Abstract
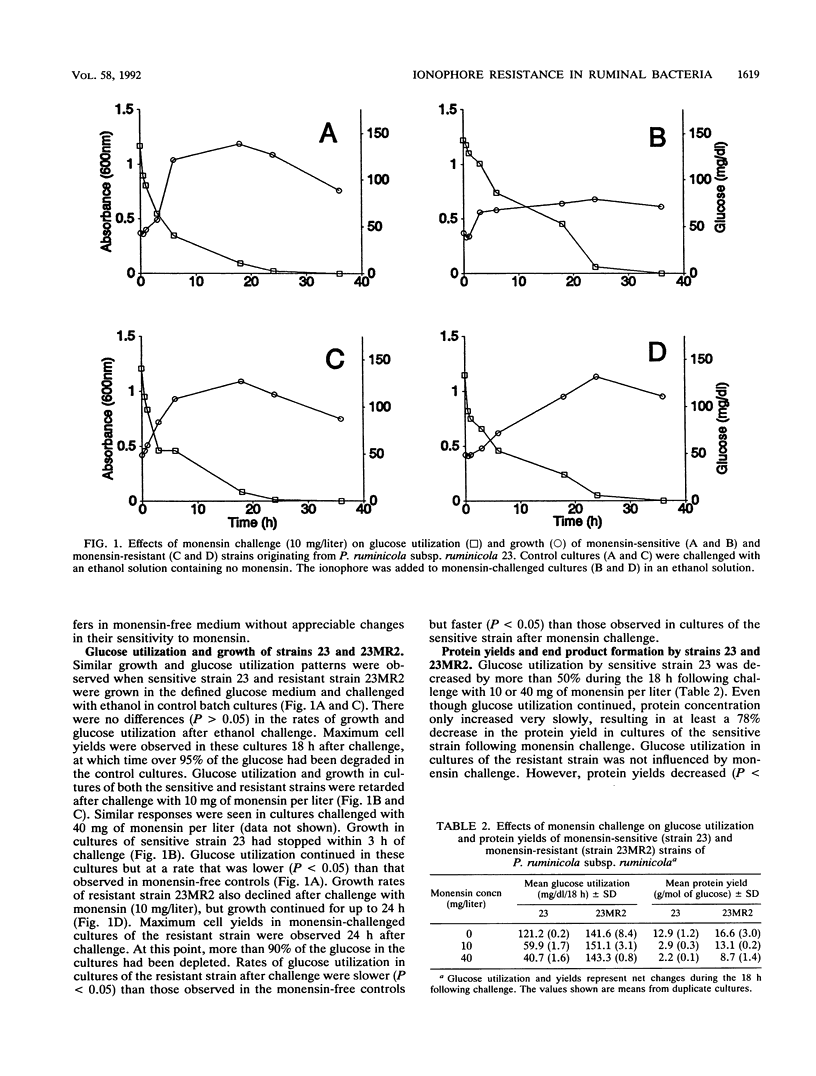
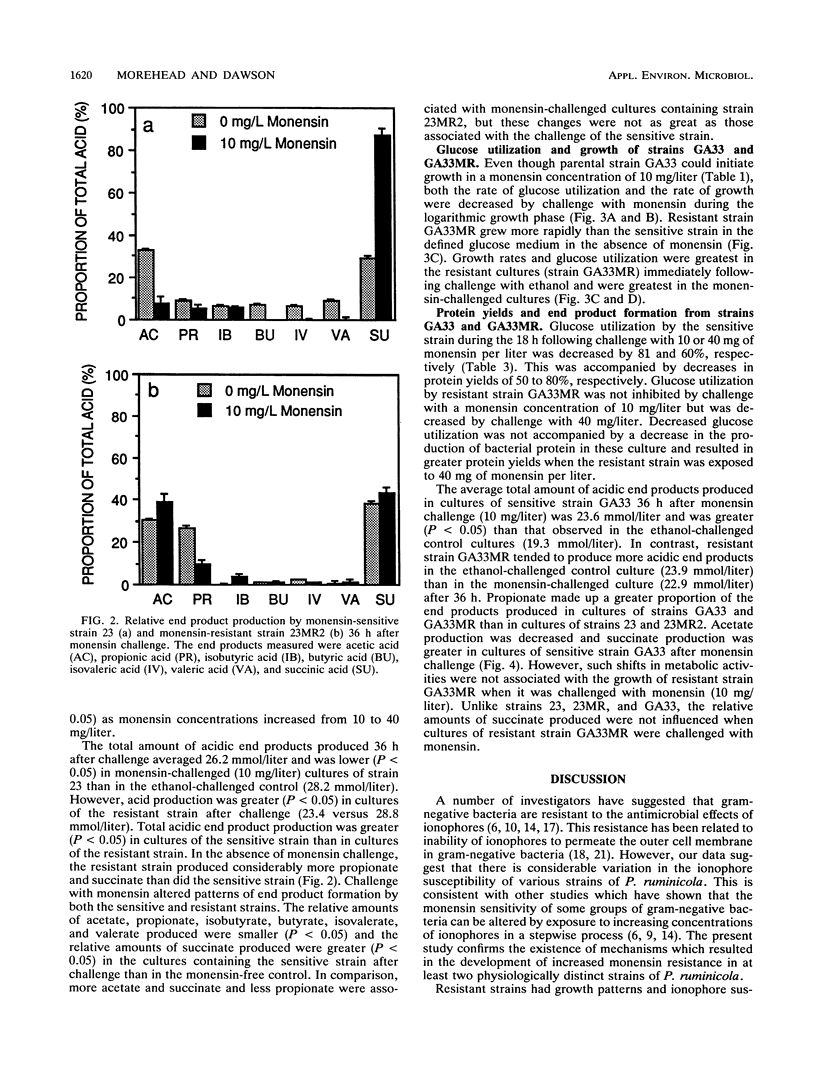
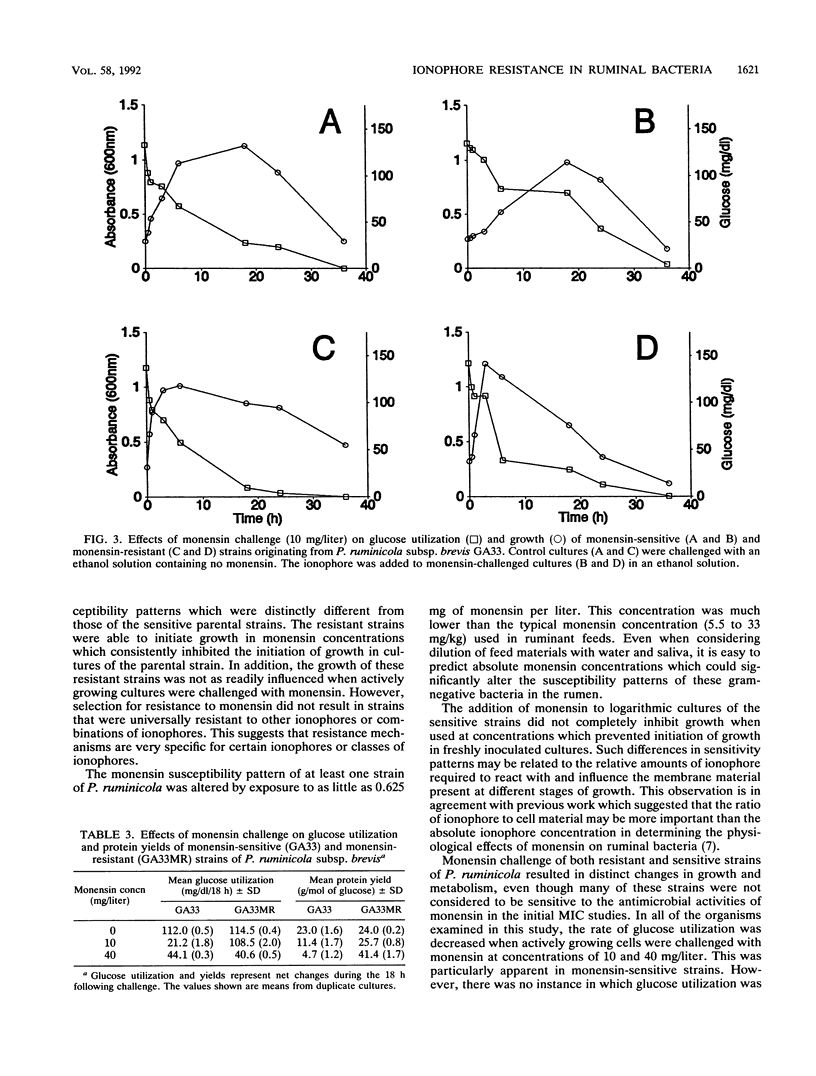
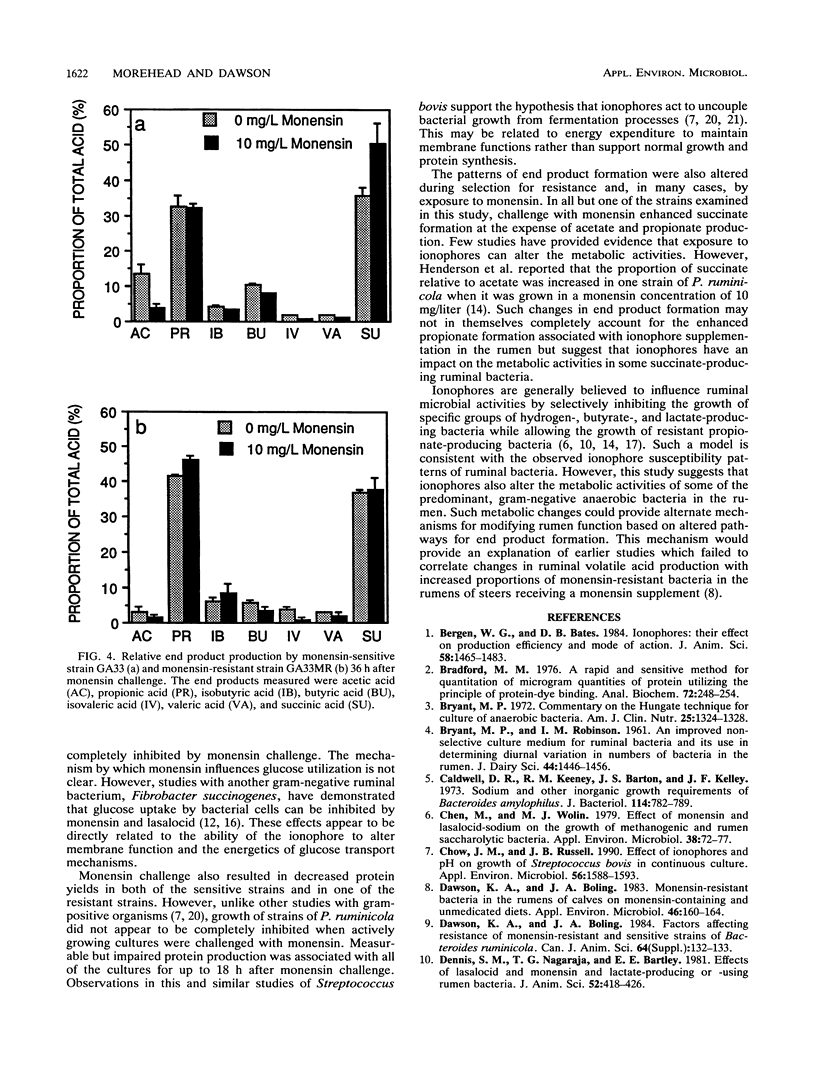
New strains with enhanced resistance to monensin were developed from Prevotella (Bacteroides) ruminicola subsp. ruminicola 23 and P. ruminicola subsp. brevis GA33 by stepwise exposure to increasing concentrations of monensin. The resulting resistant strains (23MR2 and GA33MR) could initiate growth in concentrations of monensin which were 4 to 40 times greater than those which inhibited the parental strains. Resistant strains also showed enhanced resistance to nigericin and combinations of monensin and nigericin but retained sensitivity to lasalocid. Glucose utilization in cultures of the monensin-sensitive strains (23 and GA33) and one monensin-resistant strain (23MR2) was retarded but not completely inhibited when logarithmic cultures were challenged with monensin (10 mg/liter). Monensin challenge of cultures of the two monensin-sensitive strains (23 and GA33) was characterized by 78 and 51% decreases in protein yield (milligrams of protein per mole of glucose utilized), respectively. Protein yields in cultures of resistant strain 23MR2 were decreased by only 21% following monensin challenge. Cell yields and rates of glucose utilization by resistant strains GA33MR were not decreased by challenge with 10 mg of monensin per liter. Resistant strains produced greater relative proportions of propionate and less acetate than the corresponding sensitive strains. The relative amounts of succinate produced were greater in cultures of strains 23, GA33, and 23MR2 following monensin challenge. However, only minor changes in end product formation were associate with monensin challenge of resistant strain GA33MR. These results suggest that monensin has significant effects on both the growth characteristics and metabolic activities of these predominant, gram-negative ruminal bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergen W. G., Bates D. B. Ionophores: their effect on production efficiency and mode of action. J Anim Sci. 1984 Jun;58(6):1465–1483. doi: 10.2527/jas1984.5861465x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Keeney M., Barton J. S., Kelley J. F. Sodium and other inorganic growth requirements of bacteroides amylophilus. J Bacteriol. 1973 May;114(2):782–789. doi: 10.1128/jb.114.2.782-789.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J. M., Russell J. B. Effect of ionophores and pH on growth of Streptococcus bovis in batch and continuous culture. Appl Environ Microbiol. 1990 Jun;56(6):1588–1593. doi: 10.1128/aem.56.6.1588-1593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Boling J. A. Monensin-resistant bacteria in the rumens of calves on monensin-containing and unmedicated diets. Appl Environ Microbiol. 1983 Jul;46(1):160–164. doi: 10.1128/aem.46.1.160-164.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effects of lasalocid or monensin on lactate-producing or -using rumen bacteria. J Anim Sci. 1981 Feb;52(2):418–426. doi: 10.2527/jas1981.522418x. [DOI] [PubMed] [Google Scholar]
- Franklund C. V., Glass T. L. Glucose uptake by the cellulolytic ruminal anaerobe Bacteroides succinogenes. J Bacteriol. 1987 Feb;169(2):500–506. doi: 10.1128/jb.169.2.500-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich R. D., Garrett J. E., Gast D. R., Kirick M. A., Larson D. A., Meiske J. C. Influence of monensin on the performance of cattle. J Anim Sci. 1984 Jun;58(6):1484–1498. doi: 10.2527/jas1984.5861484x. [DOI] [PubMed] [Google Scholar]
- Maas L. K., Glass T. L. Cellobiose uptake by the cellulolytic ruminal anaerobe Fibrobacter (Bacteroides) succinogenes. Can J Microbiol. 1991 Feb;37(2):141–147. doi: 10.1139/m91-021. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Taylor M. B. Susceptibility and resistance of ruminal bacteria to antimicrobial feed additives. Appl Environ Microbiol. 1987 Jul;53(7):1620–1625. doi: 10.1128/aem.53.7.1620-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Russell J. B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: effects on ion flux and protonmotive force. J Anim Sci. 1987 May;64(5):1519–1525. doi: 10.2527/jas1987.6451519x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Effect of ionophores on ruminal fermentation. Appl Environ Microbiol. 1989 Jan;55(1):1–6. doi: 10.1128/aem.55.1.1-6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelling G. T. Monensin mode of action in the rumen. J Anim Sci. 1984 Jun;58(6):1518–1527. doi: 10.2527/jas1984.5861518x. [DOI] [PubMed] [Google Scholar]
- Schwingel W. R., Bates D. B., Denham S. C., Beede D. K. Lasalocid-catalyzed proton conductance in Streptococcus bovis as affected by extracellular potassium. Appl Environ Microbiol. 1989 Jan;55(1):259–260. doi: 10.1128/aem.55.1.259-260.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nevel C. J., Demeyer D. I. Effect of monensin on rumen metabolism in vitro. Appl Environ Microbiol. 1977 Sep;34(3):251–257. doi: 10.1128/aem.34.3.251-257.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


