Abstract
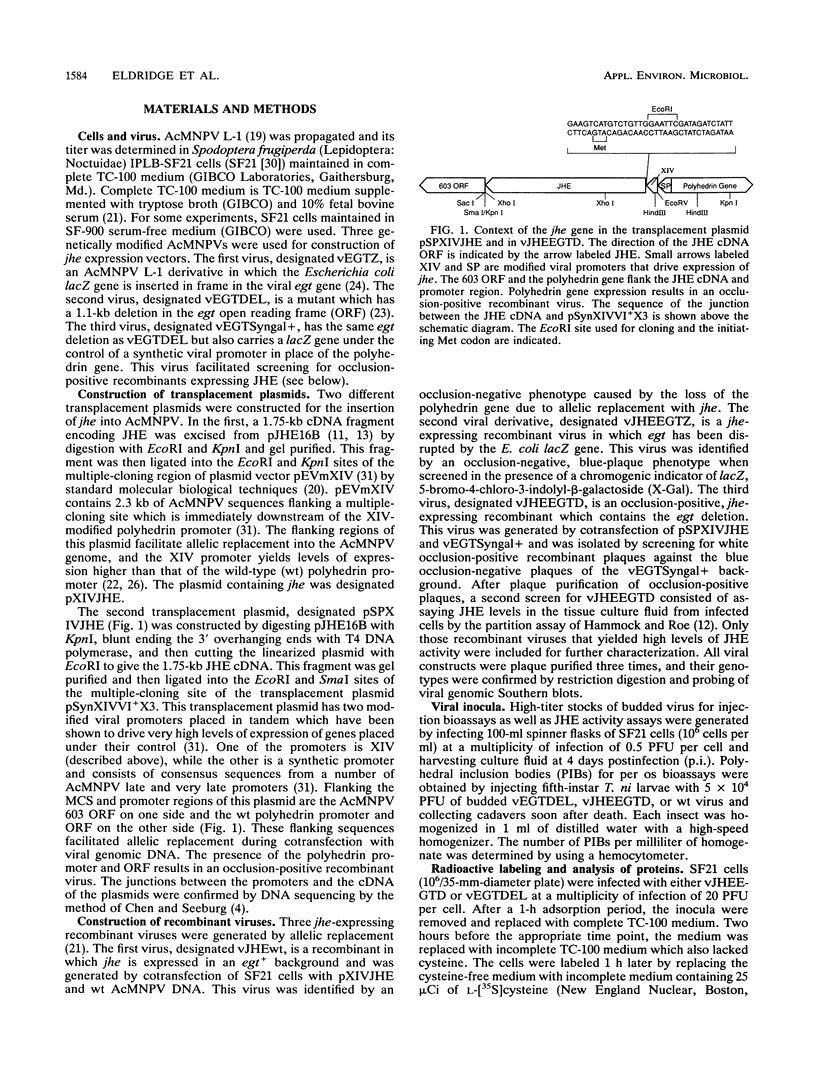
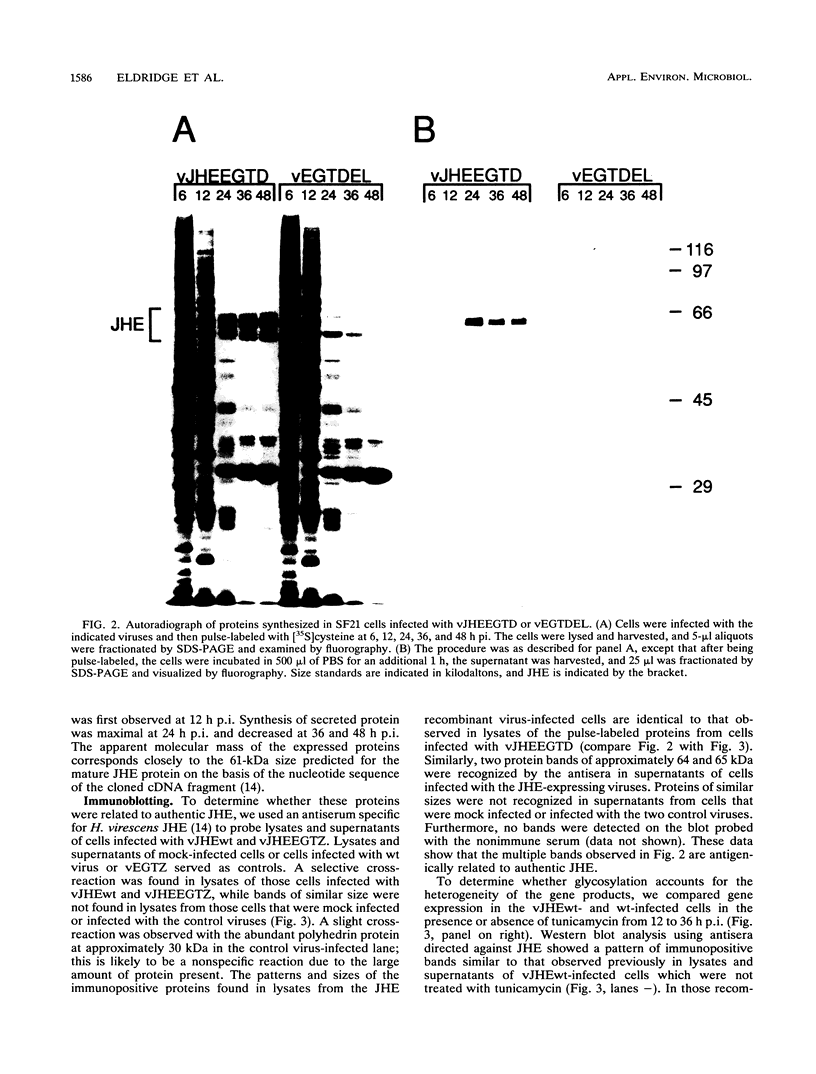
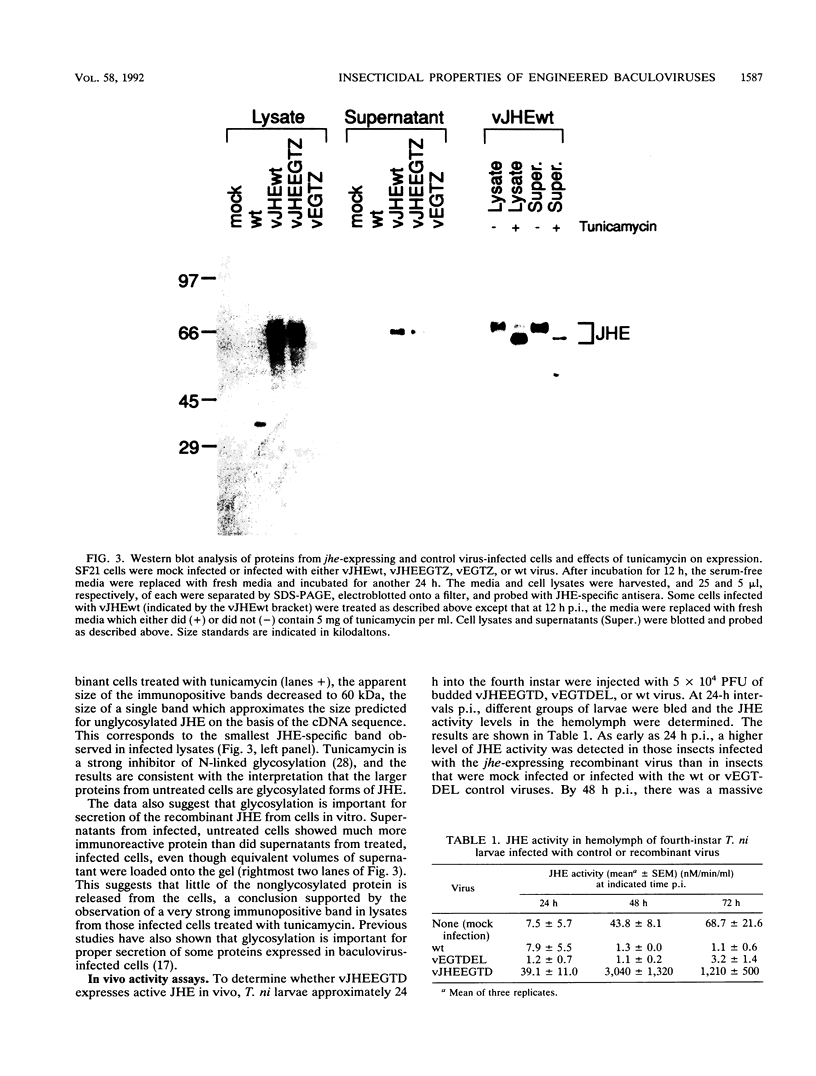
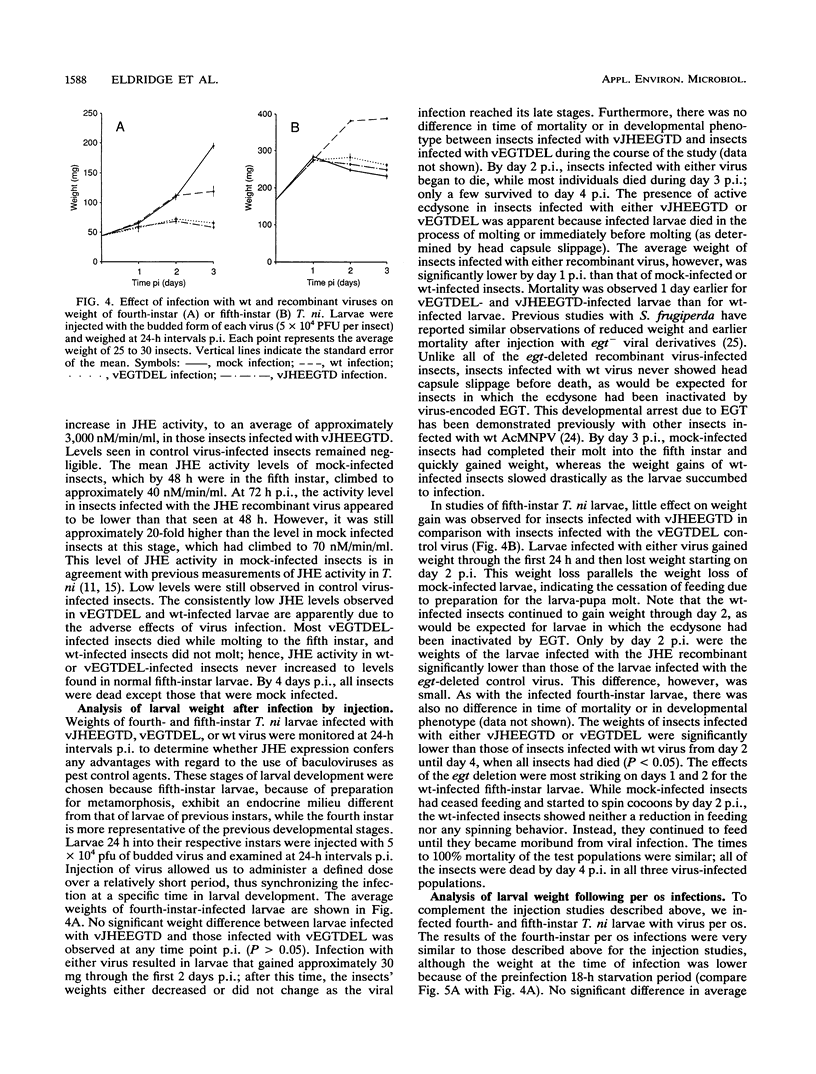
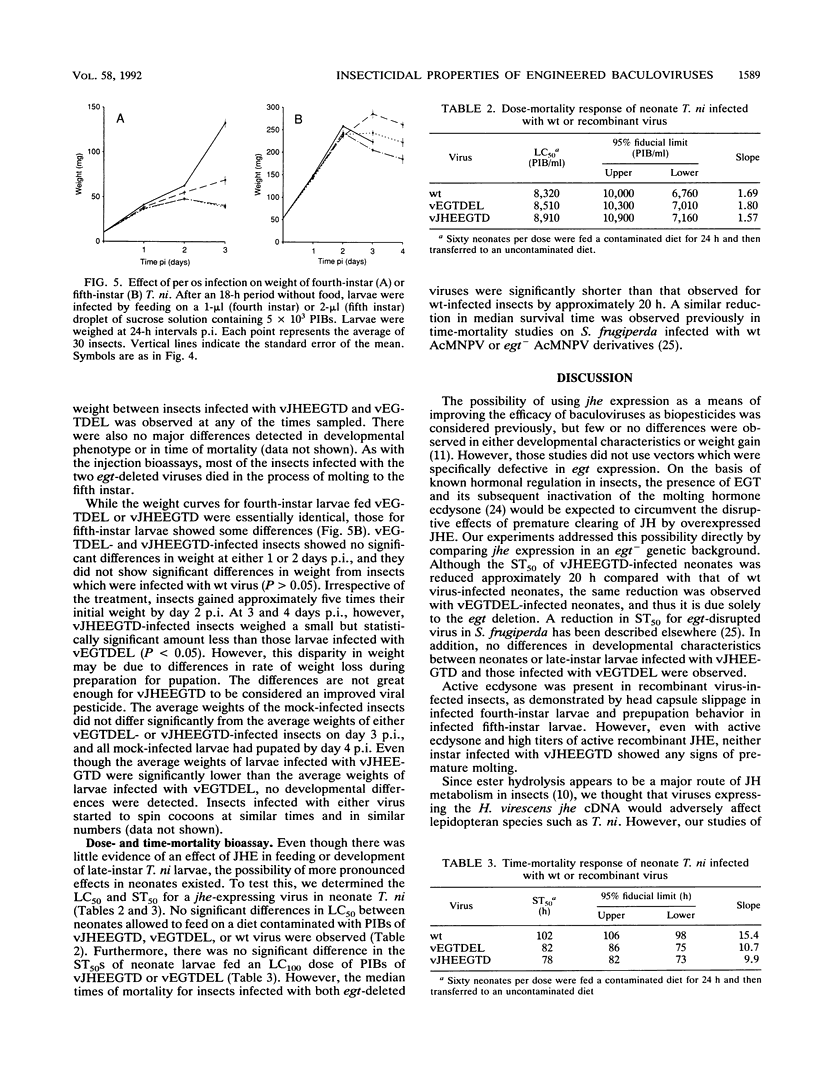
Exploring the possibility of enhancing the properties of baculoviruses as biological control agents of insect pests, we tested the effect of expressing an insect gene (jhe) encoding juvenile hormone esterase. Juvenile hormone esterase inactivates juvenile hormone, which regulates the outcome of an insect molt. A cDNA encoding the juvenile hormone esterase of Heliothis virescens was inserted into the genome of Autographa californica nuclear polyhedrosis virus such that the gene was expressed under the control of a strong, modified viral promoter. This virus, however, naturally encodes an ecdysteroid UDP-glucosyltransferase which inactivates ecdysone, the hormone which initiates molting. Since ecdysteroid UDP-glucosyltransferase could mask the effects of jhe expression by blocking molting entirely, jhe-expressing viruses in which the ecdysteroid UDP-glucosyltransferase gene was deleted or disrupted were constructed. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of proteins from infected cells revealed several intracellular proteins and two major secreted proteins which reacted with antibodies to authentic juvenile hormone esterase. Western blot analysis coupled with tunicamycin treatment indicated that differential glycosylation was responsible for the multiple products. Hemolymph of recombinant virus-infected fourth-instar Trichoplusia ni larvae contained levels of juvenile hormone esterase activity 40-fold higher than maximal levels found in uninfected larvae. However, little or no difference in developmental characteristics, weight gain, or time of mortality was observed between insects infected with the jhe-expressing viruses and control viruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Hammock B. D., Roe R. M. Analysis of juvenile hormone esterase activity. Methods Enzymol. 1985;111:487–494. doi: 10.1016/s0076-6879(85)11034-7. [DOI] [PubMed] [Google Scholar]
- Hanzlik T. N., Abdel-Aal Y. A., Harshman L. G., Hammock B. D. Isolation and sequencing of cDNA clones coding for juvenile hormone esterase from Heliothis virescens. Evidence for a catalytic mechanism for the serine carboxylesterases different from that of the serine proteases. J Biol Chem. 1989 Jul 25;264(21):12419–12425. [PubMed] [Google Scholar]
- Jarvis D. L., Summers M. D. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989 Jan;9(1):214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Miller L. K. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J Virol. 1978 Sep;27(3):754–767. doi: 10.1128/jvi.27.3.754-767.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science. 1989 Sep 8;245(4922):1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- Ooi B. G., Rankin C., Miller L. K. Downstream sequences augment transcription from the essential initiation site of a baculovirus polyhedrin gene. J Mol Biol. 1989 Dec 20;210(4):721–736. doi: 10.1016/0022-2836(89)90105-8. [DOI] [PubMed] [Google Scholar]
- Rankin C., Ooi B. G., Miller L. K. Eight base pairs encompassing the transcriptional start point are the major determinant for baculovirus polyhedrin gene expression. Gene. 1988 Oct 15;70(1):39–49. doi: 10.1016/0378-1119(88)90102-3. [DOI] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Wang X. Z., Ooi B. G., Miller L. K. Baculovirus vectors for multiple gene expression and for occluded virus production. Gene. 1991 Apr;100:131–137. doi: 10.1016/0378-1119(91)90358-i. [DOI] [PubMed] [Google Scholar]