Abstract
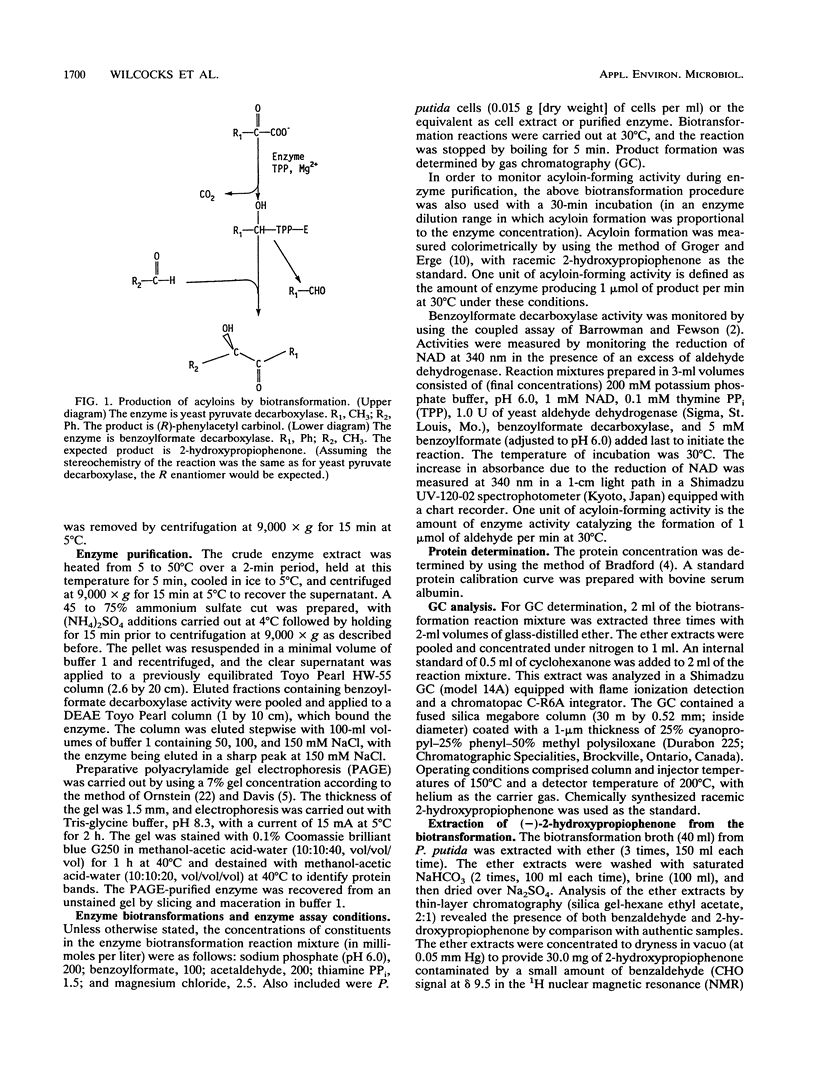
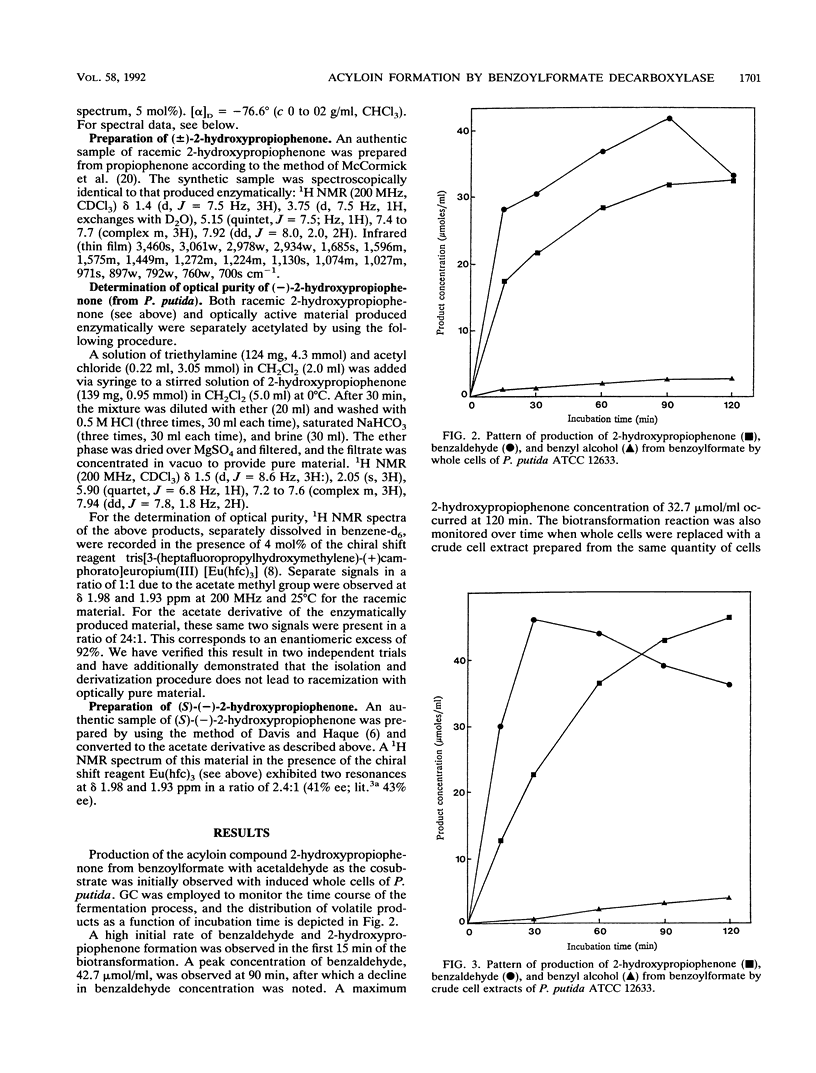
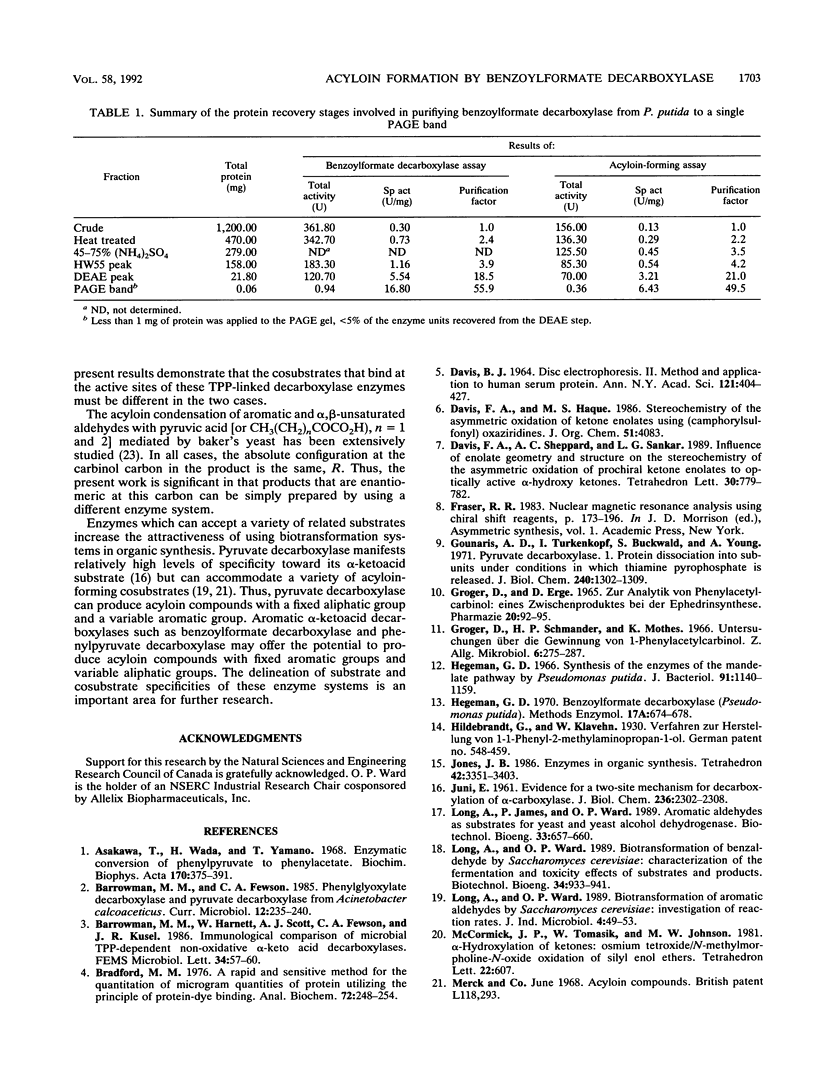
Whole cells and cell extracts of Pseudomonas putida grown in a medium containing ammonium mandelate have the capacity to produce the acyloin compound 2-hydroxypropiophenone when incubated with benzoylformate and acetaldehyde. Benzaldehyde and benzyl alcohol were formed as reaction by-products. The enantiomeric excess of the 2-hydroxypropiophenone product was found to be 91 to 92%. The absolute configuration of the enzymatically prepared product at the carbinol carbon was found to be S. The thiamine PPi-linked enzyme benzoylformate decarboxylase, purified to give a single protein band on polyacrylamide gel electrophoresis, was shown to be responsible for the catalysis of this novel condensation reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa T., Wada H., Yamano T. Enzymatic conversion of phenylpyruvate to phenylacetate. Biochim Biophys Acta. 1968 Dec 23;170(2):375–391. doi: 10.1016/0304-4165(68)90017-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
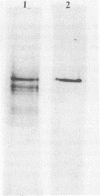
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GROEGER D., ERGE D. ZUR ANALYTIK VON PHENYLACETYLCARBINOL, EINES ZWISCHENPRODUKTS BEI DER EPHEDRINSYNTHESE. Pharmazie. 1965 Feb;20:92–95. [PubMed] [Google Scholar]
- Gounaris A. D., Turkenkopf I., Buckwald S., Young A. Pyruvate decarboxylase. I. Protein dissociation into subunits under conditions in which thiamine pyrophosphate is released. J Biol Chem. 1971 Mar 10;246(5):1302–1309. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Evidence for a two-site mechanism for decarboxylation of alpha-keto acids by alpha-carboxylase. J Biol Chem. 1961 Aug;236:2302–2308. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- SMITH P. F., HENDLIN D. Mechanism of phenylacetylcarbinol synthesis by yeast. J Bacteriol. 1953 Apr;65(4):440–445. doi: 10.1128/jb.65.4.440-445.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich J. Structure-function relationships in pyruvate decarboxylase of yeast and wheat germ. Ann N Y Acad Sci. 1982;378:287–305. doi: 10.1111/j.1749-6632.1982.tb31203.x. [DOI] [PubMed] [Google Scholar]
- Vojtísek V., Netrval J. Effect of pyruvate decarboxylase activity and of pyruvate concentration on the production of 1-hydroxy-1-phenylpropanone in Saccharomyces carlsbergensis. Folia Microbiol (Praha) 1982;27(3):173–177. doi: 10.1007/BF02877396. [DOI] [PubMed] [Google Scholar]