Abstract
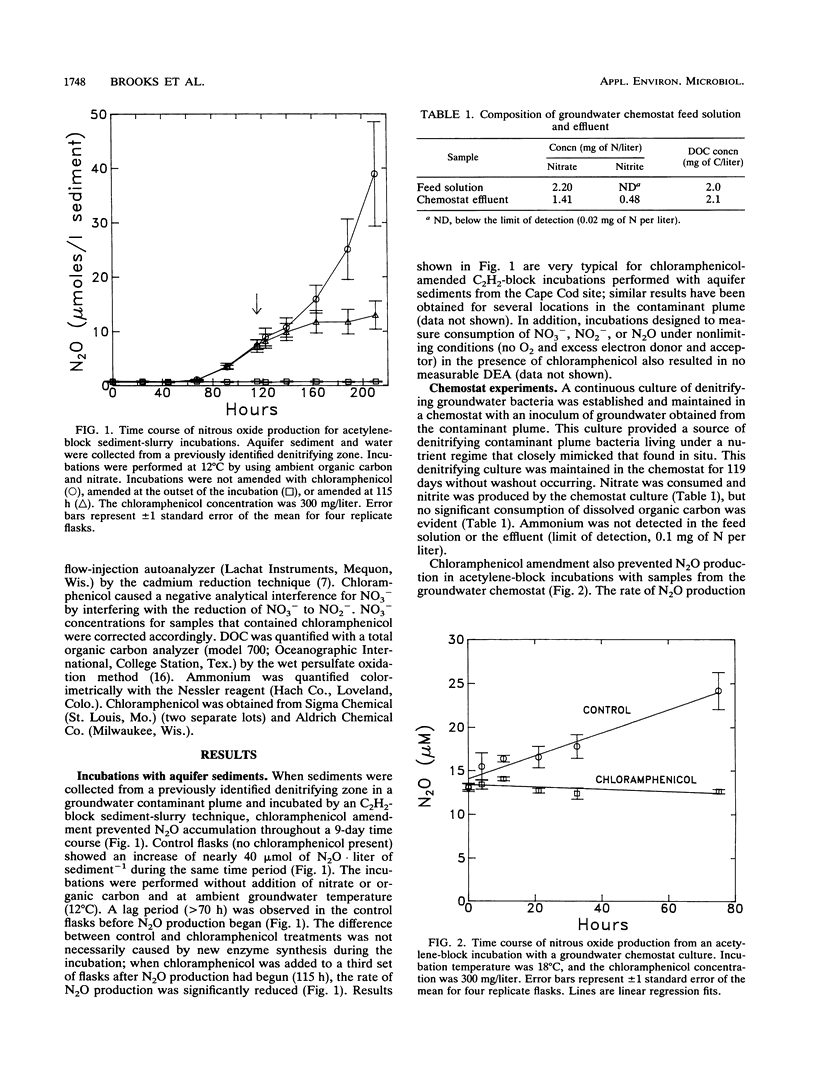
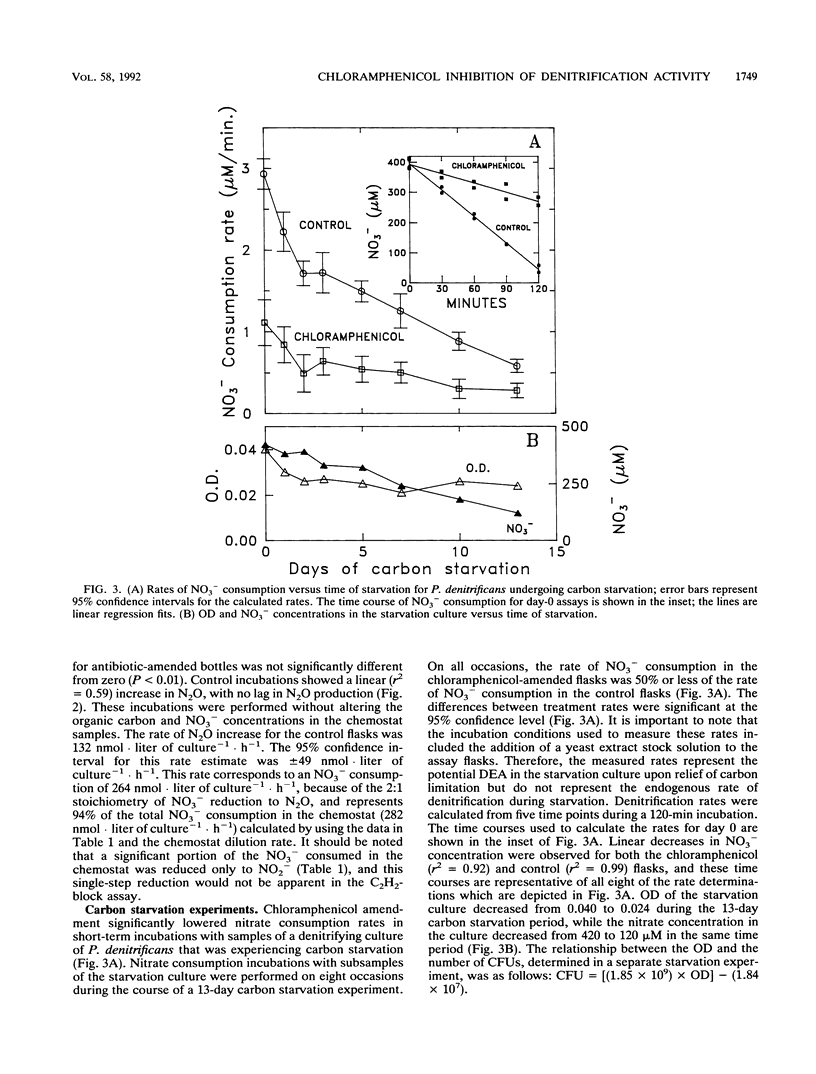
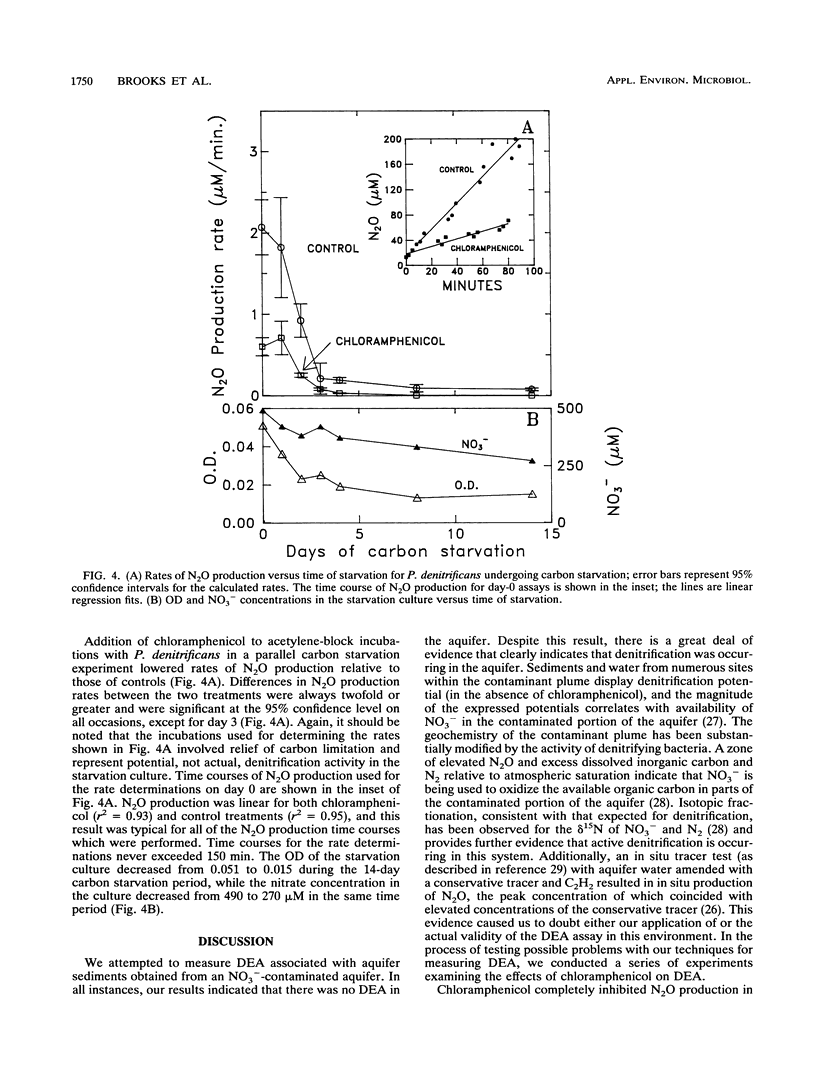
Chloramphenicol completely inhibited the activity of existing denitrification enzymes in acetylene-block incubations with (i) sediments from a nitrate-contaminated aquifer and (ii) a continuous culture of denitrifying groundwater bacteria. Control flasks with no antibiotic produced significant amounts of nitrous oxide in the same time period. Amendment with chloramphenicol after nitrous oxide production had begun resulted in a significant decrease in the rate of nitrous oxide production. Chloramphenicol also decreased (greater than 50%) the activity of existing denitrification enzymes in pure cultures of Pseudomonas denitrificans that were harvested during log-phase growth and maintained for 2 weeks in a starvation medium lacking electron donor. Short-term time courses of nitrate consumption and nitrous oxide production in the presence of acetylene with P. denitrificans undergoing carbon starvation were performed under optimal conditions designed to mimic denitrification enzyme activity assays used with soils. Time courses were linear for both chloramphenicol and control flasks, and rate estimates for the two treatments were significantly different at the 95% confidence level. Complete or partial inhibition of existing enzyme activity is not consistent with the current understanding of the mode of action of chloramphenicol or current practice, in which the compound is frequently employed to inhibit de novo protein synthesis during the course of microbial activity assays. The results of this study demonstrate that chloramphenicol amendment can inhibit the activity of existing denitrification enzymes and suggest that caution is needed in the design and interpretation of denitrification activity assays in which chloramphenicol is used to prevent new protein synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone M. K., Tiedje J. M. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl Environ Microbiol. 1979 Oct;38(4):673–679. doi: 10.1128/aem.38.4.673-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. W., George L. H. Growth determinations for unattached bacteria in a contaminated aquifer. Appl Environ Microbiol. 1987 Dec;53(12):2992–2996. doi: 10.1128/aem.53.12.2992-2996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. W., Smith R. L., George L. Effect of organic contamination upon microbial distributions and heterotrophic uptake in a Cape Cod, Mass., aquifer. Appl Environ Microbiol. 1984 Dec;48(6):1197–1202. doi: 10.1128/aem.48.6.1197-1202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K., Parsons L. L., Murray R. E., Smith M. S. Dynamics of Soil Denitrifier Populations: Relationships between Enzyme Activity, Most-Probable-Number Counts, and Actual N Gas Loss. Appl Environ Microbiol. 1988 Nov;54(11):2711–2716. doi: 10.1128/aem.54.11.2711-2716.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin T. B., Robinson J. A. Stochastic models of soil denitrification. Appl Environ Microbiol. 1989 Jan;55(1):72–77. doi: 10.1128/aem.55.1.72-77.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönner U., Sörensson F. Denitrification rates in the low-oxygen waters of the stratified baltic proper. Appl Environ Microbiol. 1985 Oct;50(4):801–806. doi: 10.1128/aem.50.4.801-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S., Parsons L. L. Persistence of denitrifying enzyme activity in dried soils. Appl Environ Microbiol. 1985 Feb;49(2):316–320. doi: 10.1128/aem.49.2.316-320.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Duff J. H. Denitrification in a sand and gravel aquifer. Appl Environ Microbiol. 1988 May;54(5):1071–1078. doi: 10.1128/aem.54.5.1071-1078.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Howes B. L., Garabedian S. P. In situ measurement of methane oxidation in groundwater by using natural-gradient tracer tests. Appl Environ Microbiol. 1991 Jul;57(7):1997–2004. doi: 10.1128/aem.57.7.1997-2004.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



