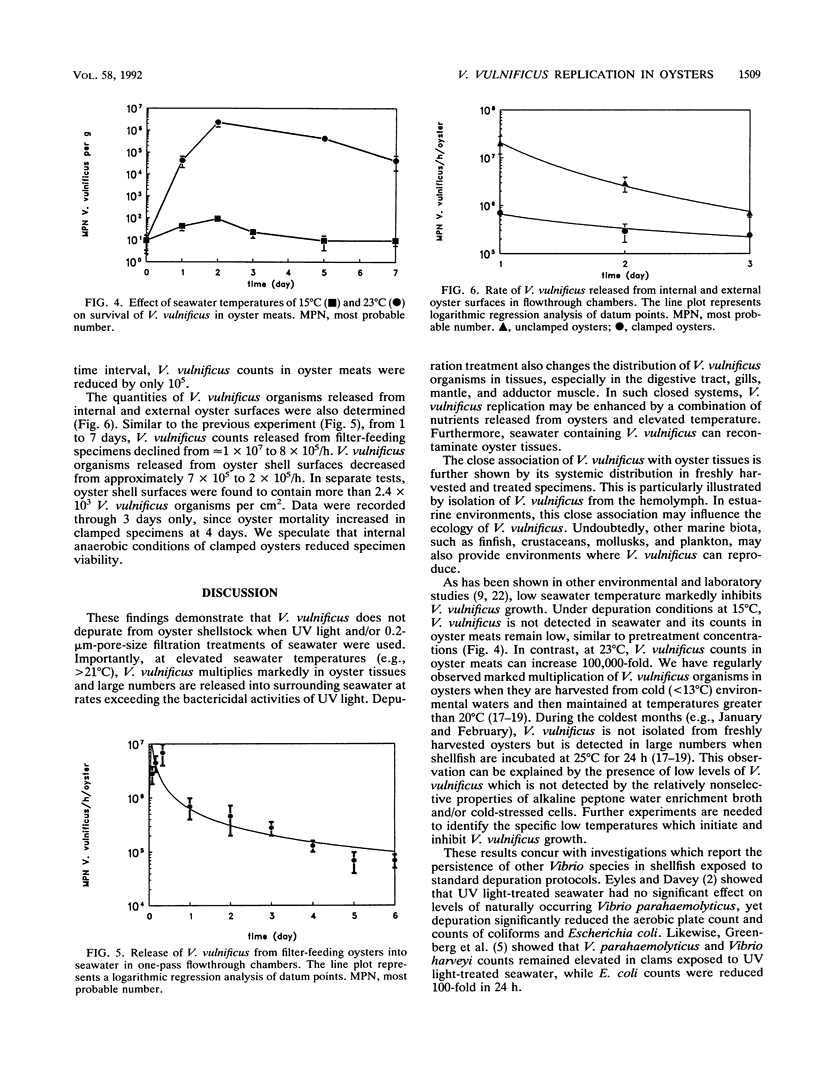
Vibrio vulnificus is an estuarine bacterium which can cause opportunistic infections in humans consuming raw Gulf Coast oysters, Crassostrea virginica. Although V. vulnificus is known as a ubiquitous organism in the Gulf of Mexico, its ecological relationship with C. virginica has not been adequately defined. The objective of the present study was to test the hypothesis that V. vulnificus is a persistent microbial flora of oysters and unamenable to traditional methods of controlled purification, such as UV light depuration. Experimental depuration systems consisted of aquaria containing temperature-controlled seawater treated with UV light and 0.2-microns-pore-size filtration. V. vulnificus was enumerated in seawater, oyster shell biofilms, homogenates of whole oyster meats, and tissues including the hemolymph, digestive region, gills, mantle, and adductor muscle. Results showed that depuration systems conducted at temperatures greater than 23 degrees C caused V. vulnificus counts to increase in oysters, especially in the hemolymph, adductor muscle, and mantle. Throughout the process, depuration water contained high concentrations of V. vulnificus, indicating that the disinfection properties of UV radiation and 0.2-microns-pore-size filtration were less than the rate at which V. vulnificus was released into seawater. Approximately 10(5) to 10(6) V. vulnificus organisms were released from each oyster per hour, with 0.05 to 35% originating from shell surfaces. These surfaces contained greater than 10(3) V. vulnificus organisms per cm2. In contrast, when depuration seawater was maintained at 15 degrees C, V. vulnificus was not detected in seawater and multiplication in oyster tissues was inhibited.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook D. W., Pabst G. S., Jr Recommended modification of dilution procedure used for bacteriological examination of shellfish. J Assoc Off Anal Chem. 1984 Jan-Feb;67(1):197–198. [PubMed] [Google Scholar]
- Kaysner C. A., Abeyta C., Jr, Wekell M. M., DePaola A., Jr, Stott R. F., Leitch J. M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987 Jun;53(6):1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T., Dinuzzo A. Uptake and clearance of Vibrio vulnificus from Gulf coast oysters (Crassostrea virginica). Appl Environ Microbiol. 1985 Dec;50(6):1548–1549. doi: 10.1128/aem.50.6.1548-1549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Effect of temperature and salinity on Vibrio (Beneckea) vulnificus occurrence in a Gulf Coast environment. Appl Environ Microbiol. 1982 Oct;44(4):820–824. doi: 10.1128/aem.44.4.820-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klontz K. C., Lieb S., Schreiber M., Janowski H. T., Baldy L. M., Gunn R. A. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981-1987. Ann Intern Med. 1988 Aug 15;109(4):318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- Massad G., Oliver J. D. New selective and differential medium for Vibrio cholerae and Vibrio vulnificus. Appl Environ Microbiol. 1987 Sep;53(9):2262–2264. doi: 10.1128/aem.53.9.2262-2264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Warner R. A., Cleland D. R. Distribution and ecology of Vibrio vulnificus and other lactose-fermenting marine vibrios in coastal waters of the southeastern United States. Appl Environ Microbiol. 1982 Dec;44(6):1404–1414. doi: 10.1128/aem.44.6.1404-1414.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M. L., Martin A. L., Ruple A. D., Cook D. W., Kaspar C. W. Enzyme immunoassay for identification of Vibrio vulnificus in seawater, sediment, and oysters. Appl Environ Microbiol. 1991 Apr;57(4):1235–1240. doi: 10.1128/aem.57.4.1235-1240.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin M. L., Rodrick G. E., Blake N. J., Bundy D. A., Alexander L. Public health aspects of halophilic Vibrios in Jamaica. West Indian Med J. 1983 Sep;32(3):147–151. [PubMed] [Google Scholar]
- Tamplin M., Rodrick G. E., Blake N. J., Cuba T. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol. 1982 Dec;44(6):1466–1470. doi: 10.1128/aem.44.6.1466-1470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


