Abstract
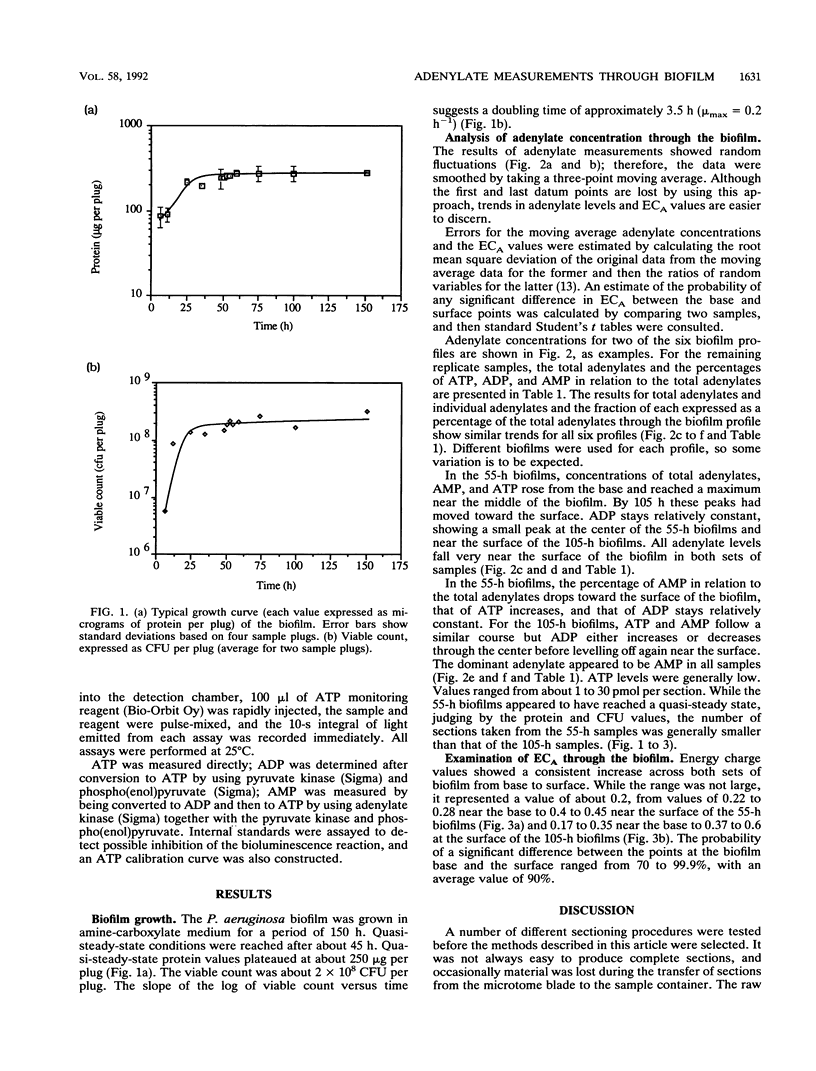
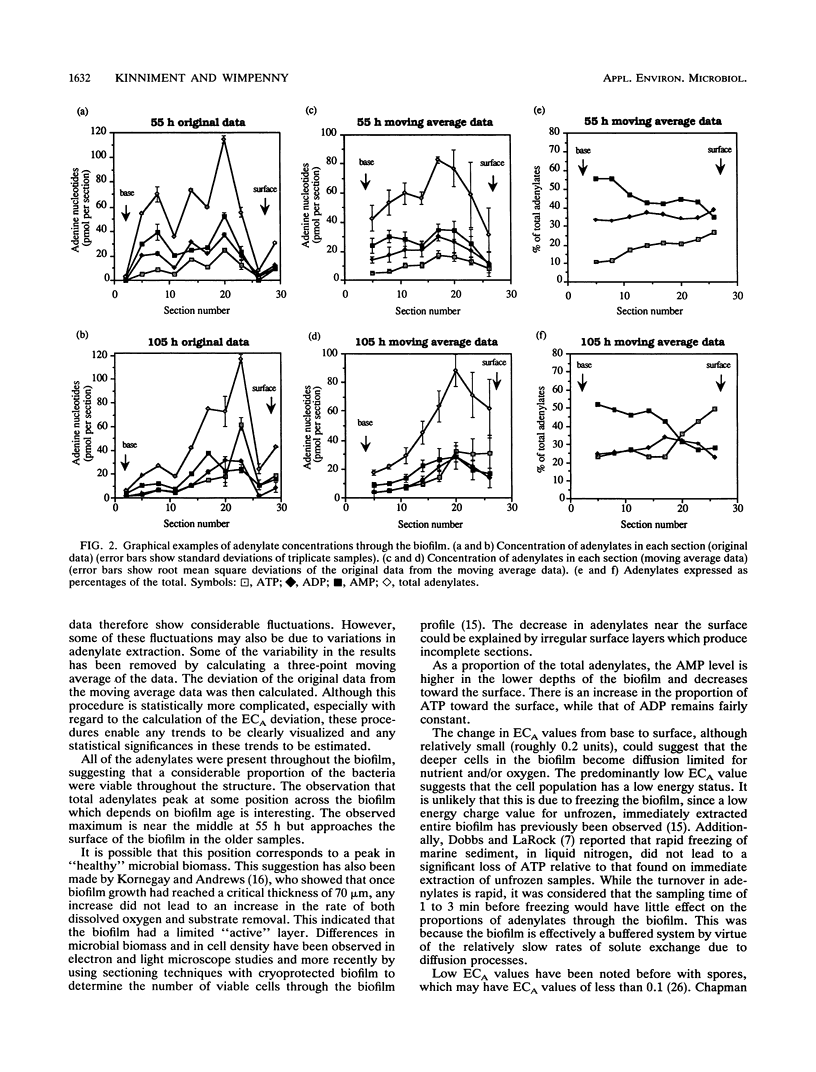
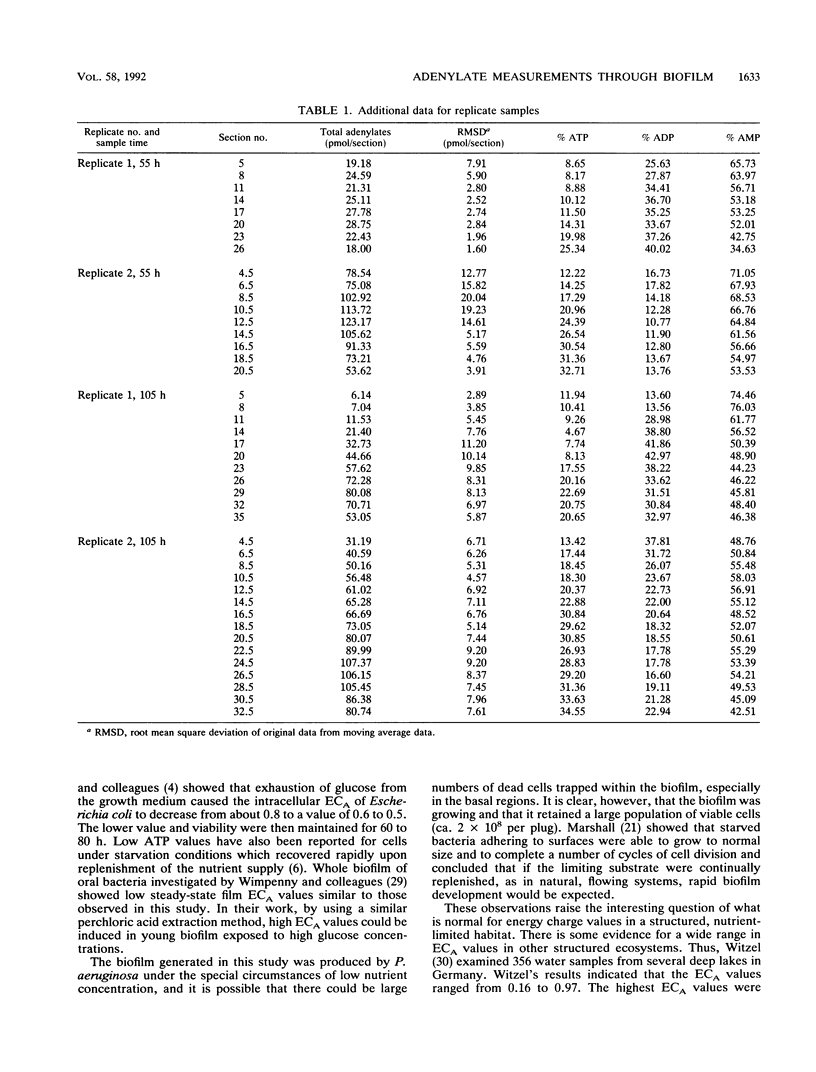
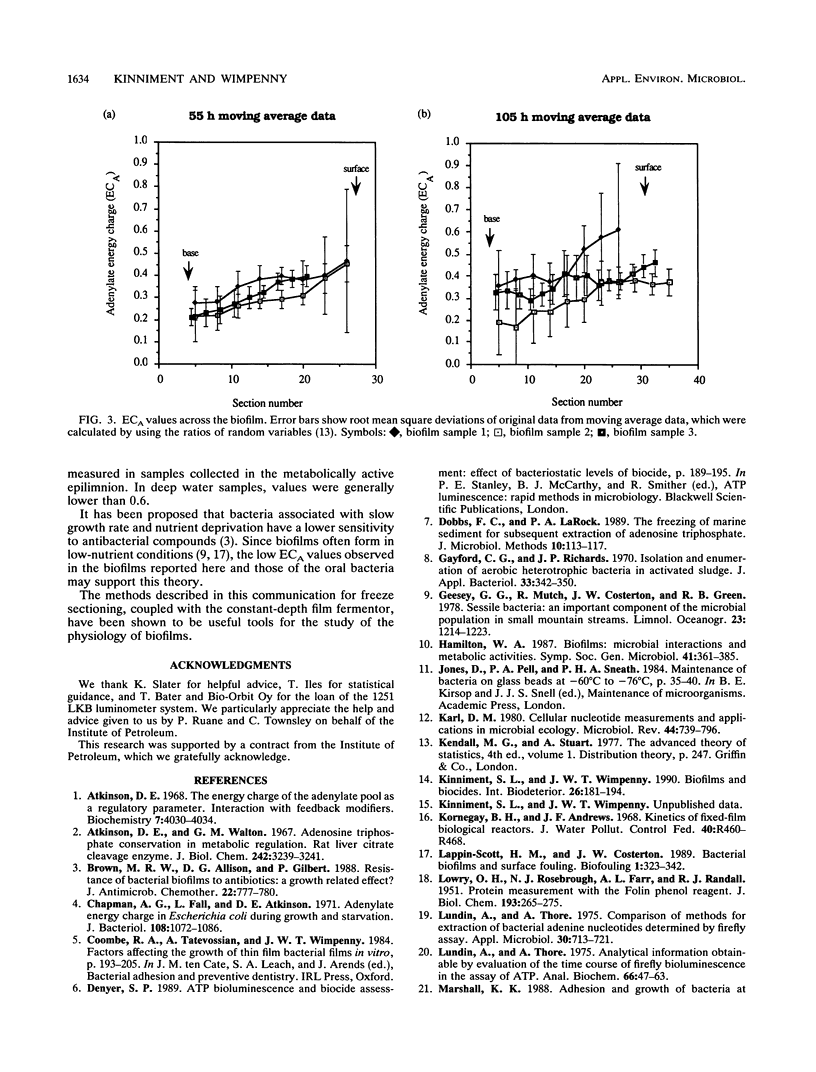
Adenine nucleotide pools and adenylate energy charge distributions were determined by using a laboratory-generated quasi-steady-state Pseudomonas aeruginosa biofilm. The method used involved freezing and sectioning of the intact biofilm, followed by extraction and assay of the adenylates in the sectioned material. Results indicated an increase in adenylate energy charge of about 0.2 units from the bottom to the surface of the biofilm. However, energy charge values were generally low throughout the biofilm, reaching a maximum of only 0.6 units. Of the adenylates measured, AMP was the predominant nucleotide, especially in the deeper parts of the biofilm profile.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- Atkinson D. E., Walton G. M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967 Jul 10;242(13):3239–3241. [PubMed] [Google Scholar]
- Brown M. R., Allison D. G., Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988 Dec;22(6):777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayford C. G., Richards J. P. Isolation and enumeration of aerobic heterotrophic bacteria in activated sludge. J Appl Bacteriol. 1970 Jun;33(2):342–350. doi: 10.1111/j.1365-2672.1970.tb02205.x. [DOI] [PubMed] [Google Scholar]
- Karl D. M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol Rev. 1980 Dec;44(4):739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lundin A., Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem. 1975 May 26;66(1):47–63. doi: 10.1016/0003-2697(75)90723-x. [DOI] [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]


