Abstract
Five peptide fragments, based on the C-terminal sequence of bombesin (BN)-(6-14) or BN-(7-14), were selected as carriers for radicals doxorubicin (DOX) and 2-pyrrolino-DOX to create hybrid cytotoxic analogs. All these compounds had a reduced peptide bond (CH2-NH or CH2-N) between positions 13 (Phe or Leu) and 14 (Phe, Leu, or Tac) (Tac = thiazolidine-4-carboxylic acid). Three pseudononapeptide carriers contained N-terminal d-Phe or d-Tpi at position 6 (Tpi = 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid). Two pseudooctapeptides had Gln7 at the N terminus. The conjugation of N-(9-fluorenylmethoxycarbonyl)doxorubicin (N-Fmoc-DOX)-14-O-hemiglutarate to the peptide carriers at the N terminus resulted in cytotoxic hybrids of BN-like peptides containing DOX. These hybrids could then be converted to analogs with 2-pyrrolino-DOX by a reaction with 4-iodobutyraldehyde. The ability of the carriers and the conjugates to inhibit the binding of 125I-labeled [Tyr4]BN to receptors for BN/gastrin releasing peptide (GRP) on Swiss 3T3 cells was determined. Cytotoxic conjugates of pseudooctapeptide carrier analogs displayed the highest binding affinity (KD ≈1 nM). The cytotoxic BN analogs and their corresponding cytotoxic radicals exerted similar inhibitory effects on the in vitro growth of CFPAC-1 human pancreatic cancer, DMS-53 human lung cancer, PC-3 human prostate cancer, and MKN-45 human gastric cancer cell lines that have receptors for BN/GRP. In DMS-53 cells, the activity of 2-pyrrolino-DOX and its conjugates was ≈2500 times higher than that of DOX and its hybrids. These highly potent cytotoxic analogs of BN have been designed as targeted anti-tumor agents for the treatment of various cancers that possess receptors for BN/GRP.
Keywords: targeted chemotherapeutic agents, hybrid molecules, receptor binding, antiproliferative activity
Following the isolation of the tetradecapeptide bombesin (BN) from frog skin, a variety of its amphibian and mammalian homologs has been isolated and identified (1). This family of BN-like peptides includes gastrin-releasing peptide (GRP), a 27-amino acid peptide, which was considered to be the mammalian counterpart of BN, the amphibian ranatensin and neuromedin B, which was found in mammals, as well as the less known class of phyllolitorins (1). All these peptides have a highly conserved C terminus that is responsible for a wide variety of pharmacological effects, ranging from the release of gastrointestinal hormones to the effects on blood pressure, body temperature, and cardiac output (1). Various studies indicate that these peptides act as neuroregulatory hormones and growth factors in normal and neoplastic tissues (2) and exert their effects through binding to multiple receptors for the BN-like peptides. The receptors are located mainly in the central nervous system, in the digestive tract, and in other target organs such as the lung (2). Thus far, four different receptor subtypes for the BN family have been cloned and characterized. One of these receptors, found in large numbers (≈100,000 per cell) on the membranes of Swiss 3T3 murine fibroblasts, binds BN and GRP with a KD in the nanomolar range (1). Another receptor subtype binds peptides of the neuromedin B family with an affinity ≈100 times higher than those peptides that have a C terminal in common with BN and GRP (1). A third subtype shows a 100 times lower affinity for BN and neuromedin B than the receptors specific for these peptides (1, 3). The fourth known receptor subtype was characterized as having a higher affinity for BN than for GRP (4).
During the past decade, extensive evidence has been gathered on the involvement of peptides of the BN family in the mitogenesis of various tumor cells including small cell lung carcinoma (SCLC) (5, 6), cancers of the gastrointestinal tract such as pancreatic cancer (7), colon cancer (8), as well as breast cancer (9). The putative role of BN-like peptides as autocrine growth factors for these tumors (5–9) prompted researchers to design and synthesize antagonists of BN and GRP in hope of finding a different approach for the treatment of certain cancers (10–16). Over the past few years, we have developed a series of powerful BN antagonists (12–16). One of these antagonists (RC-3095 = B1, Table 1) showed promising tumor inhibitory effects in various animal cancer models and in nude mice bearing xenografts of human cancer cell lines and is presently undergoing clinical trials. Antitumoral effects of BN/GRP antagonists in vivo have been demonstrated on CFPAC-1 and SW-1990 human pancreatic cancers (17, 18), nitrosamine-induced pancreatic cancers in hamsters (19), H69 human SCLC (20), MKN45 and Hs746T human gastric cancers (21, 22), HT-29 human colon cancers (23, 24), PC-82, PC-3, and DU-145 human prostate cancers (25, 26), androgen independent Dunning R-3327-AT-1 rat prostate cancers (27), estrogen dependent and independent MXT mouse mammary cancers (28), MCF-7 MIII human breast cancer (29), and U-87MG and U-373MG human glioblastomas (30). Receptor analyses of these tumors showed the presence of high-affinity binding sites for 125I[Tyr4]BN (1, 2, 17–33). Recently, we described the synthesis and evaluation of cytotoxic analogs of luteinizing hormone-releasing hormone containing doxorubicin (DOX) or 2-pyrrolino-DOX, a derivative 500-1000 times more potent (34, 35). These cytotoxic analogs were developed for therapy of cancers that contain receptors for luteinizing hormone-releasing hormone (35). The presence of receptors for BN-like peptides on a wide variety of tumors (17–33), prompted us to use some of our powerful BN/GRP antagonists as carrier molecules for targeting cytotoxic agents to tumor cells.
Table 1.
Structures of cytotoxic BN analogs and carriers and their ability to displace [125I-Tyr4]BN binding to BN/GRP receptors on Swiss 3T3 cells
| Structure | Receptor binding Ki,* nM |
|---|---|
| B1 = [d-Tpi6,13 ψ14, CH2-NH, Leu14]BN-(6-14) | 3.5 |
| AN-253 = DOX-14-O-glt-B1 | 8.0 |
| AN-254 = 2-pyrrolino-DOX-14-O-glt-B1 | 13.0 |
| B2 = [d-Phe6,13 ψ14,CH2-NH, Phe14]BN-(6-14) | 4.3 |
| AN-246 = DOX-14-O-glt-B2 | 4.9 |
| AN-247 = 2-pyrrolino-DOX-14-O-glt-B2 | 8.6 |
| B3 = [d-Phe6,13 ψ14,CH2-N, Tac14]BN-(6-14) | 2.7 |
| AN-161 = DOX-14-O-glt-B3 | 2.9 |
| AN-257 = 2-pyrrolino-DOX-14-O-glt-B3 | 3.4 |
| B4 = [13 ψ14,CH2-NH, Leu14]BN-(7-14) | >1000 |
| AN-160 = DOX-14-O-glt-B4 | 0.95 |
| AN-215 = 2-pyrrolino-DOX-14-O-glt-B4 | 1.6 |
| B5 = [13 ψ14,CH2-N, Tac14]BN-(7-14) | >1000 |
| AN-251 = DOX-14-O-glt-B5 | 0.7 |
| AN-252 = 2-pyrrolino-DOX-14-O-glt-B5 | 0.6 |
| BN = pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp- | 2.0† |
| Ala-Val-Gly-His-Leu-Met-NH2 |
Tac, thiazolidine-4-carboxylic acid; Tpi, 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]-indole-3-carboxylic acid
Varying amounts of unlabeled peptide were used to determine the ability to displace [125I-Tyr4]BN binding; mean values of two or more independent tests (each performed in triplicate) are indicated (14).
Mean value of 12 independent tests.
In this paper we report the design, synthesis, and biological evaluation in vitro of cytotoxic BN analogs containing DOX and 2-pyrrolino-DOX (34, 35). The tests in vitro included the determination of the binding affinities to BN/GRP receptors on Swiss 3T3 murine fibroblasts and of the cytotoxic activities on CFPAC-1 human pancreatic cancer, DMS-53 human lung cancer, PC-3 human prostate cancer, and MKN-45 human gastric cancer cell lines.
MATERIALS AND METHODS
Synthesis.
Pseudononapeptide and pseudooctapeptide BN-like peptide carriers were synthesized as described (12–16). Cytotoxic conjugates of these peptides with DOX or 2-pyrrolino-DOX were prepared by an improvement of the procedure reported earlier for the formation of cytotoxic luteinizing hormone-releasing hormone conjugates (35).
Preparation of N-(9-fluorenylmethoxycarbonyl)-(N-Fmoc)-DOX-14-O-hemiglutarate.
N-Fmoc-DOX (35) (1.3 g, 1.7 mmol) was dissolved in 15 ml of anhydrous pyridine and 50 ml of N,N-dimethylformamide (DMF) was added. The pyridine was then evaporated in vacuo, the DMF solution was concentrated to 30 ml to eliminate traces of water, and glutaric anhydride (750 mg, 6.6 mmol) was added followed by N,N-diisopropylethylamine (592 μl, 3.4 mmol). After 4 hr the reaction mixture contained ≈75% of the desired end product. The DMF solution was then poured into 500 ml of 5% aqueous acetic acid (AcOH) (vol/vol) on an ice bath. The precipitate formed was filtered off and washed three times with 200 ml of distilled water. After drying in a desiccator, the 1.45 g crude solid was dissolved in 10 ml of CHCl3/AcOH (4:1, vol/vol) and applied on a column (2.5 × 30 cm), packed with 75 g of silicagel (Merck grade 9385; 230–400 mesh; pore size, 60 Å) equilibrated with CHCl3/AcOH (4:1, vol/vol). Flash chromatography using this solvent system resulted in good separation of the desired end product. On TLC aluminum sheets precoated with silicagel 60 F254 (Merck Art No. 5554), using CHCl3/AcOH (4:1, vol/vol) as eluent, the desired end product shows an Rf = 0.7, whereas the unreacted starting material runs at Rf = 0.5 and the diester derivative at Rf = 0.85. After combining the fractions containing pure material, the CHCl3 was evaporated and the AcOH was concentrated to 30 ml. This solution was poured into 200 ml of water on an ice bath. The resulting precipitate was filtered off and washed three times with 200 ml of water. After drying in a desiccator, 950 mg of 98% pure N-Fmoc-DOX-14-O-hemiglutarate was obtained, representing a 56% overall yield, starting from 1 g of DOX × HCl.
This pure N-Fmoc-DOX-14-O-hemiglutarate was used for the preparation of cytotoxic conjugates of BN containing DOX, with yields higher than 60% (35). Cytotoxic BN analogs with DOX were converted to their 2-pyrrolino-DOX derivatives by a reaction with a 30-fold excess of 4-iodobutyraldehyde in DMF (35).
Analytical HPLC.
A Beckman analytical HPLC system equipped with model 168 diode array detector and System Gold chromatography software (Beckman) was used to monitor the chemical reactions and check the purity. The column used was a Dynamax C18 (250 × 4.6 mm; pore size, 300 Å; particle size, 12 μm).
Purification.
Final purification of all peptide conjugates was carried out on a Beckman model 342 semipreparative HPLC system, using an Aquapore Octyl (250 × 10 mm; pore size, 300 Å; particle size, 15 μm) column. The solvent system consisted of two components—0.1% trifluoroacetic acid in water and 0.1% trifluoroacetic acid in 70% aqueous acetonitrile—and was used in linear gradient mode.
Analysis.
Electrospray mass spectrometer Finnigan-MAT TSQ 7000 was used for the structural identification of the peptide conjugates.
Receptor Binding.
Binding affinities of the analogs to receptors for BN/GRP on Swiss 3T3 cells were determined as described (14, 16, 32).
Cytotoxicity Assay.
CFPAC-1 human pancreatic cancer, DMS-53 human SCLC, PC-3 human prostate cancer, and MKN-45 human gastric cancer cell lines were obtained from the American Type Culture Collection. These cells were cultured in media indicated in the footnotes to Tables 2 and 3. DOX, 2-pyrrolino-DOX and the cytotoxic BN/GRP analogs were dissolved in culture media and added at three different concentrations, as shown in detail in Tables 2 and 3. The determination of the cytotoxic activity of the analogs on all four cell lines was performed by using a colorimetric cytotoxicity assay in microtitration plates based on quantification of biomass by staining cells with crystal violet (36).
Table 2.
Inhibitory effects of DOX, 2-pyrrolino-DOX (AN-201), and their conjugates with BN-like peptide carriers on the growth of CFPAC-1 human pancreatic cancer cell line and on DMS-53 human lung cancer cell line in vitro
| Compound | Cell line | T/C Value
|
||||||
|---|---|---|---|---|---|---|---|---|
| 3 × 10−11 M | 10−10 M | 3 × 10−10 M | 10−9 M | 3 × 10−8 M | 10−7 M | 3 × 10−7 M | ||
| Analogs with DOX | ||||||||
| AN-253 | CFPAC-1 | 79 (32) | 18 (−7) | 6 (−7) | ||||
| DMS-53 | 67 (−12) | 33 (−15) | 6 (−15) | |||||
| AN-246 | CFPAC-1 | 89 | 28 | 6 | ||||
| DMS-53 | 70 | 23 | 2 | |||||
| AN-161 | CFPAC-1 | 95 | 35 | 10 | ||||
| DMS-53 | 80 | 39 | 5 | |||||
| AN-160 | CFPAC-1 | 85 | 22 | 0 | ||||
| DMS-53 | 68 | 24 | 0 | |||||
| AN-251 | CFPAC-1 | 88 | 26 | −2 | ||||
| DMS-53 | 60 | 3 | −12 | |||||
| DOX | CFPAC-1 | 68 | 22 | 4 | ||||
| DMS-53 | 78 | 27 | 1 | |||||
| Analogs with AN-201 | ||||||||
| AN-254 | CFPAC-1 | 95 | 13 | −7 | ||||
| DMS-53 | 45 | −6 | −15 | |||||
| AN-247 | CFPAC-1 | 90 | 27 | −7 | ||||
| DMS-53 | 61 | 4 | −14 | |||||
| AN-257 | CFPAC-1 | 85 | 16 | −7 | ||||
| DMS-53 | 58 | 0 | −18 | |||||
| AN-215 | CFPAC-1 | 97 | 42 | −7 | ||||
| DMS-53 | 52 | 2 | −15 | |||||
| AN-252 | CFPAC-1 | 91 | 56 | −6 | ||||
| DMS-53 | 72 | 13 | −19 | |||||
| AN-201 | CFPAC-1 | 76 | 8 | −5 | ||||
| DMS-53 | 34 | −7 | −19 | |||||
CFPAC-1 cells were incubated in Iscove’s modified Dulbecco’s medium with 10% fetal bovine serum (FBS) for 120 hr and DMS-53 cells were incubated for 140 hr in Waymouth’s MB 752/1 medium containing 10% FBS in 96-well plates. Relative cell number in treated and control plates was determined by crystal violet staining and expressed as T/C values where T/C = (T − C0)/(C − C0) × 100. [T = absorbance of treated cultures, C = absorbance of control cultures, C0 = absorbance of cultures at the start of incubation (t = 0). Measured absorbance is proportionate to cell number.] Negative T/C values indicate a cell number smaller than the number originally seeded at t = 0—i.e., a cytocidal effect. The structures of the compounds are shown in Table 1. The carrier peptides had no effect on cell proliferation at 10−7 M and lower concentrations. T/C values in brackets are derived from results with a sample of AN-253 containing decomposition products.
Table 3.
Inhibition of growth of CFPAC-1 human pancreatic cancer, DMS-53 human SCLC, PC-3 human prostate cancer, and MKN-45 human gastric cancer cell lines by DOX, 2-pyrrolino-DOX (AN-201), and the corresponding cytotoxic BN analogs
| Compound | IC50,* 10−10 M
|
|||
|---|---|---|---|---|
| CFPAC-1 at 120 hr | DMS-53 at 140 hr | PC-3 at 72 hr | MKN-45 at 115 hr | |
| Analogs with DOX | ||||
| AN-253 | 530 (180)† | 640 (≪300) | 2100 (760) | 2700 (500) |
| AN-246 | 650 | 490 | 2700 | 3600† |
| AN-161 | 760 | 780 | 3500† | 5100† |
| AN-160 | 580 | 530 | 3200† | 2300 |
| AN-251 | 630 | 370 | 2600 | 2000 |
| DOX | 570 | 580 | 1500 | 1800 |
| Analogs with AN-201 | ||||
| AN-254 | 1.8 | 0.33 | 6.1 | 2.1 |
| AN-247 | 2.0 | 0.37 | 6.8 | 2.9 |
| AN-257 | 1.7 | 0.35 | 6.7 | 2.1 |
| AN-215 | 2.7 | 0.41 | 6.8 | 2.4 |
| AN-252 | 3.5 | 0.46 | 13.0† | 3.7 |
| AN-201 | 1.6 | 0.22† | 3.6 | 1.5 |
Cell growth inhibition data, determined at three different concentrations as shown in Table 2, were used to calculate the drug concentration that inhibited cell growth by 50%, as compared with untreated control cultures. All data were derived from an average of three determinations each in eight replicates. CFPAC-1 and DMS-53 cells were grown under conditions described in Table 2. PC-3 cells were incubated in RPMI 1640:F12 (1:1) medium containing 1 mM pyruvate/1 μM FeSO4/0.5% bovine serum albumin. MKN-45 cells were incubated in Dulbecco’s modified Eagle medium containing 10% FBS.
Values calculated by extrapolation. IC50 values in brackets are derived from results with a sample of AN-253 containing decomposition products.
RESULTS
Design and Synthesis.
To create targeted cytotoxic analogs of BN/GRP with specific high-affinity binding to BN/GRP receptors, three pseudononapeptide BN antagonists and two pseudooctapeptide BN-like peptides were selected as carriers. The chemical structures of these carriers were based on the C-terminal sequence of BN (Table 1). To form cytotoxic analogs containing DOX, the peptides were acylated at their N terminus with N-Fmoc-DOX-14-O-hemiglutarate as described (35). In our previous effort to synthesize cytotoxic analogs of luteinizing hormone-releasing hormone containing DOX, N-Fmoc-DOX-14-O-hemiglutarate was used in a crude form (35). In this study, we used an effective purification procedure based on silica-gel chromatography with CHCl3/AcOH (4:1, vol/vol) as eluent for the separation of N-Fmoc-DOX-14-O-hemiglutarate from impurities such as N-Fmoc-DOX (unreacted starting material) and its diester that is formed because of the presence of an excess of glutaric anhydride. This improvement in the preparation and purification of N-Fmoc-DOX-14-O-hemiglutarate resulted in ≈10% higher yields in the conjugation step in comparison with our previous results (35). Cytotoxic BN analogs containing DOX were obtained after cleavage of the Fmoc protecting group. These conjugates were then converted to derivatives with 2-pyrrolino-DOX by reaction with an excess of 4-iodobutyraldehyde (34, 35).
Receptor Binding Affinity.
The carrier peptides and their cytotoxic analogs containing DOX and 2-pyrrolino-DOX were tested for their ability to displace the binding of [125I-Tyr4]BN to BN/GRP receptors on Swiss 3T3 cells. As shown in Table 1, the deletion of the hydrophobic d-amino acids such as d-Phe or d-Tpi from position 6 of carriers B1 and B2, respectively, resulted in analogs (B4 and B5) that displayed a severe loss of binding affinity. Conjugation of the bulky cytotoxic radicals, containing a very hydrophobic anthracycline moiety, to these shortened carriers led to the formation of analogs with high-binding affinity to BN/GRP receptors on Swiss 3T3 cells. Cytotoxic derivatives of BN antagonist carriers B2 and B3, containing d-Phe at position 6, virtually preserved the binding affinity of the carriers, which is in the nanomolar range. However, in the case of carrier B1 containing d-Tpi at the amino terminus, the attachment of a bulky radical reduced the binding. The binding affinity of AN-254 (2-pyrrolino-DOX-14-O-hemiglutarate linked to B1), was ≈4 times lower than that of the carrier (Table 1).
Cytotoxicity.
Antiproliferative activities of the cytotoxic hybrid molecules and their corresponding cytotoxic radicals were compared on CFPAC-1 human pancreatic cancer, DMS-53 human SCLC, PC-3 human prostate cancer, and MKN-45 human gastric cancer cell lines in vitro (Tables 2 and 3). The results indicate that the cytotoxic activity of the antineoplastic radicals was virtually preserved in most of the conjugates, the distinct structures showing small variations in their effect on different cell lines. A very high antiproliferative activity of 2-pyrrolino-DOX (AN-201) and its peptide conjugates was observed on DMS-53 cells. As shown in Table 3, AN-201 is ≈2500 times more effective in this cell line than DOX. One of the hybrid analogs, AN-253, consisting of DOX linked to [d-Tpi6, 13ψ14,CH2-NH, Leu14]BN-(6-14) (Fig. 1), showed 2–3 times higher antiproliferative activity than DOX when tested after 4 months of storage in a lyophilized form. These data are displayed in brackets in Table 3. The increased activity was found to be due to decomposition products. Freshly purified AN-253 had a similar activity to DOX. AN-254 consisting of 2-pyrrolino-DOX linked to [d-Tpi6, 13ψ14,CH2-NH, Leu14]BN-(6-14) showed a similar instability, but the cytotoxic activity of freshly purified AN-254 did not differ from that of an 80% pure sample. Other hybrid analogs were found to be stable under the same storage conditions.
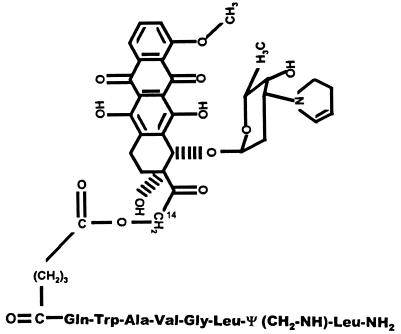
Figure 1.
Molecular structure of cytotoxic BN analog AN-215. 2-Pyrrolino-DOX-14-0-hemiglutarate is linked to the N terminal of [13Ψ14,CH2-NH, Leu14]BN-(7-14).
DISCUSSION
Chemotherapeutic agents play a major role in the management of various cancers in spite of the frequent severe toxic side effects caused by their nonselective action during systemic administration. One of the approaches aimed at improving the selectivity and reducing the toxicity of antitumor agents is drug targeting, which takes advantage of specific receptors for biologically active peptides or macromolecules on the cell membrane of cancerous cells (35, 37). BN-like peptides have properties of hormones or growth factors and are responsible for a wide variety of receptor-mediated pharmacological effects (1). Accordingly, receptors for BN-like peptides are present on normal, nonmalignant cells in the digestive tract, the central nervous system and other target organs such as the lung (1, 2, 10). Investigation of the role of BN-like peptides in the mitogenesis of various cancers revealed that high-affinity binding sites for these peptide hormones are also expressed on a wide variety of human and experimental animal tumors (1–10, 17–32). A recent study also indicates that on certain cancers, such as azaserine-induced pancreatic carcinoma in the rat, high-affinity GRP receptors are present in significantly higher numbers than on the normal pancreas (38). Thus, BN/GRP analogs, by virtue of binding to these receptors, may be used for the design of targeted cytotoxic conjugates. The hybrid molecules must preserve both the antineoplastic and specific binding character of their respective components. To create targeted BN-like cytotoxic agents, we linked DOX-14-O-hemiglutarate to the N terminal of pseudooctapeptide BN-(7-14) and pseudononapeptide BN-(6-14) analogs previously developed at our institute. The pseudooctapeptide carriers lack the bulky hydrophobic d-amino acids such as d-Phe or d-Tpi at position 6 of BN-(6-14) analogs. As expected, these shortened analogs exhibit no binding to BN/GRP receptors on Swiss 3T3 cells. As shown in Table 1, attachment of a bulky hydrophobic cytotoxic radical to the N terminals of these analogs leads to the formation of cytotoxic BN derivatives with high-binding affinity to BN/GRP receptors. These data show that replacement of the d-amino acids with hydrophobic acids at position 6 of BN-(6-14) analogs can result in BN-(7-14) derivatives with increased binding affinity. Cytotoxic conjugates of pseudononapeptide BN-(6-14) carriers, containing d-Phe at position 6, have high-binding affinity to BN/GRP receptors (Table 1), indicating a tolerance for substitution with bulky groups at the N terminal of these peptides. However, this bulk tolerance of the BN antagonists at the N terminus was not so apparent in the case of carrier B1 containing d-Tpi at position 6, which is larger than d-Phe.
Antiproliferative activity of the cytotoxic radicals is well preserved in the BN conjugates. Small variations were observed in the cytotoxic activities of different analogs, as compared with the respective cytotoxic radicals incorporated on the four cell lines tested. For instance, DOX showed an activity ≈50% higher on PC-3 prostate cancer cell line than its BN conjugate AN-251, but AN-251 was approximately twice as active on DMS-53 SCLC than DOX (Table 3). Such variations could be due to different binding affinities of the same hybrid analog to receptors on the different cell lines. A sample of AN-253, containing DOX linked to an N-terminal d-Tpi, was ≈2-3 times more potent than DOX on four cancer cell lines when tested after 4 months of storage as a lyophilizate (Tables 2 and 3, values in brackets). A purity check of this sample by HPLC revealed the presence of several decomposition products. Because a freshly purified sample of AN-253 had similar or even lower antiproliferative effect than DOX, one or more of the decomposition products must be responsible for this increased cytotoxic activity. A plausible explanation of this finding can be given by considering the following. Tpi is formed by Pictet–Spengler condensation of Trp with one equivalent of formaldehyde in dilute acid (39). The less stable N-acyl-d-Tpi, present in AN-253, can be expected to undergo decomposition to yield formaldehyde and N-acyl-d-Trp. Because vicinal amino alcohols readily react with aldehydes, the amino alcohol function of the daunosamine moiety of DOX would entrap the formaldehyde generated by the N-acyl-d-Tpi moiety. Such a byproduct might have a much higher antiproliferative activity than DOX, due to its ability to alkylate a nucleophyl at the intercalation site as clearly demonstrated by Gao et al (40). Decomposition products of AN-254 (2-pyrrolino-DOX linked to d-Tpi) could not have increased potency, as compared with the pure product, because 2-pyrrolino-DOX is a latent aldehyde derivative of DOX with increased cytotoxicity. A very high antiproliferative activity of 2-pyrrolino-DOX (AN-201) and its peptide conjugates was observed on DMS-53 SCLC cells. As shown in Table 3, AN-201 is ≈2500 times more active in this cell line than DOX. This great difference in the activity of DOX and its daunosamine-modified derivative is very interesting, because it is not caused by the resistance of DMS-53 to DOX. In fact, of the four cell lines tested, DMS-53 was the most sensitive to DOX (Tables 2 and 3). A high activity of AN-201 suggests that 2-pyrrolino-DOX and its cytotoxic BN conjugates could be used in preference to DOX or its analogs for the treatment of cancers such as SCLC typified by DMS-53.
Preliminary in vivo experiments on nitrosamine-induced pancreatic cancers in golden hamsters indicated that both cytotoxic BN analog AN-215 and cytotoxic radical AN-201 have significant antitumor activity in this experimental model. Nevertheless, in one of these pilot experiments, 16 of 18 hamsters died after intraperitoneal administration of a total dose of 100 nmol/kg of cytotoxic radical AN-201 by the 5th week after the last injection. Only 5 of 18 animals died in the group treated with the same dose of AN-215 and 5 of 20 hamsters in the untreated control group. This indicates that the BN hybrid analog AN-215 has lower toxicity than the unconjugated cytotoxic radical.
In conclusion, our in vitro studies indicate that BN/GRP analogs linked to DOX or its 2-pyrrolino derivative have high cytotoxic activity. However, additional extensive investigations in vivo are required on pancreatic, lung, prostate, gastric, and brain cancer models that possess receptors for BN/GRP, to evaluate the efficacy of these targeted cytotoxic BN analogs.
Acknowledgments
We thank Prof. J. Engel, Dr. M. Bernd, Dr. E. Busker (Degussa AG and Asta Medica AG, Frankfurt am Main) for mass spectra analyses and for their help in preparation of this manuscript. Some work described in this paper was supported by the Medical Research Service of the Veterans Affairs.
Footnotes
Abbreviations: DOX, doxorubicin; BN, bombesin; Tpi, 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid; GRP, gastrin-releasing peptide; SCLC, small cell lung carcinoma.
References
- 1.Spindel E R, Giladi E, Segerson T P, Nagalla S. Rec Prog Horm Res. 1993;48:365–391. doi: 10.1016/b978-0-12-571148-7.50017-8. [DOI] [PubMed] [Google Scholar]
- 2.Sunday M E, Kaplan L M, Motoyama E, Chin W W, Spindel E R. Lab Invest. 1988;59:5–23. [PubMed] [Google Scholar]
- 3.Fathi Z, Corjay M H, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville E A, Battey J F. J Biol Chem. 1993;268:5979–5984. [PubMed] [Google Scholar]
- 4.Nagalla S R, Barry B J, Creswick K C, Eden P, Taylor J T, Spindel E R. Proc Natl Acad Sci USA. 1995;92:6205–6209. doi: 10.1073/pnas.92.13.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuttitta F, Carney D N, Mulshine J, Moody T W, Fedorko J, Fischler A, Minna J D. Nature (London) 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 6.Alexander R W, Upp J R, Poston G J, Gupta V, Townsend C M, Thompson J C. Cancer Res. 1988;48:1439–1441. [PubMed] [Google Scholar]
- 7.Hajri A, Balboni G, Koenig M, Garaud J C, Damgé C. Cancer Res. 1992;52:3726–3732. [PubMed] [Google Scholar]
- 8.Narayan S, Guo Y S, Townsend C M, Singh P. Cancer Res. 1990;50:6772–6778. [PubMed] [Google Scholar]
- 9.Yano T, Pinski J, Groot K, Schally A V. Cancer Res. 1992;52:4545–4547. [PubMed] [Google Scholar]
- 10.Thomas F, Mormont C, Morgan B. Drugs Future. 1994;19:349–359. [Google Scholar]
- 11.Jensen R T, Coy D H. Trends Pharmacol Sci. 1991;12:13–19. doi: 10.1016/0165-6147(91)90483-9. [DOI] [PubMed] [Google Scholar]
- 12.Radulovic S, Cai R-Z, Serfozo P, Groot K, Redding T W, Pinski J, Schally A V. Int J Peptide Protein Res. 1991;38:593–600. doi: 10.1111/j.1399-3011.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 13.Cai R-Z, Radulovic S, Pinski J, Nagy A, Redding T W, Olsen D B, Schally A V. Peptides. 1992;13:267–271. doi: 10.1016/0196-9781(92)90107-e. [DOI] [PubMed] [Google Scholar]
- 14.Cai R-Z, Reile H, Armatis P, Schally A V. Proc Natl Acad Sci USA. 1994;91:12664–12668. doi: 10.1073/pnas.91.26.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai R-Z, Qin Y, Ertl T, Schally A V. Int J Oncol. 1995;6:1165–1172. doi: 10.3892/ijo.6.6.1165. [DOI] [PubMed] [Google Scholar]
- 16.Reile H, Cai R-Z, Armatis P, Schally A V. Int J Oncol. 1995;7:749–754. doi: 10.3892/ijo.7.4.749. [DOI] [PubMed] [Google Scholar]
- 17.Qin Y, Ertl T, Cai R-Z, Halmos G, Schally A V. Cancer Res. 1994;54:1035–1041. [PubMed] [Google Scholar]
- 18.Qin Y, Ertl T, Cai R-Z, Horvath J E, Groot K, Schally A V. Int J Cancer. 1995;63:257–262. doi: 10.1002/ijc.2910630219. [DOI] [PubMed] [Google Scholar]
- 19.Szepeshazi K, Schally A V, Groot K, Halmos G. Int J Cancer. 1993;54:282–289. doi: 10.1002/ijc.2910540220. [DOI] [PubMed] [Google Scholar]
- 20.Pinski J, Schally A V, Halmos G, Szepeshazi K, Groot K, O’Byrne K, Cai R-Z. Br J Cancer. 1994;70:886–892. doi: 10.1038/bjc.1994.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinski J, Halmos G, Yano T, Szepeshazi K, Qin Y, Ertl T, Schally A V. Int J Cancer. 1994;57:574–580. doi: 10.1002/ijc.2910570422. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Halmos G, Cai R-Z, Szoke B, Ertl T, Schally A V. Cancer Res Clin Oncol. 1994;120:519–528. doi: 10.1007/BF01221028. [DOI] [PubMed] [Google Scholar]
- 23.Radulovic S, Miller G, Schally A V. Cancer Res. 1991;51:6006–6009. [PubMed] [Google Scholar]
- 24.Radulovic S, Schally A V, Reile H, Halmos G, Szepeshazi K, Groot K, Milovanovic S, Miller G, Yano T. Acta Oncol. 1994;33:693–701. doi: 10.3109/02841869409121784. [DOI] [PubMed] [Google Scholar]
- 25.Milovanovic S R, Radulovic S, Groot K, Schally A V. Prostate. 1992;20:269–280. doi: 10.1002/pros.2990200403. [DOI] [PubMed] [Google Scholar]
- 26.Pinski J, Halmos G, Schally A V. Cancer Lett. 1993;71:189–196. doi: 10.1016/0304-3835(93)90115-p. [DOI] [PubMed] [Google Scholar]
- 27.Pinski J, Reile H, Halmos G, Groot K, Schally A V. Cancer Res. 1994;54:169–174. [PubMed] [Google Scholar]
- 28.Szepeshazi K, Schally A V, Halmos G, Groot K, Radulovic S. J Natl Cancer Inst. 1992;84:1915–1922. doi: 10.1093/jnci/84.24.1915. [DOI] [PubMed] [Google Scholar]
- 29.Shirahige Y, Cai R-Z, Szepeshazi K, Halmos G, Pinski J, Groot K, Schally A V. Biomed Pharmacother. 1994;48:465–472. doi: 10.1016/0753-3322(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 30.Pinski J, Schally A V, Halmos G, Szepeshazi K, Groot K. Cancer Res. 1994;54:5895–5901. [PubMed] [Google Scholar]
- 31.Reile H, Armatis P E, Schally A V. Prostate. 1994;25:29–38. doi: 10.1002/pros.2990250105. [DOI] [PubMed] [Google Scholar]
- 32.Halmos G, Pinski J, Szoke B, Schally A V. Cancer Lett. 1994;85:111–118. doi: 10.1016/0304-3835(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 33.Halmos G, Wittliff J L, Schally A V. Cancer Res. 1995;55:280–287. [PubMed] [Google Scholar]
- 34.Nagy A, Armatis P, Schally A V. Proc Natl Acad Sci USA. 1996;93:2464–2469. doi: 10.1073/pnas.93.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagy A, Schally A V, Armatis P, Szepeshazi K, Halmos G, Kovacs M, Zarandi M, Groot K, Miyazaki M, Jungwirth A, Horvath J. Proc Natl Acad Sci USA. 1996;93:7269–7273. doi: 10.1073/pnas.93.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reile H, Birnböck H, Bernhardt G, Spruss T, Schönenberger H. Anal Biochem. 1990;187:262–267. doi: 10.1016/0003-2697(90)90454-h. [DOI] [PubMed] [Google Scholar]
- 37.Schally A V, Nagy A, Szepeshazi K, Pinski J, Halmos G, Armatis P, Miyazaki M, Comaru-Schally A-M, Yano T, Emons G. In: Treatment with GnRH Analogs: Controversies and Perspectives. Filicori M, Flamigni C, editors. Carnforth, U.K.: Parthenon; 1996. pp. 33–44. [Google Scholar]
- 38.Hajri A, Koenig M, Balboni G, Damgé C. Pancreas. 1996;12:25–35. doi: 10.1097/00006676-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Brossi A, Focella A, Teital S. J Med Chem. 1973;16:418–425. doi: 10.1021/jm00262a027. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y-G, Liaw Y-C, Li Y-K, van der Marel G A, van Boom J H, Wang A H-J. Proc Natl Acad Sci USA. 1991;88:4845–4849. doi: 10.1073/pnas.88.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]