Abstract
Interferon (IFN)-α/β-mediated negative regulation of interleukin 12 (IL-12) and IFN-γ proteins is reported here. Both IFN-α and IFN-β inhibited fixed Staphylococcus aureus Cowan strain induction of IL-12 and IFN-γ production by mouse splenic leukocytes in culture. Extended studies with IFN-α demonstrated that inhibition was at the level of biologically active IL-12 p70. Effects were selective, as induction of tumor necrosis factor was unaffected and induction of IL-6 was enhanced. Neutralization of IFN-α/β expressed endogenously during infections with murine cytomegalovirus (MCMV) enhanced early IL-12 and IFN-γ protein production. Furthermore, during infections of mice with lymphocytic choriomeningitis virus (LCMV), this treatment revealed a previously undetected early IL-12 and IFN-γ protein expression, and mice deficient in IFN-α/β receptor function, but not control mice, also expressed endogenous LCMV-induced IL-12. The effects of IFN-α/β neutralization on production of IL-12 and IFN-γ during the viral infections were detected in both serum samples and medium conditioned with splenic leukocytes isolated from infected animals. In vitro studies demonstrated that splenic leukocytes isolated from LCMV-infected mice were primed to produce IL-12 in response to stimulation with Staphylococcus aureus Cowan strain, but that this responsiveness was sensitive to added IFN-α. Moreover, endogenous IFN-α/β induced by LCMV inhibited in vivo lipopolysaccharide stimulation of IL-12 production. These results demonstrate a new pathway for regulating cytokine responses, and suggest a mechanism for inhibition of IL-12-dependent immune responses during viral infections.
Keywords: cytokine regulation, lymphocytic choriomeningitis virus, murine cytomegalovirus
Interleukin 12 (IL-12) is a heterodimeric cytokine with many functions including induction of interferon (IFN)-γ by natural killer (NK) cells and generation of T helper 1 (Th1) cells producing IFN-γ (1). The role of IL-12 in promoting endogenous protective immune responses to viral infections, however, is only beginning to be understood (2, 3). Our studies of IL-12 administration during lymphocytic choriomeningitis virus (LCMV) infections of mice have demonstrated that high concentrations of the factor are detrimental to expansion of protective CD8+ cytotoxic T lymphocytes (CTL) and synergize with endogenous immune responses to promote systemic toxicities (4, 5). These results suggest that, if endogenous IL-12 is to play a role in viral infections requiring CD8+ T cell responses for defense, the levels of expression would have to be tightly regulated. Interestingly, HIV infections are also associated with protective CD8+ CTL (6–9), and cells from HIV-infected individuals are inhibited in their ability to produce IL-12 (10, 11).
Studies from this laboratory evaluating endogenous expression and function of IL-12 indicate that the factor is differentially regulated in contrasting viral infections of mice. Although early IL-12 protein expression is induced during murine cytomegalovirus (MCMV) infection (12) and results in NK cell IFN-γ production (12–14), detectable levels of IL-12 and NK cell IFN-γ production are not observed during LCMV infection (12). The presence of IL-12 inversely correlates with induction of CTL function; in contrast to MCMV, LCMV infections readily induce high levels of CD8+ CTL activity. In addition, treatments with antibodies neutralizing IL-12 do not significantly modify the prominent late T cell CTL and IFN-γ responses during LCMV infection (12). These results are in striking contrast to the demonstrated IL-12 dependency of T cell IFN-γ responses during bacterial and parasitic infections (1–3, 15, 16). Taken together, the studies suggest that IL-12 responses are differentially regulated during particular viral infections, and that IL-12-induced effects under the conditions of viral infections are distinct from those observed during other challenges.
One of the conditions distinguishing viral infections is early induction of systemic IFN-α/β production (17–19). The experiments presented here were undertaken to evaluate the effects of these factors on IL-12 and IFN-γ expression. The results show that production of the cytokines is inhibited by IFN-α/β. They demonstrate that IFN-α/β inhibition acts on expression in response to known exogenous inducers of IL-12 and is an endogenous component shaping cytokine expression during viral infections.
MATERIALS AND METHODS
Animals.
Male pathogen-free C57BL/6 (C57BL/6NTacfBR), defined flora C57BL/6 athymic nude (C57BL/6NTac-nufDF N9), and 129 (129SvEvTacFBR) mice were purchased from Taconic Farms (Germantown, NY). Mice defective in IFN-α/β receptor function as a result of genetic mutation (IFN-α/βR KO) on the 129 genetic background (19), originally purchased from B & K Universal (North Humberside, U.K.), were bred in strict isolation with sterile food, water, and caging in the animal facility at Brown University. Male C57BL/6 severe combined immunodeficient (SCID) (C57BL/6J-scid/SzJ) mice were obtained from The Jackson Laboratory. All mice were between 4 and 12 weeks of age when used in experiments and were handled in accordance with institutional guidelines for animal care and use.
In Vivo Treatment Protocols.
Animals were treated according to the following protocols, or combinations thereof, as specified. Infections were initiated on day 0 with 2 × 104 plaque-forming units of LCMV, Armstrong strain clone E350, or 2 × 105 plaque-forming units of salivary gland-extracted MCMV, Smith strain. Under these conditions, serum IFN-α/β was detected in a vesicular stomatitis virus biological assay as early as 1 day after either LCMV or MCMV infection and reached peak levels of 2000 units/ml. IFN-α/β induction was such that production declined after day 1 during MCMV infection, but continued to increase through day 2 during LCMV infection. In vivo neutralization of IFN-α/β was carried out by treating mice with 0.15 mg of sheep anti-murine IFN-α/β or control sheep IgG (20) at 3–12 hr before infection (gifts of Ion Gresser, Centre National de la Recherche Scientifique, Villejuif, France), resulting in >98% inhibition of endogenous IFN-α/β bioactivity during either MCMV or LCMV infections. To stimulate IL-12 production in vivo with a known IL-12 inducer (21), mice were injected i.p. with PBS or 5 μg lipopolysaccharide (LPS) from Salmonella enteriditis (Sigma) in PBS 4 hr before being killed.
Preparation of Samples for Analysis of in Vivo Cytokine Induction.
Mice were anesthetized with methoxyflurane and bled retro-orbitally into tubes with small amounts of heparin, before sacrifice by cervical dislocation and spleen harvest. Serum samples were prepared by spinning whole blood for 30 min at 6000 rpm. Splenic leukocytes were isolated by teasing apart whole spleens and lysing erythrocytes by ammonium chloride treatment. Viable yields were determined by trypan blue exclusion. To prepare conditioned medium, splenic leukocytes were cultured for 24 hr at a density of 107 cells/ml in RPMI 1640 medium containing fetal bovine serum. Supernatants were harvested and concentrated ≈5-fold using Centricon concentrators (Amicon).
In Vitro Stimulation of Cytokine Production.
One million C57BL/6 splenic leukocytes were added to individual wells in 96-well microtiter plates with the specified wt/vol percentage of fixed Staphylococcus aureus Cowan strain (SAC) (Pansorbin; Calbiochem), a known inducer of IL-12 (22). Various amounts of one of the following were added: natural purified mouse IFN-α (specific activity of 6 × 106 units/mg; Lee BioMolecular Laboratories, San Diego), murine recombinant IFN-α (specific activity of 3 × 104 units/mg; BioSource International, Carmarillo, CA), natural purified mouse IFN-β (specific activity of 1.3 × 108 units/mg; Lee BioMolecular Laboratories), or human recombinant IL-2 (specific activity of 3 × 106 units/mg; Cetus). Mixtures of mediators were added to cells in 10% fetal bovine serum RPMI 1640 medium containing 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. After 24 hr incubation at 37°C in a 5% CO2/95% air environment, microtiter plates were centrifuged, and cell supernatant fluids were harvested.
Cytokine Analyses.
Culture supernatants after in vitro stimulations, serum samples, or conditioned medium from splenic leukocytes isolated after in vivo manipulations were tested for IL-12 p40, IFN-γ, tumor necrosis factor (TNF), or IL-6 protein by sandwich ELISA as described (12). Briefly, the IL-12 p40 ELISA utilized C15.1 (prepared from hybridomas that were kindly provided by Georgio Trinchieri, Wistar Institute, Philadelphia) and polyclonal sheep anti-mouse IL-12 (Genetics Institute, Cambridge, MA) as the primary and secondary antibodies, respectively. The tertiary antibody was a peroxidase-conjugated donkey anti-sheep antibody (Jackson ImmunoResearch). Mouse recombinant IL-12 (23), a gift from Genetics Institute, was used a standard. The limits of detection in the assay were 90 pg/ml for serum samples and 0.9 pg per million cells for conditioned medium samples. The IFN-γ ELISA utilized XMG1.2 (gift of Robert Coffman, DNAX, Palo Alto, CA) as the capture antibody, polyclonal rabbit-anti-IFN-γ (provided by Phillip Scott, University of Pennsylvania, Philadelphia) as the secondary antibody and peroxidase-conjugated donkey-anti-rabbit antibody (Jackson ImmunoResearch) as the tertiary antibody. Mouse recombinant IFN-γ was purchased from PharMingen as a standard. The limits of detection in the assays were 39 pg/ml for serum samples and 0.2 pg/million cells for conditioned medium samples. For TNF quantitation, the factor was captured with hamster mAb TN3.19-12 (provided by Celltech, Slough, U.K. through Steven Opal, Brown University), and detected with polyclonal rabbit anti-TNF-α (Endogen, Wouburn, MA) followed by peroxidase-conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch). A standard curve generated with recombinant murine TNF purchased from R & D Systems indicated that the limit of detection for the assay was 19 pg/ml. IL-6 concentrations were tested using rat anti-mouse MP5-20F3 for capture, and biotinylated rat anti-mouse MP5-32C11 (both purchased from PharMingen), followed by 2.5 μg/ml avidin peroxidase (Sigma), and calculated based on a standard curve of recombinant murine IL-6 for which the limit of detection was 19 pg/ml. For all ELISAs, colorimetric changes upon addition of ABTS (2.2′-azino-di [3-ethyl-benzthiazoline sulfate 6]) substrate (Kierkegaard & Perry Laboratories) were detected using a Dynatech MR 4000 reader. To quantitate biologically active IL-12 p70, nonneutralizing C15.1 mAb was used to capture IL-12 on a microtiter plate. Normal splenic leukocytes, at a density of 106 per well, were added to the plate and incubated for 24 hr at 37°C in a 5% CO2/95% air environment. Microtiter plates were then centrifuged, and cell supernatant fluids transferred into an IFN-γ ELISA, as described above. IFN-γ production was compared with levels obtained from a recombinant murine IL-12 standard curve (Genetics Institute) to calculate the concentration of IL-12 p70 in each sample.
Statistical Analysis.
The Student’s t test was performed where indicated.
RESULTS
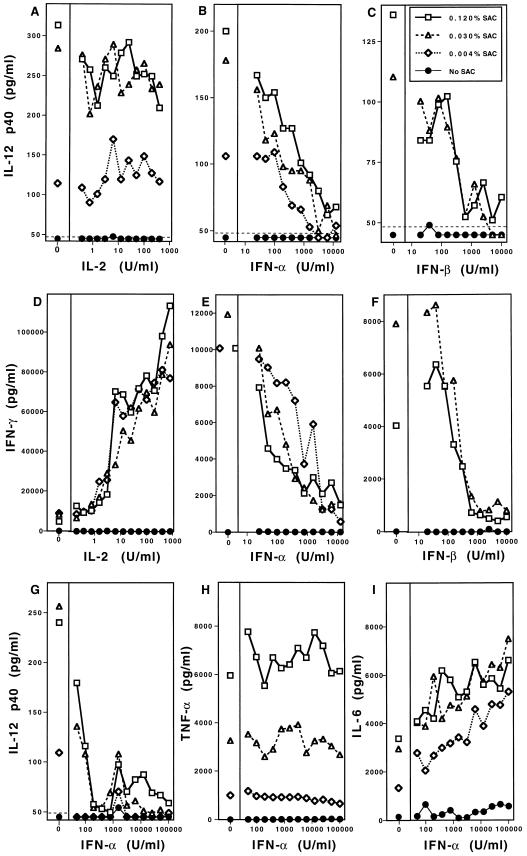
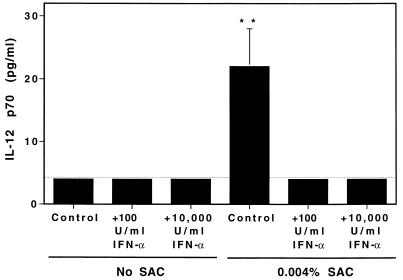
The roles of IFN-α/β in regulating IL-12 induction and IL-12-driven IFN-γ induction were evaluated in vitro using a known potent inducer of IL-12, SAC (22). Splenic leukocytes from C57BL/6 mice were incubated with various concentrations of SAC with or without natural purified murine IFN-α or IFN-β (Fig. 1). Cell supernatant fluids were collected and evaluated by ELISA for cytokine expression. Addition of either IFN-α (Fig. 1B) or IFN-β (Fig. 1C) resulted in a dose-dependent inhibition of SAC-induced IL-12. IFN-α (Fig. 1E) or IFN-β (Fig. 1F) also inhibited SAC-induced IFN-γ in a dose-dependent manner. The inhibitory effects of IFN-α or IFN-β on SAC-induced IL-12 were specific for IFN-α/β as IL-2 did not alter IL-12-induction by SAC (Fig. 1A) and resulted in an enhanced IFN-γ production (Fig. 1D) consistent with the previously observed synergism between IL-12 and IL-2 (24). The inhibitory activity of IFN-α/β was selective, as addition at concentrations sufficient to inhibit IL-12 p40 production (Fig. 1G) had no effect on TNF (Fig. 1H) and modestly enhanced IL-6 (Fig. 1I) proteins. Further analysis demonstrated that inhibition extended to the level of biologically active factor, as addition of either 100 or 10,000 units/ml exogenous IFN-α decreased SAC induction of IL-12 p70 to below limits of detection (Fig. 2). Inhibitory effects were also observed with recombinant murine IFN-α, as addition of 10,000 units/ml of murine recombinant IFN-α inhibited >75% of the IL-12 p40 and >95% of the IFN-γ induced in response to 0.030% SAC (data not shown). Taken together, these in vitro assays demonstrate that IFN-α and IFN-β are potent, selective inhibitors of both IL-12 production and a resulting induction of IFN-γ production.
Figure 1.
IFN-α/β inhibition of IL-12 and IFN-γ protein production in vitro. The effects of IFN-α, IFN-β, or IL-2 on SAC-induced IL-12, IFN-γ, TNF, and IL-6 production were examined in culture after 24 hr stimulation of 1 × 106 splenic leukocytes isolated from C57BL/6 mice. Stimulation with SAC was carried out with 0.12% (□), 0.03% (▵), and 0.004% (⋄), or no (•) SAC as specified. Serial dilutions of purified natural IFN-α, IFN-β, or human recombinant IL-2 were added to the test conditions as indicated. Culture supernatant fluids were prepared, and production of IL-12 p40, IFN-γ, TNF, or IL-6 was determined by specific protein ELISA. The following are shown: IL-2 effects on SAC-induced IL-12 p40 (A), IFN-α (B) and IFN-β (C) effects on SAC-induced IL-12 p40, IL-2 effects on SAC-induced IFN-γ (D), IFN-α (E) and IFN-β (F) effects on SAC-induced IFN-γ production, and IFN-α effects on SAC-induced IL-12 p40 (G), TNF (H), and IL-6 (I). The confidence limit of detection for assays is indicated (dotted line).
Figure 2.
Effect of IFN-α on production of biologically active IL-12 p70 in vitro. The effects of IFN-α on SAC-induced IL-12 p70 production were examined in culture after 24 hr of stimulation of 1 × 106 splenic leukocytes isolated from C57BL/6 mice. Stimulation with SAC was carried out with 0.004% or no SAC as specified. Purified natural IFN-α at concentrations of 100 units/ml or 10,000 units/ml were added to the test conditions as indicated. Culture supernatant fluids were prepared, and production of IL-12 p70 was determined by bioassay. Results shown are means of four mice per group ± SE. Differences between SAC stimulation of IL-12 p70 in the absence of IFN-α and in the presence of either 100 or 10,000 units/ml IFN-α are significant by the Student’s t test. ∗∗, P < 0.05.
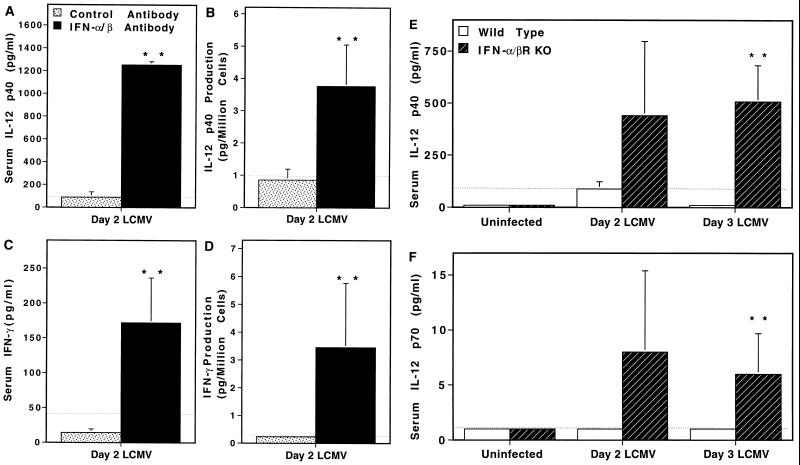
To examine the role of endogenously produced IFN-α/β on IL-12 and IFN-γ expression in the context of viral infections, studies were carried out in MCMV-infected mice. This virus induces early serum IL-12 as well as splenic leukocyte production of the factor into conditioned medium (12, 14). The effects of treatment with control antibodies or antibodies neutralizing mouse IFN-α/β before infection were evaluated in normal C57BL/6, T cell-deficient C57BL/6 nude, and T and B cell-deficient C57BL/6 SCID. In vivo neutralization of IFN-α/β resulted in IL-12 p40 increases of 1.5-, 11.2-, and 3.7-fold in C57BL/6, C57BL/6 nude, and C57BL/6 SCID, respectively, on day 2 after MCMV infection (Table 1). Increases also were observed in splenic IL-12 p40 production; cells from C57BL/6 mice produced 2.1-fold more and those from C57BL/6 nude produced 11.4-fold more IL-12 during a 24-hr culture as a result of in vivo IFN-α/β neutralization during MCMV infection (Table 1). Thus, endogenous IFN-α/β is dampening IL-12 protein production during MCMV infection.
Table 1.
Role of IFN-α/β in regulating IL-12 production during MCMV infection
| Mouse strain | Treatment | Serum IL-12 p40, pg/ml ± SE | Splenic IL-12 p40 production, pg per million cells ± SE |
|---|---|---|---|
| C57BL/6 | Control Ab | 814 ± 96 | 19.9 ± 3.8 |
| Anti-IFN-α/β | 1239 ± 272 | 42.0 ± 4.9 | |
| C57BL/6 nude | Control Ab | 271 ± 73 | 1.9 ± 3.0 |
| Anti-IFN-α/β | 3041 ± 355 | 21.7 ± 4.4 | |
| C57BL/6 SCID | Control Ab | 1055 ± 272 | ND |
| Anti-IFN-α/β | 3872 ± 589 | ND |
Mice were treated with control sheep antibody or sheep antibody neutralizing murine IFN-α/β before infection with 2 × 105 plaque-forming units salivary gland extracted MCMV Smith strain. Samples were prepared 2 days after infection. Each point is the mean ± SE of results from 3–4 replicate animals. ND, not done.
In contrast to MCMV infection, LCMV infection of normal mice does not induce detectable IL-12 protein (12). To determine whether or not endogenous IFN-α/β contributed to this apparent lack of IL-12 induction during LCMV infection, studies were carried out under conditions of IFN-α/β neutralization either as a result of treatment with antibody specific for IFN-α/β or in mice rendered IFN-α/β nonresponsive as a result of genetic mutation in the IFN-α/β receptor gene (IFN-α/βR KO) (Fig. 3). Because of the known kinetics of IFN-α/β expression (17–19), characterization of IFN-α/β responses under the conditions tested here (Materials and Methods), and kinetics of IL-12 expression during MCMV infection (12), effects were evaluated in C57BL/6 mice at early times after LCMV infection. In agreement with our previous results (12), control-treated mice did not have detectable levels of serum IL-12 on day 2 of LCMV infection (Fig. 3A). In contrast, day 2 LCMV-infected mice treated with antibodies neutralizing IFN-α/β had significant levels of the factor; serum levels of IL-12 p40 protein reached >1000 pg/ml (Fig. 3A). A similar level of IL-12 induction was also seen on day 1 after infection in anti-IFN-α/β-treated but not control-treated infected mice (data not shown). To evaluate IL-12 production by cells from infected mice, conditioned medium were prepared with splenic leukocytes isolated from day 2 LCMV-infected control-treated and IFN-α/β-neutralized mice. The levels of IL-12 p40 produced by cells isolated from control-treated day 2 LCMV-infected mice were below the limits of detection, whereas cells isolated from mice treated with antibodies neutralizing IFN-α/β released approximately 4 pg per million cells (Fig. 3B). These results were repeated in four independent experiments. As an alternative approach to examine IFN-α/β regulation of IL-12 induction, LCMV infections of normal 129 mice were compared with those of 129 mice deficient for IFN-α/β receptor function (IFN-α/βR KO). IL-12 p40 (Fig. 3E) and biologically active p70 (Fig. 3F) were detected in IFN-α/βR KO, but not in wild-type, mice on days 2 and 3 after LCMV infection. Taken together, these studies demonstrate that IL-12 protein expression is profoundly inhibited by endogenous IFN-α/β during viral infections and that these cytokines are contributing to the lack of detectable IL-12 protein during certain viral infections.
Figure 3.
Effects of endogenous IFN-α/β on IL-12 and IFN-γ expression during LCMV infection. IFN-α/β functions were evaluated by either treatment with neutralizing antibody (A–D) or in IFN-α/βR KO mice (E and F). Mice were infected with 2 × 104 plaque-forming units Armstrong strain LCMV clone E350. Serum samples were collected and splenic leukocytes were harvested from uninfected and infected mice at times indicated. Conditioned medium were prepared with splenic leukocytes at 107 cells/ml of medium for 24 hr, and supernatant fluids were harvested and concentrated. IL-12 p40 and IFN-γ protein levels were measured by specific ELISA, and IL-12 p70 was measured with a capture bioassay. The confidence limit of detection for each assay is indicated (· · ·). C57BL/6 mice were treated with neutralizing sheep antibody to IFN-α/β (▪) or control sheep IgG (□) 3 hr before infection (A–D). The following are shown: (A) IL-12 p40 in serum samples from three mice per group; (B) IL-12 p40 in conditioned medium samples from three mice per group; (C) IFN-γ in serum from six mice per group; (D) IFN-γ in conditioned medium samples from six mice per group. Wild-type 129 (□) and IFN-α/βR KO (▪) mice were infected with LCMV for 0, 2, or 3 days, four mice per group (E and F). Shown are IL-12 levels in serum samples, quantitated by IL-12 p40-specific ELISA (E) and IL-12 p70 bioassay (F). Results are means ± SE. The differences between the samples from anti-IFN-α/β- and control-treated (A–D), or IFN-α/β RKO and wild-type-infected mice (E and F) were significant by Student’s t test. ∗∗, P < 0.05.
To evaluate a functional consequence of IL-12 regulation, parallel studies were carried out examining IFN-α/β effects on IFN-γ protein production. IFN-α/β neutralization also promoted IFN-γ production (Fig. 3 C and D). Sera and conditioned medium from control-treated LCMV-infected mice did not contain detectable IFN-γ protein, however, comparable samples from anti-IFN-α/β-treated mice had significant levels of the factor—i.e., IFN-γ at 172 pg/ml in serum (Fig. 3C) and ≈3.5 pg per million cells in conditioned medium (Fig. 3D). As both the IL-12 and accompanying IFN-γ responses are revealed after IFN-α/β neutralization, the data indicate that the inhibition is functional and biologically relevant.
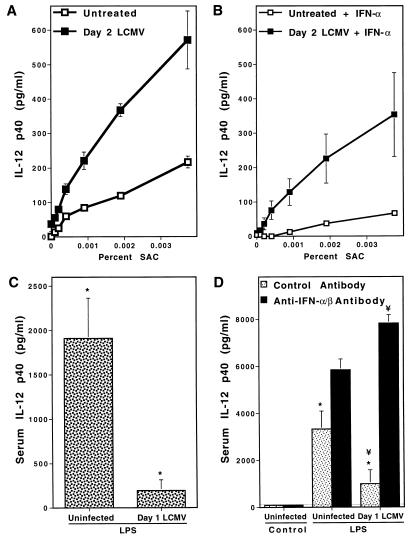
To determine if the in vivo inhibitory effects mediated by IFN-α/β occurred at the level of cell responsiveness for expression, splenic leukocytes were isolated from LCMV-infected mice and compared with those isolated from uninfected mice for their ability to produce IL-12 in response to stimulation in culture. Although cell populations from infected mice were not induced to release IL-12 p40 spontaneously, they were elevated ≈3-fold in their responsiveness to SAC for production of the protein relative to cells from uninfected mice (Fig. 4A). As addition of IFN-α blocked the elevated IL-12 production (Fig. 4B), however, this response was still sensitive to IFN-α/β-mediated inhibition. These results demonstrate that cells from LCMV-infected mice are primed to make IL-12, and suggest that inhibition of IL-12 requires the continuous presence of IFN-α/β.
Figure 4.
Effects of IFN-α/β during LCMV infection on cellular responsiveness to in vitro and in vivo stimulation of IL-12 production. Virus-induced IFN-α/β effects were examined in vitro by stimulating splenic leukocytes prepared from uninfected or LCMV-infected C57BL/6 mice with SAC, and evaluating IL-12 production (A and B). Cells, isolated from untreated (□) or day 2 LCMV-infected (▪) mice, were stimulated in vitro with 2-fold serial dilutions of SAC (0.12–0.001%) alone (A) or in the presence of 104 units/ml of IFN-α (B). Supernatant fluids were harvested after 24 hr and IL-12 p40 was measured by ELISA. In vivo responsiveness to a replication-independent stimulus of IL-12 in the context of virus-induced IFN-α/β was examined by administering 5 μg of S. enteriditis-derived LPS to uninfected or day 1 LCMV-infected C57BL/6 mice (C and D). Serum samples were prepared for IL-12 p40 by ELISA at 4 hr after LPS administration. Uninfected and infected mice treated with PBS only did not produce significant levels of serum IL-12 p40 (data not shown). Means ± SE of results from three mice per group are shown for all experiments. In vivo IL-12 induction by LPS in uninfected and day 1 LCMV-infected mice is shown (C). The role of endogenous IFN-α/β for inhibition of IL-12 stimulation in LCMV-infected mice was examined by treating uninfected and day 1 LCMV-infected mice with control antibody (□) or anti-IFN-α/β (▪) (D). Samples from uninfected and infected mice given LPS (C and D) were significantly different (∗), as were those from anti-IFN-α/β- compared with control-treated mice given LPS one day after LCMV infection (¥) as determined by a Student’s t test (P < 0.05) (D).
The endogenous virus-induced IFN-α/β effect on IL-12 induction in response to a known inducer of the factor was tested by treating LCMV-infected mice with LPS. Kinetic and dosage experiments in uninfected C57BL/6 mice demonstrated that peak production of IL-12 p40 protein in serum was observed at 4 hr after LPS treatment, that LPS doses in excess of 10 μg were saturating, and that treatment with as little as 0.2 μg of LPS induced detectable serum levels of IL-12 p40. In contrast to the 1911 ± 454 pg/ml of serum IL-12 p40 induced at 4 hr by 5 μg of LPS in uninfected mice, mice infected with LCMV 1 day before LPS treatment were inhibited by 90% and produced only 194 ± 120 pg/ml (Fig. 4C). To show that this effect was specifically mediated by IFN-α/β, LPS stimulation of IL-12 was examined in uninfected and infected mice treated with anti-IFN-α/β. IFN-α/β-neutralized, uninfected, and infected mice responded to LPS with serum IL-12 levels of 5845 ± 460 and 7827 ± 360 pg/ml, respectively (Fig. 4D). This did not appear to be a consequence of revealed LCMV induction of IL-12, as IFN-α/β neutralization on day 1 of LCMV infection only resulted in 1266 ± 115 pg/ml, whereas LPS treatment of IFN-α/β-neutralized mice one day after LCMV infection induced 6-fold more IL-12 p40. Thus, LCMV may have synergized with LPS under conditions of IFN-α/β neutralization, but viral infection alone was not sufficient to stimulate the levels of IL-12 p40 observed after LPS treatment in LCMV-infected IFN-α/β neutralized mice. As LPS is a non-replicating stimulus of IL-12, the observed effects of IFN-α/β were independent of changes in microbial burden. These experiments demonstrate that endogenous IFN-α/β impair(s) IL-12 expression in response to nonviral stimulatory signals during viral infections.
DISCUSSION
The experiments presented here conclusively demonstrate the ability of IFN-α/β to inhibit expression of IL-12 and IFN-γ in response to known inducers of these factors both in culture and in vivo. Endogenous IFN-α/β-mediated inhibition of these cytokines was shown to occur in vivo during infection with LCMV (Figs. 3 and 4). Studies of MCMV infection demonstrated that the regulatory effects of IFN-α/β were not unique to the LCMV infection because the detectable levels of MCMV-induced IL-12 (12) were further elevated after in vivo neutralization of IFN-α/β (Table 1). The elevated endogenous levels of IL-12 and IFN-γ after neutralization of IFN-α/β were not a consequence of increased viral burdens because (i) it was not possible to induce detectable levels of either factor by infection with up to 2 log higher doses of LCMV (data not shown), (ii) IFN-α mediated specific inhibitory effects on cells from LCMV-infected mice for induction of IL-12 (Fig. 4B), and (iii) LPS induction of IL-12 also was inhibited by endogenous IFN-α/β during LCMV infection (Fig. 4 C and D). Thus, the induction of IFN-α/β during an antiviral immune response has significant effects on the endogenous immune response to infection.
The novel regulatory functions of IFN-α/β reported here extends the list of cytokines able to modulate IL-12 and IL-12 function. Other known inhibitors are transforming growth factor β (TGF-β) and IL-10. Unlike IFN-α/β, TGF-β has been reported to inhibit the effect of IL-12 without appreciably altering the levels of factor production (16, 25–27). On the other hand, IL-10 is reported to inhibit NK cell IFN-γ production indirectly through its inhibition of IL-12 expression (27, 28). We show here that IFN-α/β negatively regulates IL-12 as well as the resultant IFN-γ production. In addition, ongoing studies assessing direct effects on IFN-γ expression indicate that, at high concentrations, IFN-α/β also inhibit(s) IL-12 induction of IFN-γ (data not shown). Preliminary studies with antibodies neutralizing TGF-β or IL-10 suggest that TGF-β does not contribute, and that IL-10 may account for some but not all of the inhibitory effects observed. Thus, these cytokines may regulate the IL-12 and IFN-γ pathway at several different and/or overlapping levels. As viral infections can be good inducers of IFN-α/β, however, they are likely to be the pivotal mediators of IL-12 regulation in the context of viral infection.
Although the molecular mechanisms for IFN-α/β-mediated inhibition of IL-12 and IFN-γ expression are not understood, our ability to detect both IFN-γ mRNA (29) in the absence of IFN-γ protein (ref. 12; Fig. 3C) and priming for IL-12 expression (Fig. 4) during LCMV infection suggests that the effects may be posttranscriptional. These observations underscore the importance of evaluating protein expression. Although it has been possible to document induction of IL-12 p40 mRNA during a number of different viral infections (30), it is not yet clear if the protein will be produced and/or the factor will play a biological role under all of these conditions.
As both LCMV and MCMV infection induce IFN-α/β (17–19), the kinetics and/or magnitude of endogenous IFN-α/β expression must be in balance to allow IL-12 expression during MCMV but not LCMV infection (12). Comparative studies to evaluate IFN-α/β responses to LCMV and MCMV indicate extended, higher levels of IFN-α/β induced by LCMV (Materials and Methods). Although the kinetics and magnitude of IFN-α/β production may be important, there may be other mechanisms contributing to the effect as well. MCMV could provide a stronger stimulation for IL-12 expression. Alternatively and/or additionally, infection of different cell types by these two viruses might also account for the observed differences. Targeting of infection to cells preferentially producing IFN-α/β would result in conditions inhibiting subsequent events that might induce IL-12 expression in other cell types. In contrast, early targeting of infection to cells of macrophage lineage might promote conditions for concurrent expression of IL-12 and IFN-α/β. This would be consistent with reported IFN-α- and IL-12-dependent effects induced by the chemical polyinosinic acid: polycytidylic acid (31). However, even during MCMV infection, IL-12 is only observed over a narrow period of time (12) and is decreased by endogenous IFN-α/β (Table 1). Thus, IL-12 expression is being regulated by IFN-α/β under these conditions as well.
The IFN-α/β-mediated inhibition of IL-12 has the potential to contribute to both beneficial and detrimental consequences. IL-12 facilitates certain protective immune responses as well as promotes toxic shock in the host (3). Previous work in this laboratory has shown that LCMV-infected mice are extremely sensitive to IL-12-mediated effects and that IL-12 administration during LCMV infection can result in profound immunotoxicities, with reductions in responding CD8+ T cells and increased viral burden (4, 5). Given this potential for IL-12-induced toxicity, a beneficial consequence of IFN-α/β inhibition of this factor would be protection of developing antiviral CD8+ T cells.
Alternatively, however, the IFN-α/β pathway of IL-12 inhibition might render the host more susceptible to pathogenic challenge if IL-12 is required for defense—i.e., intracellular bacterial and parasitic infections. Continued IFN-α/β production during chronic viral infection could be extremely problematic. Serum levels of IFN-α are known to be present during various persistent viral infections including human HIV infection (32). Presumably the constant presence of virus induces consistent elevation of IFN-α/β. This chronic induction of IFN-α/β during HIV infection is potentially beneficial as HIV replication has been shown to be sensitive to IFN-α or IFN-β (33, 34). Based upon the evidence presented here, a disadvantage of chronic IFN-α/β induction might be the subsequent inhibition of IL-12 induction and responsiveness. Individuals infected with HIV are more susceptible to infectious agents that require IL-12 for development of protective immunity, and cells from HIV-infected individuals are reduced in their ability to produce IL-12 in response to stimulation (10, 11). Thus, the chronic expression of IFN-α/β could be contributing to similar effects in HIV-infected patients. In support of this hypothesis, the inhibitory role of IFN-α during HIV infection has been demonstrated in experiments showing that anti-IFN-α prevents suppressive effects of serum from HIV-infected individuals on in vitro cell proliferation (35). The regulatory mechanisms mediated by IFN-α/β presented in this report may have general and significant ramifications in situations associated with virus persistence and chronic levels of IFN-α/β.
Acknowledgments
We thank Drs. P. Scott, G. Trinchieri, S. Opal, and I. Gresser for antibodies, and Genetics Institute for antibodies and murine recombinant IL-12. The work was supported by National Institutes of Health Grants RO1 CA41268 and T32 ES07272. H.C.S. is an Howard Hughes Medical Institute predoctoral fellow.
Footnotes
Abbreviations: IFN, interferon; IL, interleukin; SAC, Staphylococcus aureus Cowan strain; MCMV, murine cytomegalovirus; LCMV, lymphocytic choriomeningitis virus; LPS, lipopolysaccharide; CTL, cytotoxic T lymphocyte; TNF, tumor necrosis factor.
References
- 1.Trinchieri G. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 2.Biron C A. Curr Opin Immunol. 1994;6:530–538. doi: 10.1016/0952-7915(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 3.Biron C A, Gazzinelli R T. Curr Opin Immunol. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 4.Orange J S, Wolf S F, Biron C A. J Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 5.Orange J S, Salazar-Mather T P, Opal S M, Spencer R L, Miller A H, McEwen B S, Biron C A. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalams S A, Walker B D. Clin Lab Med. 1994;14:271–299. [PubMed] [Google Scholar]
- 7.Levy J A. AIDS. 1993;7:1401–1410. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ennen J, Findeklee H, Dittmar M T, Norley S, Ernst M, Kurth R. Proc Natl Acad Sci USA. 1994;91:7207–7211. doi: 10.1073/pnas.91.15.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baier M, Werner A, Bannert N, Metzner K, Kurth R. Nature (London) 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 10.Chehimi J, Starr S E, Frank I, D’Andrea A, Ma X, MacGreggor R R, Sennelier J, Trinchieri G. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chehimi J, Trinchieri G. J Clin Immunol. 1994;14:149–161. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 12.Orange J S, Biron C A. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 13.Orange J S, Wang B, Terhorst C, Biron C A. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orange J S, Biron C A. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 15.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scharton-Kersten T, Afonso L C C, Wysocka M, Trinchieri G, Scott P. J Immunol. 1995;154:5320–5330. [PubMed] [Google Scholar]
- 17.Welsh R M. J Exp Med. 1978;148:161–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy (Chalmer) J E, Trapman J, Allan J E, Shellam G R, Melier C J M. Infect Immun. 1982;37:143–150. doi: 10.1128/iai.37.1.143-150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller U, Steinhof U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 20.Gresser I, Tovey M G, Bandu M-T, Maury C, Broty-Boye D. J Exp Med. 1976;144:1305–1323. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzel F P, Rerko R M, Ling P, Hakimi J, Schoenhaut D S. Infect Immun. 1994;62:4224–4249. doi: 10.1128/iai.62.10.4244-4249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kumin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenhaut D S, Chua A O, Wolitzky A, Quinn G P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 24.Kobayashi M, Fitz L, Ryan M, Hewick R M, Clark S C, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U. J Immunol. 1995;155:1066–1073. [PubMed] [Google Scholar]
- 26.Hunter C A, Bermudez L, Beernink H, Waegell W, Remington J S. Eur J Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 27.D’Andrea A, Ma X, Aste-Amezaga M, Pagain C, Trinchieri G. J Exp Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biron C A, Young H A, Kasaian M T. J Exp Med. 1990;171:173–188. doi: 10.1084/jem.171.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coutelier J P, Van Broeck J, Wolf S F. J Virol. 1994;69:1955–1958. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manetti R, Annunziato F, Tomasevic L, Gianno V, Parronchi P, Romagnani S, Maggi E. Eur J Immunol. 1995;25:2656–2660. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 32.Von Sydow M, Sonnerborg A, Gaines H, Strannegard O. AIDS Res Hum Retroviruses. 1991;7:375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 33.Gessani S, Puddu P, Varano B, Borghi P, Conti L, Fantuzzi L, Belardelli F. J Virol. 1994;68:1983–1986. doi: 10.1128/jvi.68.3.1983-1986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirazi Y, Pitha P M. J Virol. 1992;66:1321–1328. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachgar A, Bizzini B. Biomed Pharmacother. 1994;48:73–77. doi: 10.1016/0753-3322(94)90079-5. [DOI] [PubMed] [Google Scholar]