Abstract
Rheumatoid factor (RF) B cells proliferate during secondary immune responses to immune complexed antigen and antigen specific T cells, but higher affinity RFs are not detected except in patients with rheumatoid arthritis and other autoimmune diseases. Consequently, there must exist highly efficient mechanisms for inactivation of these higher-affinity RF B cell clones under normal circumstances. Exposure of transgenic mice expressing a human IgM RF to soluble human IgG in the absence of T cell help causes antigen specific B cell deletion in 2–3 days. The deletion is independent of the Fas/Fas ligand (FasL) pathway of apoptosis and is preceded by a phase of partial activation involving increase in cell size and expression of B7 and ICAM-1, and transient release of low levels of immunoglobulin. Complete B cell activation involving the formation of germinal centers and sustained high level RF secretion only occurs if T cell help is provided simultaneously. RF B cells exposed to tolerogen remain competent to secrete RF in vitro if provided with an appropriate antigenic stimulus and T cell help. Consequently, death of these cells is not preceded by anergy. Abortive activation/deletion of B cells by antigen in the absence of T cell-derived survival signals may represent the major mechanism for maintaining peripheral tolerance in B cells expressing higher affinity RF. The lack of anergy, and the potential for reactivation before death, provide a means for maintaining RF production under pathologic circumstances, such as may occur in the inflamed rheumatoid synovium.
Keywords: transgenic mice, B cell deletion, peripheral tolerance, Fas
Normal individuals express low affinity IgM rheumatoid factors (RFs) on peripheral B cells, but fail to express the higher affinity “pathologic” RFs that are associated with diseases such as rheumatoid arthritis (RA) (1). Evidence has shown that RF-expressing B cells are highly efficient antigen-presenting cells for multivalent, immune complexed antigen (2, 3) and may play a role in normal secondary immune responses (4, 5). Although T cells reactive to human IgG (hIgG) are lacking due to T cell tolerance, there are abundant T cells present during a secondary immune response able to recognize peptides derived from the antigen portion of an immune complex. Under normal circumstances, the provision of antigen together with T cell help results in clonal expansion, somatic mutation, and affinity maturation of B cells (6). The absence of higher-affinity RFs in normal individuals suggests that there exist very efficient mechanisms for the peripheral elimination of B cells expressing such higher affinity, somatically mutated RFs. However, the precise mechanisms of removal of these cells have not been established. Transgenic models of autoimmunity have supported and elaborated on previously proposed mechanisms for induction of B cell tolerance. These include physical elimination of self-reactive clones (deletion) and functional inactivation (anergy) followed by reduced life span (7–15). The current study shows that in vivo encounter of transgenic B cells which express a somatically mutated, monospecific, human IgM (hIgM) RF with soluble, deaggregated human IgG (DHGG) results in abortive activation of the RF B cells followed by cell death by a Fas-independent mechanism between 2 and 3 days after antigen encounter. RF B cells show no evidence of anergy after exposure to soluble hIgG, as they are unaffected in their ability to differentiate into antibody producing cells in the presence of antigen and T cell help. Indeed, the kinetics of abortive activation/deletion and the lack of anergy provide an interval of opportunity for reactivation and survival of some RF B cells. This could potentially occur within the inflamed synovia of RA joints where the concentration of soluble IgG is lower than in plasma, and where both immune complexed antigen and antigen-specific T cells are present together with cytokines that promote B cell differentiation. We hypothesize that such stimulation within the inflammatory synovial environment may interfere with the normal process of activation induced deletion of higher affinity RF B cells in RA.
MATERIALS AND METHODS
Transgenic Mice.
The AB29 transgenic mice expressing the rearranged Ig heavy and light chain genes encoding the human Les RF have been described (3). This line is maintained by backcross mating with C57BL/6 mice or B6/lpr mice (The Jackson Laboratories). Positive progeny of transgenic matings are identified by measuring the level of hIgM RF in the serum of 4- to 5-week-old mice by ELISA (3).
Induction of Tolerance by Injection of DHGG.
hIgG1 was derived from the serum of a myeloma patient and purified using a protein A-Sepharose column and ultracentrifugation (16). A myeloma protein, as opposed to pooled hIgG, was used to avoid the possibility of aggregate formation due to anti-allotype reactions. Mice were then injected i.p. with 2 mg IgG.
Provision of T Cell Help by Coinjection of Bm12 Spleen Cells.
AB29 mice or their nontransgenic littermates were injected i.v. with 2 × 107 C57BL/6bm12 (bm12) allogeneic splenocytes in 100 μl of serum-free RPMI 1640 medium (BioWhittaker), on the same day as i.p. injection of soluble, DHGG. C57BL/6bm12 splenocytes differ from C57BL/6 by three point mutations in their I-Ab molecules, which result in allorecognition (17).
Fluorescence Analysis.
Two- or three-color fluorescence analysis was carried out on all lymphoid populations. Cell phenotypes were determined using the following reagents: rabbit anti-hIgM–fluorescein isothiocyanate (FITC) or –biotin (Jackson ImmunoResearch); rat anti-mouse B220–phycoerythrin (PE) or –CyChrome; rat anti-mouse B7.2 (CD86)–biotin, rat anti-mouse ICAM-1 (CD54)–biotin (PharMingen); mouse anti-human κ–PE, rat anti-mouse Thy1–FITC (Caltag); goat anti-mouse κ–PE (Fisher); streptavidin–PE (Molecular Probes) and peanut agglutinin (PNA)–FITC (Vector Laboratories). Samples were analyzed on a FACScan flow cytometer and data processed using lysys analysis programs (Becton Dickinson).
Immunohistochemistry.
Spleen samples were snap frozen in optimal cutting temperature medium (Miles). Sections (4 μm) were prepared from the tissue blocks and sequentially stained with affinity-purified rabbit anti-hIgM-biotin and affinity-purified goat anti-mouse IgM-biotin (Jackson ImmunoResearch) or rat anti-mouse CD4 and CD8-biotin (PharMingen) or PNA-biotin (Vector Laboratories). The first antibody was detected by saturating amounts of streptavidin-peroxidase (Kirkegaard & Perry Laboratories) and AEC (3-Amino-9 ethyl-carbazole) and the second by streptavidin-alkaline phosphatase and HistoMark Blue (Kirkegaard & Perry Laboratories). Slides were then counter stained with Mayer’s hematoxylin before mounting with Aqua Mount (Lerner, Pittsburgh).
B Cell Proliferation Assays.
Spleen cell suspensions were prepared from transgenic mice or nontransgenic littermates and proliferation measured in response to F(ab′)2 fragments of affinity-purified, species-specific goat anti-hIgM (Jackson ImmunoResearch) at 10 μg/ml or aggregated hIgG (Cappel) at 50 ng/ml or medium as control as described (16).
In Vitro Antibody Secretion.
To assay in vitro secretion of hIgM in response to antigen stimulation in the presence or absence of T cell help, triplicate cultures were set up in 200 μl final volume RP10 (RPMI 1640 medium supplemented with penicillin/streptomycin, glutamine, 2-mercaptoethanol, and 10% fetal bovine serum) and added to 96-well flat-bottom microtiter plates. Each well contained 2 × 105 responder splenocytes ± 100 ng/ml deaggregated hIgG ± T cell help (5 × 105 C57BL/6bm12 splenocytes) or filler splenocytes (5 × 105 C57BL/6 splenocytes). Cultures were incubated at 37°C/5% CO2 and samples of supernatant were harvested 5 days later. Samples from triplicate cultures were pooled, and hIgM levels were measured by ELISA (3).
ELISPOT Assays.
Numbers of hIgM RF-secreting cells present in fresh spleen were measured by ELISPOT. Briefly, 96-well flat-bottomed microtiter plates were coated with a solution of hIgG (Cappel) at 20 μg/ml in PBS, overnight at 4°C. After washing, non-specific binding was blocked using PBS/1% BSA. Fresh spleen cells were washed and resuspended in RP10 medium and added at various dilutions to the microtiter plates. After incubation at 37°C/5% CO2 for 4 h, antibody-producing cells were detected by sequential addition of goat anti-hIgM Fc5μ-biotin (Accurate Chemical) and streptavidin-alkaline phosphatase (Kirkegaard & Perry Laboratories) diluted in BBS (borate buffered saline)/1% BSA, followed by 5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate (Sigma). After developing, wells containing 5–100 spots were counted and mean numbers of spots per 106 input spleen cells and mean number of spots per 106 hIgM+ cells (number calculated from percent of hIgM+ cells in permeabilized spleen samples) were calculated.
Data from all experiments were analyzed by ANOVA and significance expressed as a P value.
RESULTS
Soluble hIgG Induces Down-Regulation of Surface IgM on RF B Cells and Loss from Secondary Lymphoid Tissue.
AB29 mice were injected i.p. with 2 mg soluble DHGG and then bled and killed at various times postinjection. Peripheral blood leukocytes, splenocytes, lymph node cells, peritoneal lavage, and bone marrow were isolated and analyzed by fluorescence-activated cell sorter (FACS) for surface phenotype. As shown in Table 1, RF B cells rapidly down-regulate surface hIgM to ≈30% of normal levels. Within 12 h of soluble hIgG injection, numbers of these hIgM low cells are reduced in the blood but increased transiently in the spleen, peaking at 24–48 h postinjection. By 4 days after injection of soluble hIgG, hIgM-expressing B cells are reduced to 15–40% of pretreatment levels in all secondary lymphoid tissues and maintained at this level for at least a further 16 days (16). A more detailed analysis of the spleen shows that loss of RF B cells starts to occur between days 2 and 3 postinjection (data not shown). This loss is not an artifact created by continuous modulation and internalization of surface IgM by antigen, as indicated by a proportionate decrease in the total B cell levels and also by the demonstration that permeabilization of splenic lymphocytes before staining shows a similar decrease in hIgM+ cells (Table 1). Neither can this loss be explained through relocalization of RF B cells to the site of antigen injection or to the bone marrow, as RF B cell numbers were also reduced in peritoneal exudate and bone marrow (data not shown).
Table 1.
Effect of soluble hIgG on the phenotype of lymphocytes from secondary lymphoid tissue
| Treatment | Blood
|
Lymph node
|
Spleen
|
Spleen (permeabilized)
|
|||
|---|---|---|---|---|---|---|---|
| % hIgM (mean fl) | % B220 | % hIgM (mean fl) | % B220 | % hIgM (mean fl) | % B220 | % hIgM (mean fl) | |
| None | 9.4 ± 0.7 | 15.1 ± 1.3 | 13.1 ± 0.9 | 16.1 ± 1.8 | 20.9 ± 1.5 | 37.9 ± 2.4 | 29.9 ± 4.1 |
| (320) | (680) | (751) | (283) | ||||
| 12 h | 4.1 ± 0.6* | 8.3 ± 0.7 | 14.9 ± 4.0 | 21.1 ± 3.5 | 23.0 ± 4.3 | 36.6 ± 3.5 | 33.7 ± 4.5 |
| (105)* | (138)* | (200)* | (381) | ||||
| 24 h | 3.0 ± 0.5* | 6.8 ± 0.7 | 15.4 ± 2.1 | 27.5 ± 5.7* | 32.3 ± 3.2* | 42.8 ± 1.9 | 39.3 ± 4.9 |
| (121)* | (177)* | (279)* | (551) | ||||
| 4 days | 2.9 ± 0.6* | 6.5 ± 0.5 | 5.1 ± 1.5* | 12.2 ± 0.7 | 8.7 ± 1.5* | 25.6 ± 3.1* | 14.4 ± 3.4* |
| (134)* | (203)* | (257)* | (367) | ||||
| 7 days | 3.4 ± 1.9* | 6.1 ± 2.4 | 4.7 ± 1.6* | 9.4 ± 2.9 | 6.8 ± 1.8* | 22.2 ± 2.4* | 9.9 ± 1.2* |
| (232)* | (283)* | (365)* | (276) | ||||
AB29 mice were injected i.p. with 2 mg DHGG and killed at various times after treatment. Lymphoid cells from spleen and blood were stained for expression of either B220 (total B cells) or hIgM and then analyzed on the cytofluorograph. Data are expressed as mean percentage positive cells ± SE and mean fluorescence (in parentheses) for groups of three mice.
P < 0.05.
Loss of hIgM RF B Cells Is Preceded by Activation.
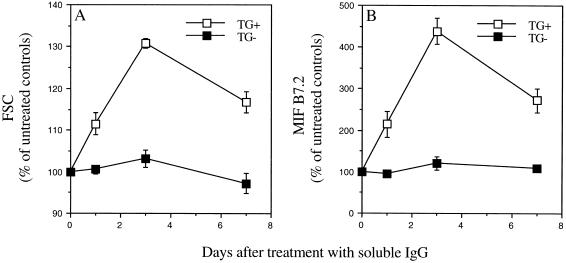
Spleen cells were analyzed by FACS at various times posttreatment for both cell size and levels of the activation antigens B7.2 (CD86) and ICAM-1 (CD54) (Fig. 1A). Significant increases in both cell size and levels of B7.2 expression were observed at days 1, 3, and 7 after i.p. injection of soluble IgG. By day 3, the cell size of the hIgM+ population had increased to 131% and levels of B7.2 to 438% of untreated controls. In contrast the levels in the B cell population that did not express surface hIgM (either mouse Ig-expressing cells or very low level hIgM-expressing cells) were not significantly increased. The level of ICAM-1 on cells from treated mice increased in parallel with B7.2 and remained elevated for at least 7 days (data not shown). The larger size of hIgM+ cells together with the up-regulation of surface B7.2 and ICAM-1 in treated mice indicate that RF-expressing B cells are activated by encountering soluble IgG before deletion.
Figure 1.
Loss of hIgM RF-B cells is preceded by activation. AB29 mice were injected i.p. with 2 mg DHGG. Spleen cells were removed 1, 3, or 7 days after treatment, stained for surface expression of B220 (total B cells), hIgM, and the activation antigen B7.2 and then analyzed on the cytofluorograph. (A) Forward scatter (FSC) values of hIgM+/B220+ cells (TG+, □) and hIgM−/B220+ cells (TG−, ▪) from the same mice, as a measure of size. (B) Mean intensity of fluorescence (MIF) of B7.2 staining for hIgM+/B220+ cells (TG+, □) and hIgM−/B220+ cells (TG−, ▪) from the same mice, as a measure of B cell activation. Data shown are means ± SE for groups of three or four mice and represent a percentage of control levels seen on untreated AB29 mice. All time points were significantly different from controls for TG+ cells, no time points were significantly different from controls for TG− cells; P < 0.05.
Redistribution of RF B Cells After Encounter with Soluble hIgG.
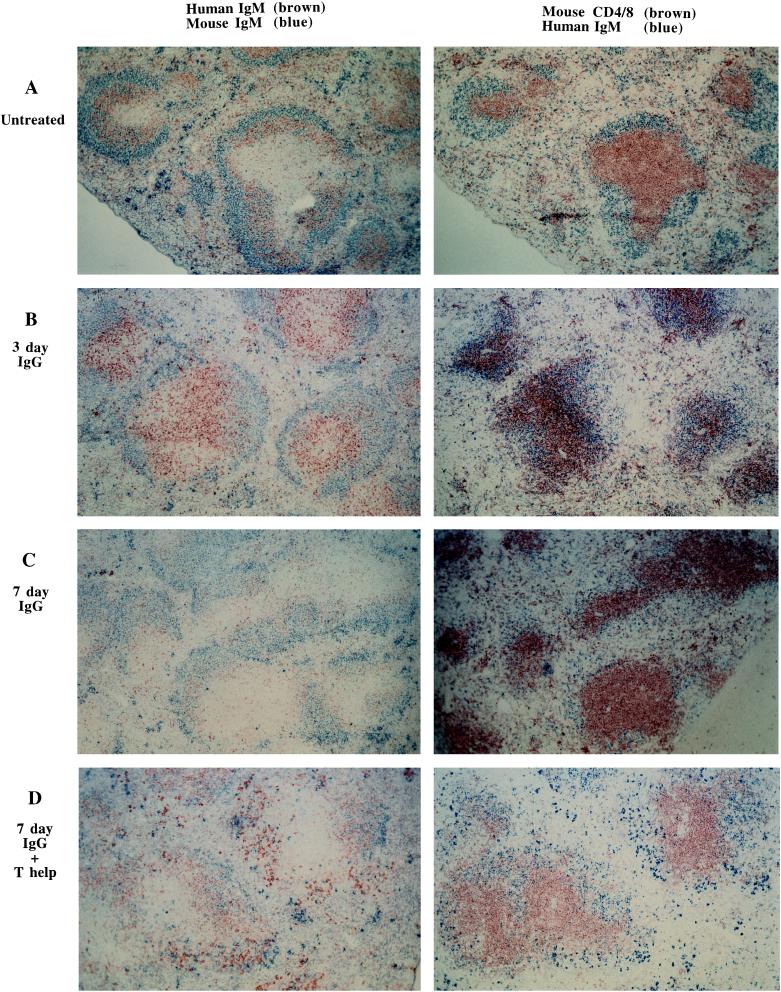
Unmanipulated AB29 mice show characteristic localization of RF B cells to primary follicles and the mantle zones of secondary follicles in the spleen (Fig. 2A). By day 3 postinjection (Fig. 2B) many of the RF B cells are relocated to the B/T cell junction of follicle and periarteriolar lymphatic sheath (PALS). Seven days after injection of soluble hIgG, the reduction in RF B cell numbers is obvious (Fig. 2C).
Figure 2.
Effect of encounter with soluble hIgG on the distribution of hIgM RF-expressing B cells in the spleen. (A–D) Immunohistochemical analysis of spleen sections from either uninjected AB29 mice (A) or AB29 mice, 3 days (B), or 7 days (C) after i.p. injection of 2 mg DHGG or 7 days after i.p. injection of DHGG and i.v. injection of 20 × 106 C57BL/6bm12 splenocytes. (D) (Low power views, ×100.)
Cell Death Occurs Through a Fas-Independent Pathway of Apoptosis.
To determine the involvement of the Fas/Fas ligand (FasL) pathway of apoptosis, AB29 mice were bred onto the B6/lpr background. AB29/lpr mice showed an identical loss of hIgM+ B cells by FACS analysis as the AB29 wild-type controls when measured 7 days after injection of DHGG, indicating that ligation of Fas on the RF B cells surface was not responsible for the observed cell death (Table 2).
Table 2.
Fas is not involved in RF B cell deletion
| Strain | Injection of hIgG | hIgM
|
B220, % | |
|---|---|---|---|---|
| % | Mean | |||
| AB29 | − | 26.6 (3.4) | 436 (66) | 41.4 (4.8) |
| AB29 | + | 6.9 (3.9)* | 182 (56)* | 20.5 (3.7)* |
| AB29/lpr | − | 21.3 (7.0) | 394 (37) | 41.4 (9.2) |
| AB29/lpr | + | 5.9 (2.5)* | 199 (34)* | 24.0 (3.3)* |
AB29 mice or AB29/lpr mice were injected i.p. with 2 mg DHGG and killed 7 days after treatment. Spleen cells were stained for surface expression of either B220 (total B cells) or hIgM and then analyzed on the cytofluorograph. Data are expressed as mean percentage positive cells and mean fluorescence (SD is shown in parentheses) for groups of three or four mice.
P < 0.05.
RF B Cells Are Not Rendered Anergic by Encounter with Antigen.
Soluble hIgG exposed RF B cells were tested in vitro for their functional capacity. Such cells were unaffected in their ability to differentiate to antibody-secreting cells, although their ability to proliferate in response to antigen was reduced.
Splenocytes were removed from mice 1 and 3 days after in vivo exposure to soluble hIgG and tested in vitro for their proliferative response to aggregated hIgG or F(ab′)2 fragments of anti-hIgM antibodies (Table 3). Proliferative responses were significantly reduced compared with splenocytes from untreated mice even when counts were adjusted to compensate for the reduced numbers of input cells in the cultures from mice exposed to soluble hIgG. In contrast, the ability of soluble hIgG exposed RF B cells to produce antibody was not significantly reduced (Table 3). In these experiments splenocytes were removed 1 and 3 days after soluble hIgG injection and incubated with antigen (aggregated hIgG) ± T cell help in the form of allogeneic C57BL/6bm12 splenocytes or filler C57BL/6 splenocytes as control. Supernatants were collected 5 days later and levels of hIgM were measured by ELISA. Data are expressed as the amount of hIgM secreted by 2 × 105 input splenocytes or as an adjusted value for the amount of hIgM secreted by 2 × 105 input hIgM+ B cells (calculation based on the percent of surface IgM+ B cells present in the spleen). These data show that on a per cell basis, RF B cells tested 1 and 3 days after exposure in vivo to soluble hIgG are not significantly affected in their ability to differentiate into antibody-producing cells.
Table 3.
Functional capacity of hIgG exposed RF B cells
| Time after hIgG injection, days | Proliferation (cpm) in response to
|
HIgM secreted (ng/ml) in response to
|
||||
|---|---|---|---|---|---|---|
| Aggregated hIgG
|
F(ab′)2 anti-hIgM
|
agg. hIgG + B6 spleen | agg. hIgG + bm12 spleen | |||
| Day 1-2 | Day 2-3 | Day 1-2 | Day 2-3 | |||
| Response per 2 × 105 spleen cells | ||||||
| 34,867 (6,632) | 25,112 (5,705) | 77,514 (14,652) | 65,440 (3,854) | 1120 (140) | 2,692 (782) | |
| 1 | 21,603* (7,777) | 15,427* (4,276) | 67,711 (46,194) | 56,247 (31,433) | 1226 (855) | 1,706 (641) |
| 3 | 8,037* (2,280) | 7,451* (2,273) | 16,236* (17,999) | 30,446* (2,865) | 529 (45) | 1,093* (324) |
| Adjusted response per 2 × 105 hIgM+ spleen cells | ||||||
| 147,241 (30,298) | 106,859 (30,740) | 323,993 (43,054) | 276,697 (35,303) | 4520 (1084) | 11,157 (5,273) | |
| 1 | 81,028* (26,232) | 59,413* (23,316) | 257,116 (174,305) | 216,553 (132,944) | 4744 (3381) | 6,639 (2,765) |
| 3 | 64,422* (22,055) | 61,450* (29,741) | 131,298* (113,719) | 241,340 (32,903) | 3856 (709) | 8,077 (3,014) |
Mice were injected i.p. with 2 mg DHGG and killed at various times after treatment. Proliferation of splenocytes was measured in response to either aggregated hIgG at 50 ng/ml or F(ab′)2 fragments of affinity-purified goat anti-hIgM at 10 μg/ml or medium as control. Data are expressed as mean cpm incorporated for groups of three or four mice (after the subtraction of individual medium background cpm) and standard deviations from the mean (in parentheses) per 2 × 105 input spleen cells or as an adjusted value calculated for 2 × 105 hIgM+ B cells. Secretion of human IgM in vitro by 2 × 105 splenocytes stimulated with aggregated (agg) hIgG at 100 ng/ml final concentration or medium ± either 5 × 105 C57Bl/6bm12 splenocytes (T cell help) or 5 × 105 C57Bl/6 splenocytes (filler cells) as control. Triplicate samples of supernatants were harvested 5 days after the initiation of culture and pooled, and their hIgM levels were measured by standard ELISA. Data are shown as means and standard deviations (in parentheses) of hIgM produced (ng/ml) by 2 × 105 input spleen cells or as an adjusted value calculated for 2 × 105 input hIgM+ B cells. Data are shown for groups of three or four mice.
P < 0.05.
RF B Cells Are Activated by Encounter with Soluble IgG, but Do Not Enter Germinal Centers in the Absence of T Cell Help.
The formation of germinal centers is characteristic of T cell-dependent B cell responses (18, 19). PNA binding is a hallmark of B cells in the germinal centers of secondary follicles. While injection of soluble hIgG alone leads to expression of activation antigens on the RF B cell, it does not enable them to accumulate in germinal centers. In contrast, when T cell help is provided at the same time as exposure to soluble hIgG by i.v. injection of allogeneic splenocytes (20 × 106) from C57BL/6bm12, hIgM+ B cells show a significant increase (P < 0.05) in the percentage of cells that bind PNA. This number increases from 0.1% in untreated animals to 3% by 1 week after treatment and only occurs in mice injected with both DHGG and C57BL/6bm12 T cells.
Provision of T cell help at the time of soluble hIgG injection also affected the redistribution of RF B cells within lymphoid tissue, as seen from immunohistological analysis of spleen sections. As shown in Fig. 2 A–C, soluble hIgG injection resulted in movement of RF B cells from the follicle into the PALS, which is primarily a T cell area. Over the next few days RF B cell numbers declined and by day 7 (Fig. 2C) after hIgG injection the sparse remaining hIgM+ cells were located at the B/T cell junction of the follicle and PALS, and few, if any, antibody-forming cells were observed in the red pulp. In contrast, when allogeneic T cell help was provided in vivo, an increase in the number of germinal centers was observed, some of which contained hIgM+ cells (data not shown) and numerous hIgM+ antibody producing cells were apparent within the interfollicular regions of the spleen (Fig. 2D). Mice injected with C57BL/6bm12 splenocytes alone were indistinguishable histologically from untreated AB29 mice, except for the appearance of a number of germinal centers which appeared to involve mainly hIgM− cells (data not shown).
RF Secretion Analysis of the serum levels of hIgM and numbers of hIgM RF secreting cells (as detected by ELISPOT) present in the spleen at the time of harvest confirmed the histology data.
In spite of inducing transient activation of hIgM RF-bearing B cells, DHGG failed to induce a detectable increase in serum hIgM levels in the absence of T cell help (Table 4). In contrast, the provision of T cell help resulted in a 15-fold increase in serum levels of hIgM. When numbers of hIgM RF-secreting B cells were measured by ELISPOT (Table 4), soluble IgG-treated mice did show an increase in the numbers of hIgM+ B cells secreting hIgM; however, this number was dramatically increased (8- to 18-fold) by the provision of T cell help. In addition, the splenocytes from mice treated with DHGG alone produced only small spots in the ELISPOT assay, whereas splenocytes from mice in which T cell help had been provided produced large spots. This indicates that abortive activation leads to low level release of IgM RF in soluble hIgG-exposed RF cells, but that germinal center formation and high level secretion are not achieved in the absence of T cell help.
Table 4.
In vivo secretion of hIgM
| Serum levels of hIgM
|
No. of IgM RF-secreting B cells
|
|||
|---|---|---|---|---|
| Time after hIgG Injection, days | T cell help (bm12) | Serum hIgM, mg/ml | No./106 spleen cells | No./106 hIgM+ B cells |
| − | 12.7 ± 4.2 | 12 ± 9 | 44 ± 32 | |
| 1 | − | 12.7 ± 3.1 | 1,059 ± 657 | 4,403 ± 3567 |
| 3 | − | 11.2 ± 12.1 | 1,310 ± 1138 | 10,562 ± 8370 |
| 7 | − | 8.2 ± 1.4 | 119 ± 70 | 1,824 ± 1237 |
| 1 | + | 23.7 ± 3.1 | 10,104 ± 1986* | 36,061 ± 9999* |
| 7 | + | 201.3 ± 43.6* | 4,087 ± 3670* | 33,094 ± 32,819* |
AB29 mice were injected i.p. with 2 mg DHGG and killed at either 1, 3 or 7 days after treatment. Additional groups included mice injected i.p. with 2 mg DHGG and i.v. with 20 × 106 C57Bl/6bm12 splenocytes (T cell help) 7 days before sacrifice and mice injected i.p. with 2 mg DHGG and i.v. with 20 × 106 C57Bl/6bm12 splenocytes (T cell help) 1 and 4 days, respectively, before sacrifice. Mice were bled at sacrifice and serum human IgM levels measured by ELISA, and numbers of human IgM RF secreting splenocytes were measured by ELISPOT using fresh spleen. Data are expressed as the means ± SD of groups of three or four mice. Numbers per 106 hIgM+ cells were calculated based on numbers of permeabilized splenocytes which stained positive for hIgM.
P < 0.05.
DISCUSSION
Deletion of RF B cells in response to exposure to soluble hIgG occurs through abortive B cell activation followed by cell death. Within 24 h of injection of soluble hIgG, RF B cells are lost from the peripheral blood and increase in number in the spleen. Normally RF B cells are found in primary follicles and mantle zones of secondary follicles. However, after IgG injection, the RF B cells start to relocalize to the T cell zone. This redistribution is complete 3 days after injection, when the majority of RF B cells are oriented toward the B/T region junctions. A similar phenomenon has recently been demonstrated in the hen egg white lysozyme/anti-hen egg white lysozyme transgenic model of autoreactive B cells (20, 21). Analysis of both cell size and expression of activation markers such as B7.2 (CD86) and ICAM (CD54) shows that encounter with a tolerogenic soluble form of their specific antigen leads to B cell activation. However, such activation is followed by a loss of RF B cells which starts to occur between days 2 and 3 after IgG exposure. By day 3, the numbers of RF B cells are reduced by 40–70%. The observed loss of RF B cells is neither an artifact created by continuous modulation and internalization of surface IgM by antigen, nor is it due to redistribution to other tissues such as lymph nodes, peritoneum, or bone marrow. RF B cell loss is maximal (numbers reduced by 70–75%) by 7 days after injection and remains low for a number of weeks, only returning to normal levels by 7 weeks after injection (16). This phenotypic profile is the basis for the functional inhibition documented in the classical experiments of soluble hIgG tolerance in which B cell tolerance extended from 3 to 50 days after exposure (22).
The basis for the ability of soluble IgG to induce B cell activation can be explained by x-ray crystallographic studies of an IgM RF–IgG complex which show that each hIgG Fc is capable of being bound simultaneously by two IgM RF-combining sites (23), although these findings contrast with earlier studies using analytic ultracentrifugation which show a single RF Fab fragment interacting with a single hIgG molecule (24, 25). Human IgG is naturally encountered in concentrations reaching 10−4 to 10−5 M. Soluble IgG could, therefore, have the ability to dimerize those RF molecules on the B cell surface that have affinities for antigen in the micromolar range or greater.
Anergic B cells have been shown to express reduced levels of surface IgM after encounter with soluble antigen (14, 26), and in the continuous presence of soluble antigen remain unreactive even when provided with T cell help (27). Initially believed to be long-lived cells, more recent experiments have shown them to have a reduced life span (28, 29). Our data show that activation induced deletion of RF B cells in response to soluble hIgG is not preceded by a stage of anergy, as such cells do not appear to be impaired in their ability to differentiate to antibody secreting cells in vitro.
Because T cells play an essential role in the normal RF response (30), it was important to determine if T cell help could overcome activation-induced B cell deletion in the transgenic RF model. The injection of allogeneic splenocytes as a source of nonspecific T cell help led to the formation of germinal centers containing hIgM-expressing B cells (as demonstrated by increased numbers of hIgM+/PNA+ cells) and large numbers of hIgM-secreting B cells in the red pulp of the spleen. As a result, the numbers of splenic hIgM+ B cells secreting hIgM increased 8- to 18-fold and the serum levels of hIgM rose 15-fold. Control animals injected only with allogeneic splenocytes had no such increases. These results show that T cell help not only rescued a proportion of RF B cells from cell death triggered by abortive activation by IgG but also induced them to differentiate into RF-secreting plasma cells.
Expression of both Fas and FasL increase in T cells on activation and are involved in activation induced T cell death. Resting B cells also express low numbers of Fas molecules, and the levels of both Fas and its ligand increase several fold after activation (31–35), resulting in the development of susceptibility to Fas-mediated lysis (34). To determine the role of the Fas system in the deletion of RF B cells by IgG, RF transgenic mice were bred onto the lpr background for more than two backcross generations. The administration of IgG to the Fas-deficient animals still caused an identical deletion of the RF B cells when measured 7 days after treatment. Consequently, death does not occur through Fas/FasL-mediated apoptosis but rather through lack of T cell-derived helper factors necessary for further growth and differentiation.
During a normal secondary immune response, RF precursors expand in number and serum IgM RF levels may rise as a consequence (4, 5, 36, 37). However, RF production in normal subjects is transient and regulated by both immune complexed antigen and by T cells specific for the antigen present in the immune complex (4, 30, 36, 38). Such clonal regulation can be explained by the ability of RF B cells to act as highly efficient antigen presenting cells for immune-complexed antigen (2, 3). It is likely that early in a secondary response, most of the antigen arrives in draining lymph nodes in the form of an immune complex. At very low concentrations, antigen presentation by macrophages and dendritic cells may be suboptimal, and presentation by antigen-specific B cells may be prevented by negative signaling through cross-linking of Fc receptors on the B cell surface (39). Under these circumstances, RF B cells, while unable to bind or recognize soluble IgG due to low affinity, may be able to take up and process immune complexes because of the increased avidity of this multivalent interaction. Cross-linking of surface Ig on the RF B cells induces expression of costimulatory molecules such as B7.2 (CD86) (H.T., unpublished data). Such activated RF B cells are then highly efficient at presenting immune-complexed antigen to antigen-specific T cells (3), which may in turn induce production of RF by the B cell and provide help for antigen-specific B cells. Studies of RFs derived from normal individuals following secondary immunization reveal evidence for extensive somatic mutation (40). However, these antibodies show no evidence of affinity maturation and display an apparent selection against amino acid replacements in the antibody-combining site (40). The accumulated data strongly imply that under normal circumstances there are efficient peripheral mechanisms for the silencing of higher affinity, potentially pathologic, RF-expressing B cells that arise by mutation of lower affinity RF genes, or by random somatic mutation in other antibody genes that generate antibodies cross reactive with hIgG. The data presented here indicate that an important mechanism of tolerance induction in these RF B cells may be deletion by abortive activation followed by cell death in response to encounter with antigen in the absence of necessary T cell-derived signals, and emphasize the importance of antigen-specific T cells in the maturation of the RF response.
RFs derived from patients with RA show evidence of antigen-induced selection but an apparent failure in peripheral tolerance mechanisms as indicated by the presence of higher affinity RFs with many somatic mutations. The target tissues in RA are the synovial membranes and other extravascular sites where the concentrations of IgG are far lower than in the plasma. It is possible that high-affinity RF B cells that lodge in extravascular compartments where the IgG concentration is low escape activation-induced deletion caused by soluble divalent IgG. Clones of both B and T cells reactive with exogenous antigens are found within the RA joint. Consequently, once inflammation is initiated, an immune response to any antigen within the joint, either exogenous or endogenous, together with an imbalance in the ratio of soluble to immune complexed IgG, could interfere with tolerance induction of RF B cells at this site.
Acknowledgments
We thank Patty Charos and Lynn Quach for their expert technical assistance and Nancy Noon and Jane Uhle for their excellent secretarial assistance. K.W. was the recipient of a fellowship from the Deutscher Akademischer Austauschdienst. H.T. was a recipient of an Arthritis Foundation Investigators Award. This research was supported in part by grants from the National Institutes of Health (AR42153 and AR25443).
Footnotes
Abbreviations: RF, rheumatoid factor; RA, rheumatoid arthritis; PNA, peanut agglutinin; DHGG, deaggregated human IgG; FACS, fluorescence-activated cell sorter; hIgG, human IgG; hIgM, human IgM; PALS, periarteriolar lymphatic sheath.
References
- 1.Tighe H, Carson D A. In: Textbook of Rheumatology. Kelley W N, Harris E D, Ruddy S, Sledge C B, editors. Philadelphia: Saunders; 1996. pp. 241–249. [Google Scholar]
- 2.Roosnek E, Lanzavecchia A. J Exp Med. 1991;173:487–489. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tighe H, Chen P P, Tucker R, Kipps T J, Roudier J, Jirik F R, Carson D A. J Exp Med. 1993;177:109–118. doi: 10.1084/jem.177.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemazee D A, Sato V L. J Exp Med. 1983;158:529–545. doi: 10.1084/jem.158.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch M J, Fong S, Vaughan J, Carson D. Clin Exp Immunol. 1983;51:299–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Berek C, Ziegner M. Immunol Today. 1993;14:400–404. doi: 10.1016/0167-5699(93)90143-9. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Nature (London) 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 8.Nemazee D A, Burki K. Nature (London) 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 9.Erikson J, Radic M Z, Camper S A, Hardy R R, Carmack C, Weigert M. Nature (London) 1991;349:331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 10.Hartley S B, Crosbie J, Brink R, Kantor A B, Basten A, Goodnow C C. Nature (London) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 11.Hartley S B, Cooke M P, Fulcher D A, Harris A W, Cory S, Basten A, Goodnow C C. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto M, Murakami M, Shimizu A, Ozaki S, Tsubata T, Kumagai S, Honjo T. J Exp Med. 1992;175:71–79. doi: 10.1084/jem.175.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Radic M Z, Erikson J, Camper S A, Litwin S, Hardy R R, Weigert M. J Immunol. 1994;152:1970–1782. [PubMed] [Google Scholar]
- 14.Goodnow C C, Crosbie J, Adelstein S, Lavoie T B, Smith-Gill S J, Brink R A, Pritchard-Briscoe H, Wotherspoon J S, Loblay R H, Raphael K, Trent R J, Basten A. Nature (London) 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 15.Goodnow C C, Adelstein S, Basten A. Science. 1990;248:1373–1379. doi: 10.1126/science.2356469. [DOI] [PubMed] [Google Scholar]
- 16.Tighe H, Heaphy P, Baird S, Weigle W O, Carson D A. J Exp Med. 1995;181:599–606. doi: 10.1084/jem.181.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mengle-Gaw L, Conner S, McDevitt H O, Fathman C G. J Exp Med. 1984;160:1184–1194. doi: 10.1084/jem.160.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berek C, Milstein C. Immunol Rev. 1988;105:5–26. doi: 10.1111/j.1600-065x.1988.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 19.Klaus G G, Humphrey J H. Immunology. 1977;33:31–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Cyster J G, Goodnow C C. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 21.Fulcher D A, Lyons A B, Korn S L, Cook M C, Koleda C, Parish C, Fazekas de St., Groth B, Basten A. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiller J M, Habicht G S, Weigle W O. Science. 1971;171:813. [PubMed] [Google Scholar]
- 23.Corper A L, Sohi M K, Bonagura V R, Steinitz M, Jefferis R, Feinstein A, Beale D, Taussig M J, Sutton B J. Immunology. 1996;88:636–641. doi: 10.1046/j.1365-2567.1996.d01-692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chavin S I, Franklin E C. J Biol Chem. 1969;244:1345–1352. [PubMed] [Google Scholar]
- 25.Stone M J, Metzger H. J Biol Chem. 1968;243:5977–5984. [PubMed] [Google Scholar]
- 26.Goodnow C C, Crosbie J, Jorgensen H, Brink R A, Basten A. Nature (London) 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 27.Goodnow C C, Brink R, Adams E. Nature (London) 1991;352:532–536. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 28.Cyster J G, Hartley S B, Goodnow C C. Nature (London) 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 29.Fulcher D A, Basten A. J Exp Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemazee D A. J Exp Med. 1985;161:242–256. doi: 10.1084/jem.161.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trauth B C, Klas C, Peters A M, Matzku S, Moller P, Falk W, Debatin K M, Krammer P H. Science. 1989;245:301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 32.Drappa J, Brot N, Elkon K B. Proc Natl Acad Sci USA. 1993;90:10340–10344. doi: 10.1073/pnas.90.21.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandik L, Nguyen K A, Erikson J. Eur J Immunol. 1995;25:3148–3154. doi: 10.1002/eji.1830251124. [DOI] [PubMed] [Google Scholar]
- 34.Onel K B, Tucek-Szabo C L, Ashany D, Lacy E, Nikolic-Zugic J, Elkon K B. Eur J Immunol. 1995;25:2940–2947. doi: 10.1002/eji.1830251034. [DOI] [PubMed] [Google Scholar]
- 35.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornard T, MacDonald H R, Tschopp J. Eur J Immunol. 1996;26:721–724. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 36.Van Snick J, Coulie P. Eur J Immunol. 1983;13:890–894. doi: 10.1002/eji.1830131106. [DOI] [PubMed] [Google Scholar]
- 37.Levine P R, Axelrod D A. Clin Exp Rheumatol. 1985;3:147–149. [PubMed] [Google Scholar]
- 38.Coulie P G, Van Snick J. J Exp Med. 1985;161:88–97. doi: 10.1084/jem.161.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips N E, Parker D C. J Immunol. 1983;130:602–606. [PubMed] [Google Scholar]
- 40.Borretzen M, Randen I, Zdarsky E, Forre O, Natvig J B, Thompson K M. Proc Natl Acad Sci USA. 1994;91:12917–12921. doi: 10.1073/pnas.91.26.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]