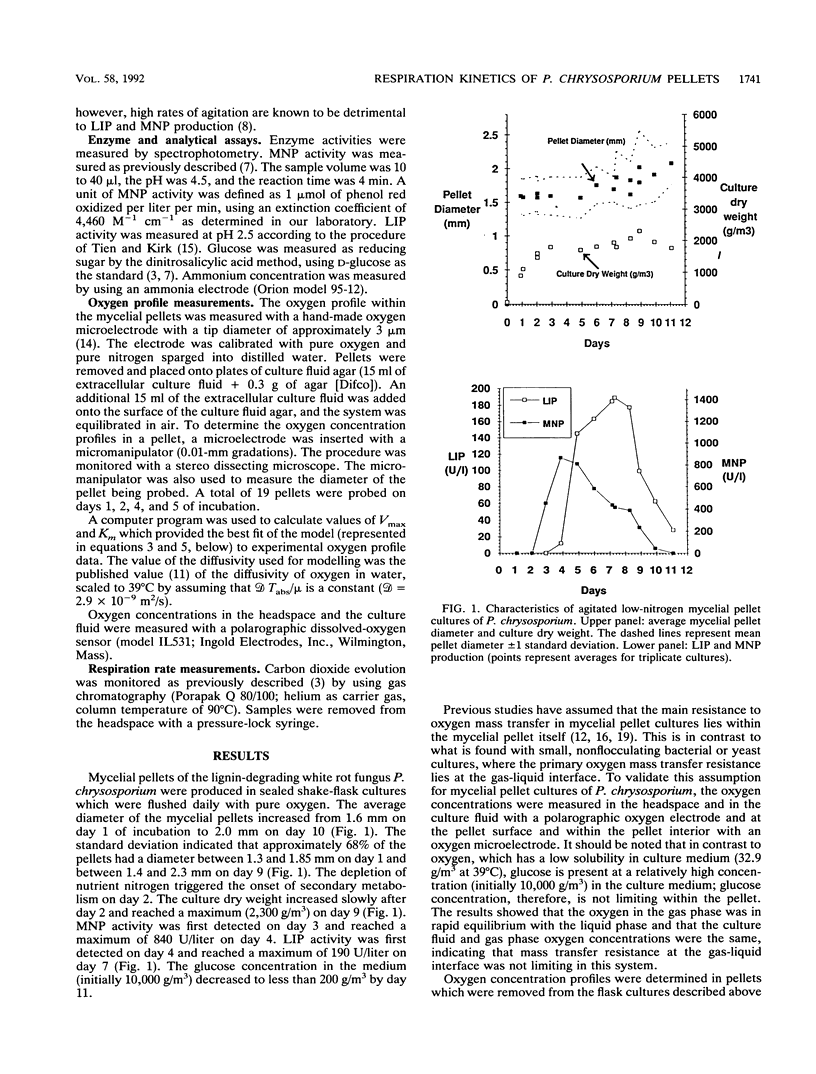
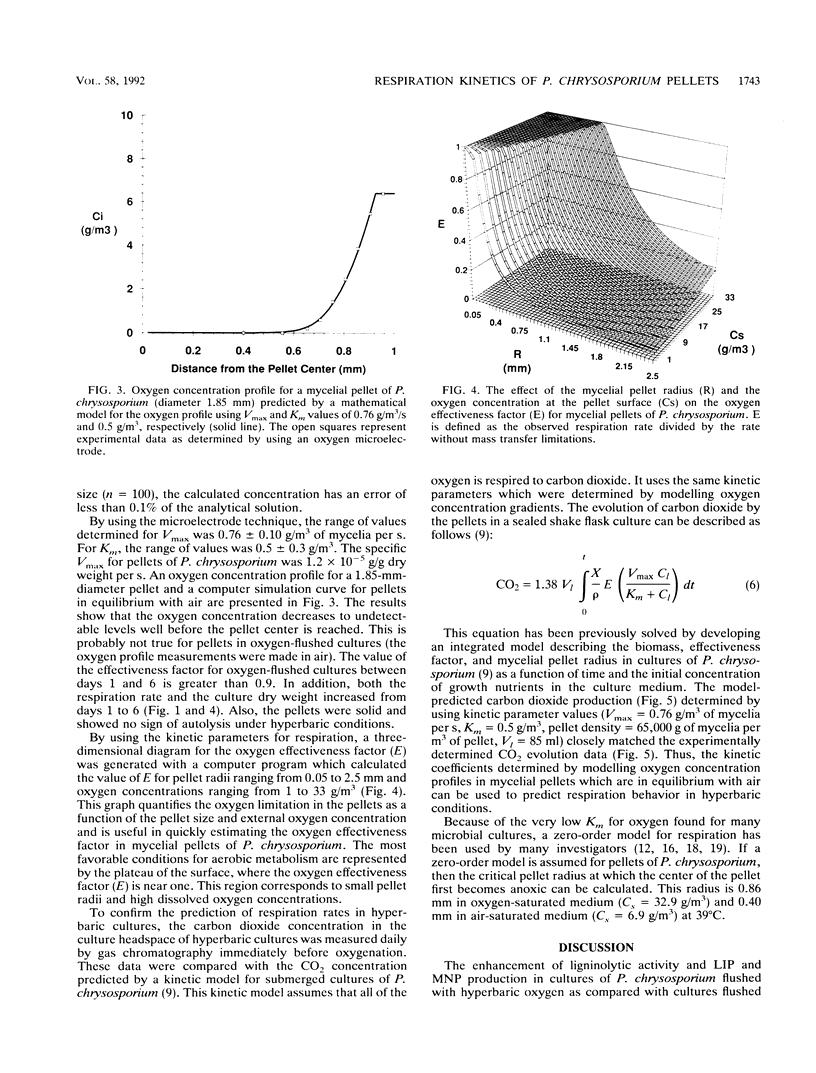
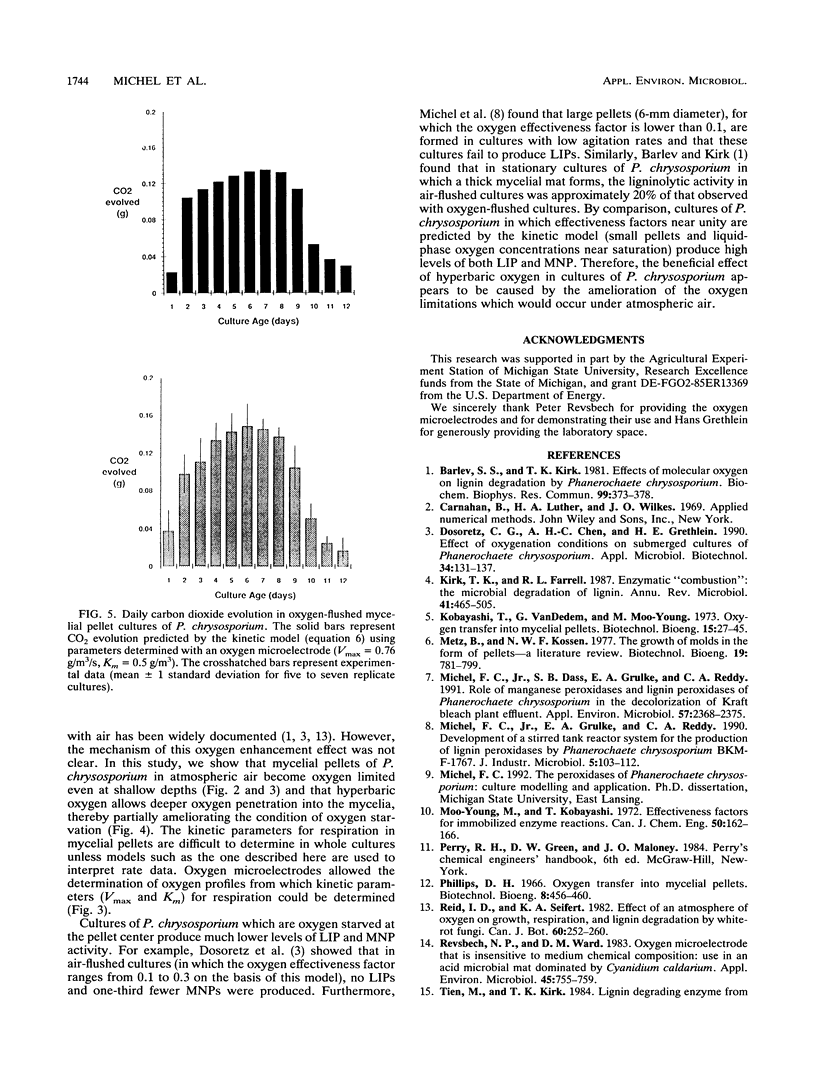
Abstract
In mycelial pellet cultures of the white rot basidiomycete Phanerochaete chrysosporium, low oxygen concentration negatively affects the production of the extracellular lignin peroxidases and manganese peroxidases which are key components of the lignin-degrading system of this organism. To test the hypothesis that oxygen limitation in the pellets is responsible for this effect, oxygen microelectrodes were used to determine oxygen concentration gradients within the mycelial pellets of P. chrysosporium. Pellets were removed from oxygenated cultures, allowed to equilibrate with air, and probed with oxygen microelectrodes. The oxygen profiles were modelled assuming that O2 uptake follows a Michaelis-Menten relationship. The Vmax and Km values for oxygen uptake were 0.76 +/- 0.10 g/m3 of pellet per s and 0.5 +/- 0.3 g/m3, respectively. These kinetic values were used to predict respiration rates in air-flushed cultures, oxygen-flushed cultures, and cultures with large pellets (diameter greater than 6 mm). The predicted respiration rates were independently validated by experimentally measuring the evolution of carbon dioxide from whole cultures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Lev S. S., Kirk T. K. Effects of molecular oxygen on lignin degradation by Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1981 Mar 31;99(2):373–378. doi: 10.1016/0006-291x(81)91755-1. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Ven Dedem G., Moo-Young M. Oxygen transfer into mycelial pellets. Biotechnol Bioeng. 1973 Jan;15(1):27–45. doi: 10.1002/bit.260150104. [DOI] [PubMed] [Google Scholar]
- Michel F. C., Jr, Dass S. B., Grulke E. A., Reddy C. A. Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of kraft bleach plant effluent. Appl Environ Microbiol. 1991 Aug;57(8):2368–2375. doi: 10.1128/aem.57.8.2368-2375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revsbech N. P., Ward D. M. Oxygen Microelectrode That Is Insensitive to Medium Chemical Composition: Use in an Acid Microbial Mat Dominated by Cyanidium caldarium. Appl Environ Microbiol. 1983 Mar;45(3):755–759. doi: 10.1128/aem.45.3.755-759.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]



