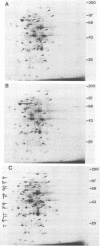
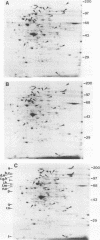
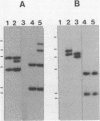
Abstract
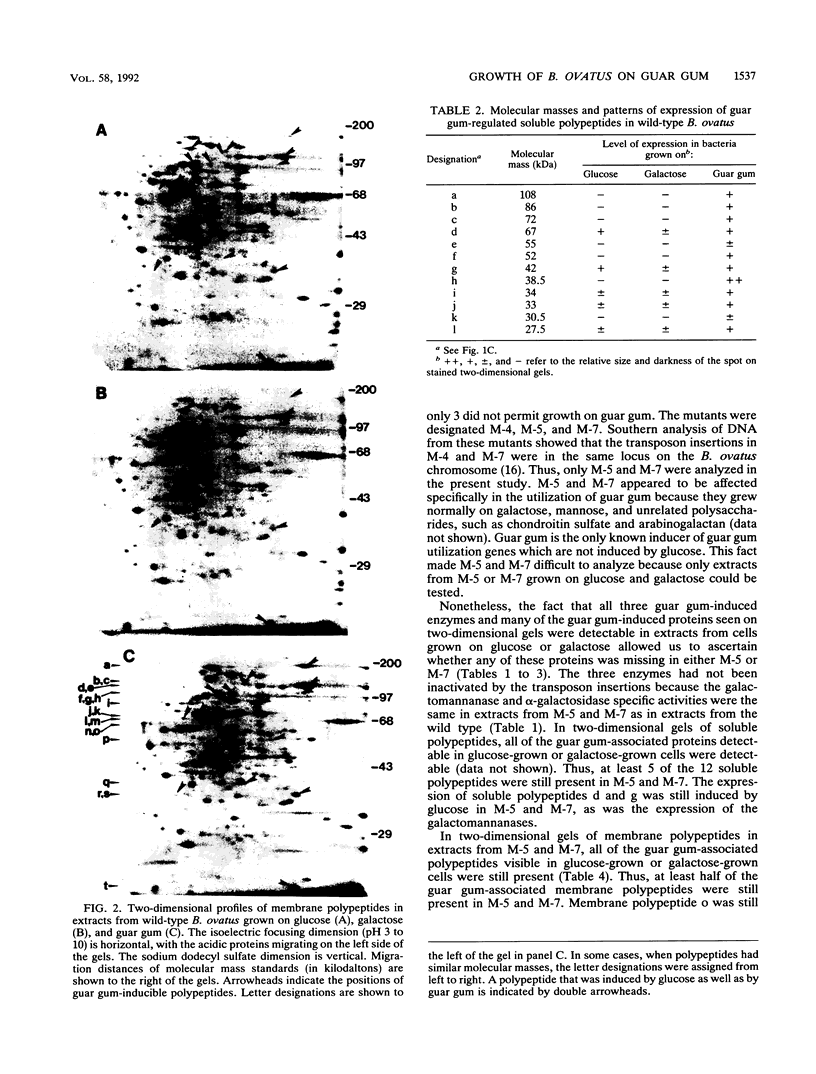
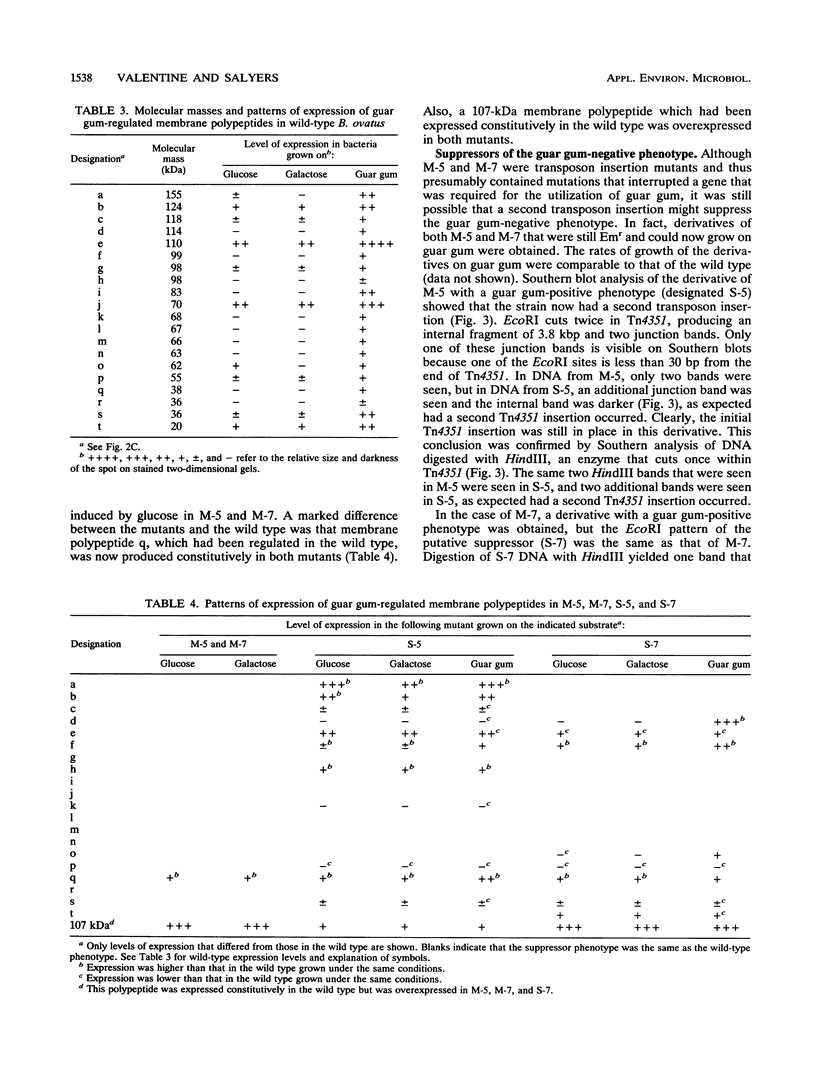
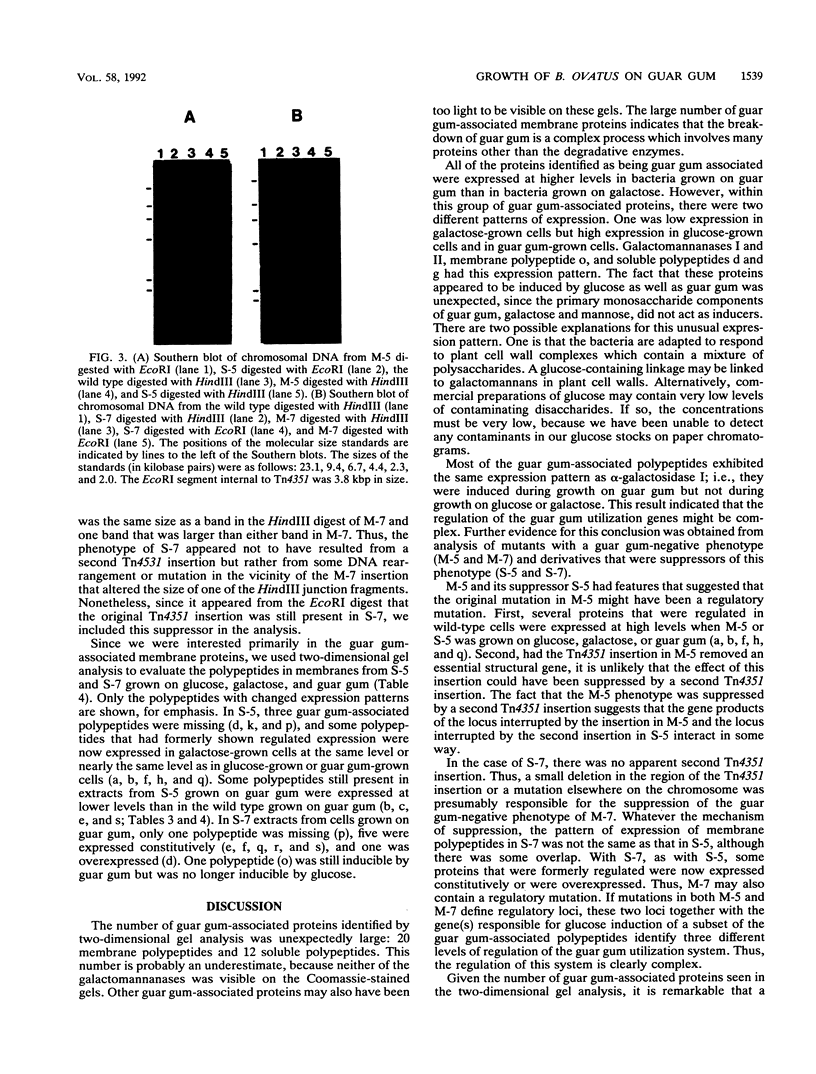
Bacteroides ovatus, a gram-negative obligate anaerobe from the human colon, can ferment the branched galactomannan guar gum. Previously, three enzymes involved in guar gum breakdown were characterized. The expression of these enzymes appeared to be regulated; i.e., specific activities were higher in extracts from bacteria grown on guar gum than in extracts from bacteria grown on the monosaccharide constituents of guar gum, mannose and galactose. In the present study, we used two-dimensional gel analysis to determine the total number of B. ovatus proteins enhanced during growth on guar gum. Twelve soluble proteins and 20 membrane proteins were expressed at higher levels in guar gum-grown cells than in galactose-grown cells. An unexpected finding was that the expression of the two galactomannanases was induced by glucose as well as guar gum. Three other proteins, one membrane protein and two soluble proteins, had this same expression pattern. The remainder of the guar gum-associated proteins seen on two-dimensional gels and the guar gum-associated alpha-galactosidase were induced in cells grown on guar gum but not in cells grown on glucose. Two transposon-generated mutants (M-5 and M-7) that could not grow on guar gum were isolated. Both mutants still expressed the galactomannanases and the alpha-galactosidase. They also still expressed all of the guar gum-associated proteins that could be detected in two-dimensional gels of glucose-grown or galactose-grown cells. A second transposon insertion that suppressed the guar gum-negative phenotype of M-5 was isolated and characterized. The characteristics of this suppressor mutant indicated that the original transposon insertion was probably in a regulatory locus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Anderson K. L., Salyers A. A. Genetic evidence that outer membrane binding of starch is required for starch utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1989 Jun;171(6):3199–3204. doi: 10.1128/jb.171.6.3199-3204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini F. C., Salyers A. A. Characterization of an outer membrane mannanase from Bacteroides ovatus. J Bacteriol. 1987 May;169(5):2031–2037. doi: 10.1128/jb.169.5.2031-2037.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini F. C., Salyers A. A. Purification and characterization of a cell-associated, soluble mannanase from Bacteroides ovatus. J Bacteriol. 1987 May;169(5):2038–2043. doi: 10.1128/jb.169.5.2038-2043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini F., Babcock M., Salyers A. A. Purification and characterization of two alpha-galactosidases associated with catabolism of guar gum and other alpha-galactosides by Bacteroides ovatus. J Bacteriol. 1985 Feb;161(2):500–506. doi: 10.1128/jb.161.2.500-506.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa V., Shoemaker N. B., Salyers A. A. Direct repeats flanking the Bacteroides transposon Tn4351 are insertion sequence elements. J Bacteriol. 1988 Jan;170(1):449–451. doi: 10.1128/jb.170.1.449-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Linz J., Braun D. M., Salyers A. A. Analysis of outer membrane proteins which are associated with growth of Bacteroides thetaiotaomicron on chondroitin sulfate. J Bacteriol. 1985 Sep;163(3):1080–1086. doi: 10.1128/jb.163.3.1080-1086.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine P. J., Arnold P., Salyers A. A. Cloning and partial characterization of two chromosomal loci from Bacteroides ovatus that contain genes essential for growth on guar gum. Appl Environ Microbiol. 1992 May;58(5):1541–1548. doi: 10.1128/aem.58.5.1541-1548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]