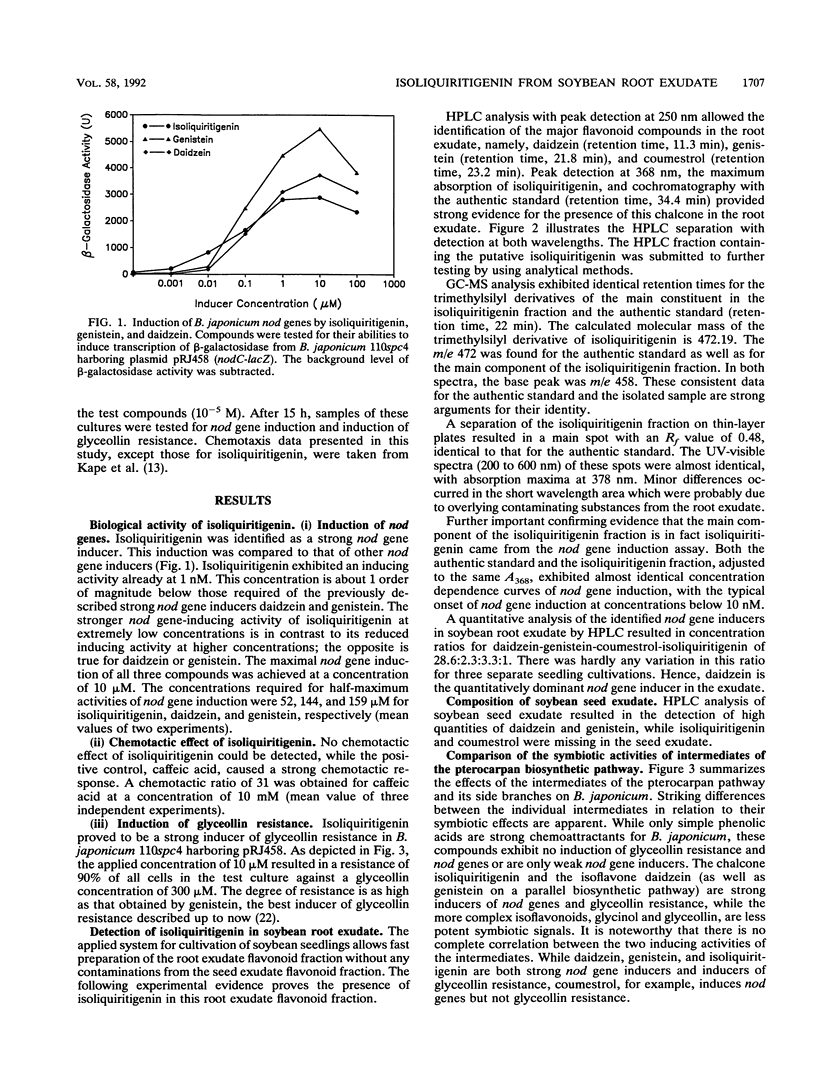
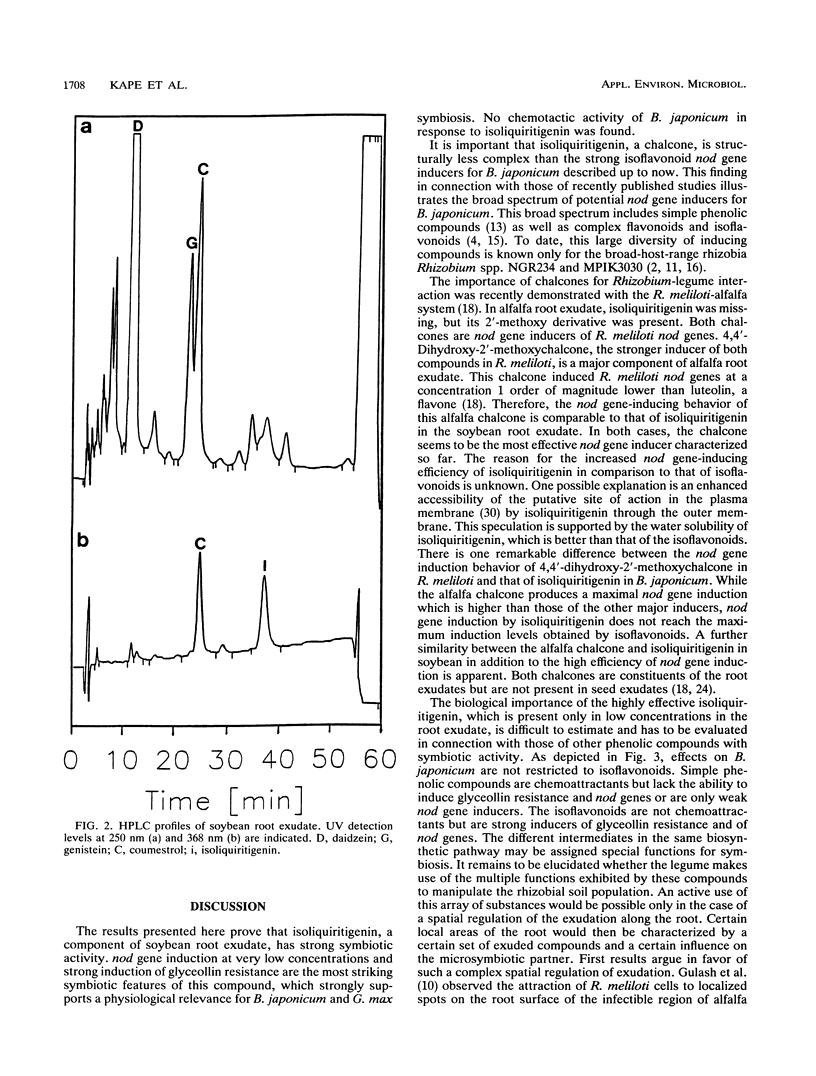
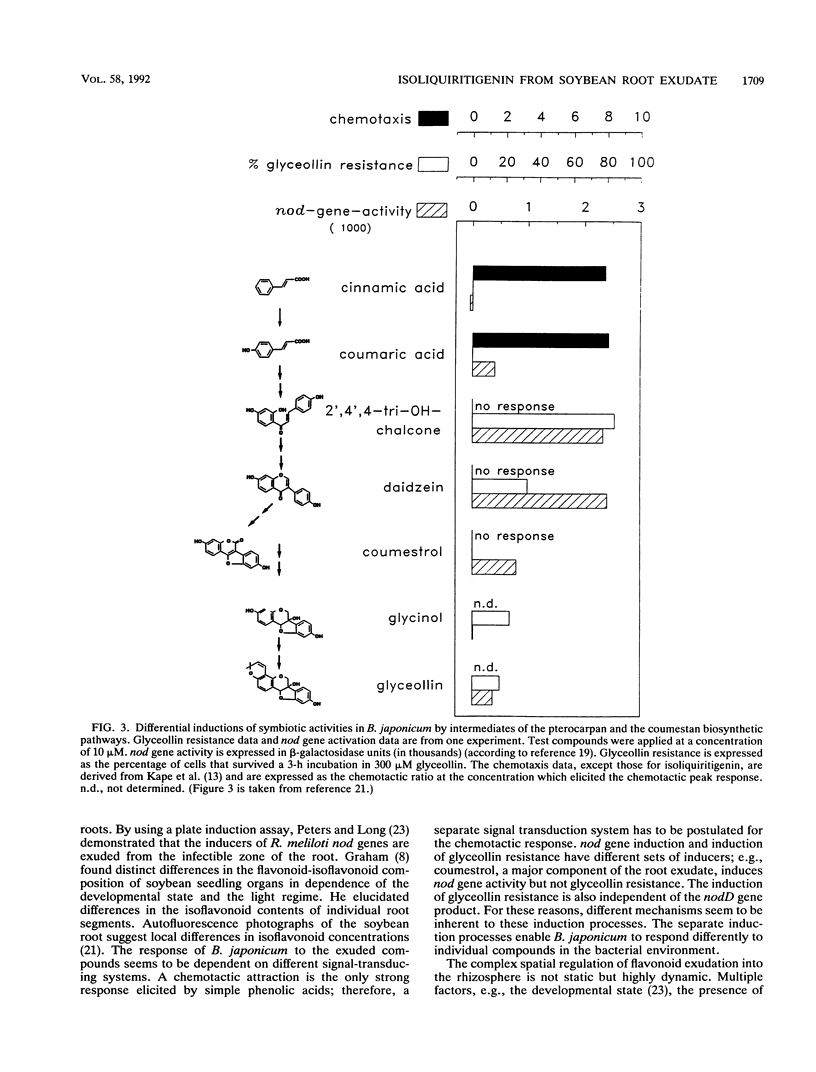
Abstract
Isoflavonoid signal molecules from soybean (Glycine max (L.) Merr.) seed and root exudate induce the transcription of nodulation (nod) genes in Bradyrhizobium japonicum. In this study, a new compound with symbiotic activity was isolated from soybean root exudate. The isolated 2',4',4-trihydroxychalcone (isoliquiritigenin) is characterized by its strong inducing activity for the nod genes of B. japonicum. These genes are already induced at concentrations 1 order of magnitude below those required of the previously described isoflavonoid inducers genistein and daidzein. Isoliquiritigenin is also a potent inducer of glyceollin resistance in B. japonicum, which renders this bacterium insensitive to potentially bactericidal concentrations of glyceollin, the phytoalexin of G. max. No chemotactic effect of isoliquiritigenin was observed. The highly efficient induction of nod genes and glyceollin resistance by isoliquiritigenin suggests the ecological significance of this compound, although it is not a major flavonoid constituent of the soybean root exudate in quantitative terms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour W. M., Hattermann D. R., Stacey G. Chemotaxis of Bradyrhizobium japonicum to soybean exudates. Appl Environ Microbiol. 1991 Sep;57(9):2635–2639. doi: 10.1128/aem.57.9.2635-2639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anollés G., Crist-Estes D. K., Bauer W. D. Chemotaxis of Rhizobium meliloti to the plant flavone luteolin requires functional nodulation genes. J Bacteriol. 1988 Jul;170(7):3164–3169. doi: 10.1128/jb.170.7.3164-3169.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham S., Kollmeyer W. D., Stacey G. Chemical control of interstrain competition for soybean nodulation by Bradyrhizobium japonicum. Appl Environ Microbiol. 1991 Jul;57(7):1886–1892. doi: 10.1128/aem.57.7.1886-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. L. Flavonoid and isoflavonoid distribution in developing soybean seedling tissues and in seed and root exudates. Plant Physiol. 1991 Feb;95(2):594–603. doi: 10.1104/pp.95.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulash M., Ames P., Larosiliere R. C., Bergman K. Rhizobia are attracted to localized sites on legume roots. Appl Environ Microbiol. 1984 Jul;48(1):149–152. doi: 10.1128/aem.48.1.149-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttfert M., Hitz S., Hennecke H. Identification of nodS and nodU, two inducible genes inserted between the Bradyrhizobium japonicum nodYABC and nodIJ genes. Mol Plant Microbe Interact. 1990 Sep-Oct;3(5):308–316. doi: 10.1094/mpmi-3-308. [DOI] [PubMed] [Google Scholar]
- Hartwig U. A., Phillips D. A. Release and Modification of nod-Gene-Inducing Flavonoids from Alfalfa Seeds. Plant Physiol. 1991 Mar;95(3):804–807. doi: 10.1104/pp.95.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kape R., Parniske M., Werner D. Chemotaxis and nod Gene Activity of Bradyrhizobium japonicum in Response to Hydroxycinnamic Acids and Isoflavonoids. Appl Environ Microbiol. 1991 Jan;57(1):316–319. doi: 10.1128/aem.57.1.316-319.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M., Ahlborn B., Werner D. Isoflavonoid-inducible resistance to the phytoalexin glyceollin in soybean rhizobia. J Bacteriol. 1991 Jun;173(11):3432–3439. doi: 10.1128/jb.173.11.3432-3439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Long S. R. Alfalfa Root Exudates and Compounds which Promote or Inhibit Induction of Rhizobium meliloti Nodulation Genes. Plant Physiol. 1988 Oct;88(2):396–400. doi: 10.1104/pp.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recourt K., Schripsema J., Kijne J. W., van Brussel A. A., Lugtenberg B. J. Inoculation of Vicia sativa subsp. nigra roots with Rhizobium leguminosarum biovar viciae results in release of nod gene activating flavanones and chalcones. Plant Mol Biol. 1991 May;16(5):841–852. doi: 10.1007/BF00015076. [DOI] [PubMed] [Google Scholar]
- Regensburger B., Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983 Aug;135(2):103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- Rolfe B. G. Flavones and isoflavones as inducing substances of legume nodulation. Biofactors. 1988 Jan;1(1):3–10. [PubMed] [Google Scholar]
- Schlaman H. R., Spaink H. P., Okker R. J., Lugtenberg B. J. Subcellular localization of the nodD gene product in Rhizobium leguminosarum. J Bacteriol. 1989 Sep;171(9):4686–4693. doi: 10.1128/jb.171.9.4686-4693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Wilcockson J., Zimmermann E. Adsorption and selection of rhizobia with ion-exchange papers. Arch Microbiol. 1975 Sep 30;105(1):27–32. doi: 10.1007/BF00447108. [DOI] [PubMed] [Google Scholar]


