Abstract
Nonsteroidal antiinflammatory drugs reduce the risk of colon cancer, possibly via cyclooxygenase (COX) inhibition. The growth factor-inducible COX-2, which is overexpressed in neoplastic colonic tissue, is an attractive target to mediate this effect. Herein we have exploited the ability of a human colon cancer cell line, HCA-7 Colony 29, to polarize when cultured on Transwell (Costar) filters to study COX-2 production and the vectorial release of prostaglandins (PGs). Administration of type α transforming growth factor to the basolateral compartment, in which the epidermal growth factor receptor (EGFR) resides, results in a marked induction of COX-2 immunoreactivity at the base of the cells and the unexpected appearance of COX-2 in the nucleus. The increase in COX-2 protein is associated with a dose- and time-dependent increase in PG levels in the basolateral, but not apical, medium. Amphiregulin is the most abundantly expressed EGFR ligand in these cells, and the protein is present at the basolateral surface. EGFR blockade reduces baseline COX-2 immunoreactivity, PG levels, and mitogenesis in a concentration-dependent manner. Two specific COX-2 inhibitors, SC-58125 and NS 398, also, in a dose-dependent manner, attenuate baseline and type α transforming growth factor-stimulated mitogenesis, although PG levels are decreased >90% at all concentrations of inhibitor tested. These findings show that activation of the EGFR stimulates COX-2 production and its translocation to the nucleus, vectorial release of PGs, and mitogenesis in polarized HCA-7 Colony 29 cells.
In the gastrointestinal tract, prostaglandins (PGs) mediate important functions, including motility, vascular tone, angiogenesis, mucosal protection, and immune responsiveness (1). Inasmuch as epithelial cells are capable of PG synthesis, it is feasible that PGs synthesized in the gastrointestinal epithelium regulate these functions by paracrine pathways in response to luminal or serosal stimuli. Although data exist in support of vectorial release of PGs in the isolated rat colon (2), as well as in other tissues and polarized kidney-derived cells (3–5), regulatory mechanisms have not been defined more precisely.
Cyclooxygenases (COXs) are key enzymes in the conversion of arachidonic acid (AA) to PGs and other eicosanoids. Two isoforms of the enzyme have been characterized. COX-1 in most cells is expressed constitutively, and a second inducible form known as COX-2 has been identified (refs. 6–8; for review see ref. 9). Recent observations indicate that many colonic polyps and cancers overexpress COX-2 (10–12) and that inhibition of this enzyme by nonsteroidal antiinflammatory drugs decreases the risk of colonic neoplasia (13–20), emphasizing the importance of defining potential autocrine and paracrine pathways for regulation of gastrointestinal epithelial growth by COX. Signaling through the epidermal growth factor receptor (EGFR) induces COX-2 expression, and unregulated overexpression of COX-2 results in a tumorigenic phenotype in the rat intestinal epithelial cell line, RIE-1 (21, 22), suggesting that growth factor and COX-related signaling pathways interact to regulate cellular proliferation.
Epithelial cells in vivo polarize with discrete apical and basolateral compartments. A limited number of epithelial cells polarize in vitro when cultured on Transwell (Costar) filters. We have identified a polarizing human colon cancer cell line, HCA-7 Colony 29, that has allowed us to examine the vectorial trafficking and release of biomolecules in vitro (ref. 23; R.J.C., unpublished data). Because of the accumulating data that COX-2-mediated PG production may be involved in colorectal tumorigenesis, we have examined spatial regulation of COX-2 and its products by the EGFR axis in the context of epithelial cell polarity. In polarized HCA-7 cells, EGFR activation leads to induction of COX-2 protein, which translocates to the nucleus, the release of PGs exclusively into the basolateral medium, and increased mitogenesis. Blockade of the basolateral EGFR results in decreased COX-2 immunoreactivity, basolateral PG levels, and mitogenesis; endogenous amphiregulin (AR) is the EGFR ligand most likely to be mediating these EGFR-related events, based on its relative abundance and basolateral localization. Interestingly, two specific COX-2 inhibitors, SC-58125 and NS 398, decrease baseline and type α transforming growth factor (TGFα)-stimulated mitogenesis, but this decrease does not correlate with reductions in PG levels, nor can administration of the two most abundant PGs produced by these cells (PGE2 and PGF2α) restore mitogenesis in SC-58125-treated HCA-7 cells.
MATERIALS AND METHODS
Reagents.
All culture reagents were purchased from GIBCO, and all chemicals were purchased from Sigma, unless otherwise indicated. Monoclonal antibodies to human EGFR (mAb 528) and human AR (6RIC2.4) were generously provided by Hideo Masui (Memorial Sloan–Kettering Cancer Center, New York) and Greg Plowman and Barbara Thorne (Bristol-Myers Squibb Pharmaceutical Research Institute, Seattle), respectively. COX-1 and COX-2 antibodies were purchased from Cayman Chemicals (Ann Arbor, MI). SC-58125 was generously provided by Peter Isakson (Searle). Sulindac sulfide and NS 398 were purchased from Merck Sharp & Dohme and Cayman Chemicals, respectively.
Cell Culture.
HCA-7 cells, passage 20–35, were grown in DMEM supplemented with 10% fetal calf serum (Intergen, Purchase, NY), glutamine, nonessential amino acids, 100 units/ml penicillin, and 100 μg/ml streptomycin in a 5% CO2 atmosphere with constant humidity at 37°C. HCA-7 cells were cultured on 12-mm Transwell filters (pore size 0.4 μM). Transepithelial electrical resistance across the Transwell filter was measured using a Millicell Electrical Resistance System (Millipore) to evaluate the fidelity of tight junctions. Experiments were conducted 7–10 days postconfluence, when the resistance was >400 Ω·cm2. RIE-1 cells were cultured on plastic in DMEM with 10% fetal calf serum, as previously described (24).
PG Analysis.
Eicosanoids were quantified by gas chromatographic/negative ion chemical ionization mass spectrometric assays using stable isotope dilution techniques (25).
Immunofluorescence of COX-2 and AR.
HCA-7 cells were grown on Transwell filters and serum-deprived for 24 h and either immediately fixed or treated with TGFα (10 ng/ml) for 24 or 48 h, with or without pretreatment with mAb 528 (0.6–15 μg/ml), before fixation in 2.0% paraformaldehyde/PBS on ice for 30 min. Cells were then washed in PBS plus 0.2% BSA and treated with 50 mM ammonium chloride/PBS for 10 min, permeabilized with 0.2% saponin/PBS for 20 min, and blocked with 5% normal donkey serum plus 1.0% BSA for 30 min. In some sequential immunostaining experiments, AR monoclonal antibody (6RIC2.4) was incubated with cells for 1 h before the fixation step. Following blocking, cells were incubated overnight at 4°C in 1:500 dilution of rabbit anti-human COX-2. After they were washed in PBS/BSA, cells were incubated in a 1:1000 dilution of donkey anti-rabbit IgG–CY3 and a 1:500 dilution of donkey anti-mouse IgG–CY5 conjugate (Jackson ImmunoResearch) for 1 h. Nuclei were counterstained with a 1:5000 dilution of YO-PRO1 (Molecular Probes). Transwell membranes were excised and mounted on a microslide in Aqua-Poly/Mount (Polysciences). Confocal images of xy and xz planes were acquired on a Zeiss confocal microscope running Zeiss lsm version 3.56β software and assembled and formatted on a Silicon Graphics Indigo workstation.
Northern Blot Analysis.
HCA-7 cells on Transwell filters were rinsed once in PBS (pH 7.5; containing 1 mM MgCl2 and 0.1 mM CaCl2) and incubated with 10 and 5 ml of lysis buffer (10 mM Tris·HCl, pH 7.6/100 mM NaCl/2 mM EDTA/0.5% SDS) in the apical and basal compartment, respectively. After gentle rocking for 15 min at room temperature, the lysate was transferred to a 50-ml conical tube and both sides of the filter were scraped. Oligo(dT)-selected mRNA was separated on a 1.0% agarose/formaldehyde gel and transferred to nitrocellulose filters (Micron Separations), as described previously (26). Hybridization with a 32P-labeled human AR cRNA probe was performed, as described previously (27). As a control for loading and transfer, the blot was probed with 1B15 (cyclophillin; ref. 28).
Western Blot Analysis.
Western blotting was performed as described previously (21), using a rabbit anti-mouse COX-2 antibody (Oxford Biomedical Research, Oxford, MI) at a 1:200 dilution.
RESULTS
Initially, we found that nonpolarized HCA-7 cells cultured on plastic in serum-containing conditions produce large amounts of eicosanoids compared with nontransformed RIE-1 cells grown in a similar manner. In addition, the eicosanoid profiles differed significantly between the two cell lines (Fig. 1A). In all subsequent studies, PGE2 levels were assayed as an index of eicosanoid generation.
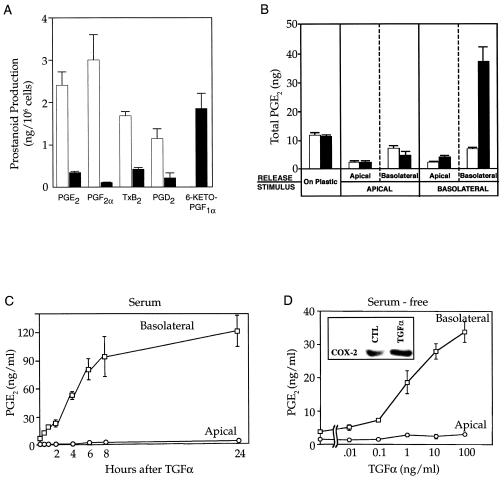
Figure 1.
Eicosanoid production by HCA-7 and RIE-1 cells. (A) Rapidly growing HCA-7 (open bars) and RIE-1 (filled bars) cells cultured on plastic in 10% fetal calf serum were stimulated by AA (20 μM) for 30 min before harvest, and prostanoid levels in the medium were quantified by gas chromatography/negative ion chemical ionization mass spectrometry using stable isotope dilution techniques (25). (B) HCA-7 cells, cultured as a flat monolayer on plastic or as a polarizing monolayer on Transwell filters in 10% fetal calf serum, were exposed to TGFα (100 ng/ml; filled bars) or diluent (open bars). A 30-min pulse of AA (20 μM) was given 7.5 h later, and PGE2 in the medium was measured. (C) TGFα (10 ng/ml) was added to the basal medium of HCA-7 cells cultured in 10% fetal calf serum on Transwell filters. Thirty minutes before each time point, AA (20 μM) was added and PGE2 was measured in the apical or basolateral medium. (D) TGFα at varying concentrations was added to the basal medium of HCA-7 cells that had been serum-free for 48 h. A 30-min pulse of AA (20 μM) was given 7.5 h later, and PGE2 in the apical or basolateral medium was measured. (Inset) COX-2 immunoblot of HCA-7 cells grown on Transwell filters that had been serum-free for 48 h and then treated with TGFα (10 ng/ml) for 24 h. TGFα stimulated PGE2 release, following addition of exogenous AA (20 μM) and endogenous stores of AA.
HCA-7 cells form polarizing monolayers with functional tight junctions and target EGFR to the basolateral membrane when grown on Transwell filters (ref. 23; R.J.C., unpublished data). We took advantage of this property to examine the vectorial release of PGE2 by polarizing HCA-7 cells. The stimulus consisted of diluent or TGFα (100 ng/ml) added to the apical or basolateral compartment of polarized HCA-7 cells in serum-containing conditions, and PGE2 levels were measured in the apical or basolateral medium (Fig. 1B). Only when TGFα was added to the basolateral compartment was there a significant increase (≈7-fold) in PGE2 levels. Moreover, these increased levels of PGE2 were confined to the basolateral medium. PGE2 levels were then measured in the apical or basolateral medium over time following addition of 100 ng/ml TGFα in serum (Fig. 1C). PGE2 levels increased at 30 min and continued to increase at 24 h in the basolateral, but not apical, medium. Under serum-free conditions, there was a dose-dependent increase in PGE2 levels only in the basolateral medium 8 h after TGFα administration (Fig. 1D). To establish that accumulation of PGs in the basolateral compartment occurred as a result of increased synthesis and release of PG, [3H]PGF2α (0.4 μCi/ml; 1 Ci = 37 GBq) was added selectively to the apical or basolateral medium, and the HCA-7 cells were incubated for 8 h with either no factor or with TGFα (10 ng/ml), and radioactivity was measured in both compartments. The lack of any detectable change in concentrations of [3H]PG in the two compartments indicates that the increased levels of PGs found in the basolateral medium are due to increased synthesis and release.
Release of PGE2 into the basolateral compartment increased over time, beginning at ≈1 h (Fig. 1C), consistent with a mechanism involving induction of enzyme synthesis. Experiments were conducted to identify whether COX-1 or COX-2 increased PG synthesis and release. TGFα (10 ng/ml) was administered to the basolateral compartment of polarizing HCA-7 cells that had been maintained serum-free for 48 h. TGFα increased COX-2 protein levels significantly 24 h after treatment (Fig. 1D Inset), but no change occurred in COX-1 protein levels (data not shown).
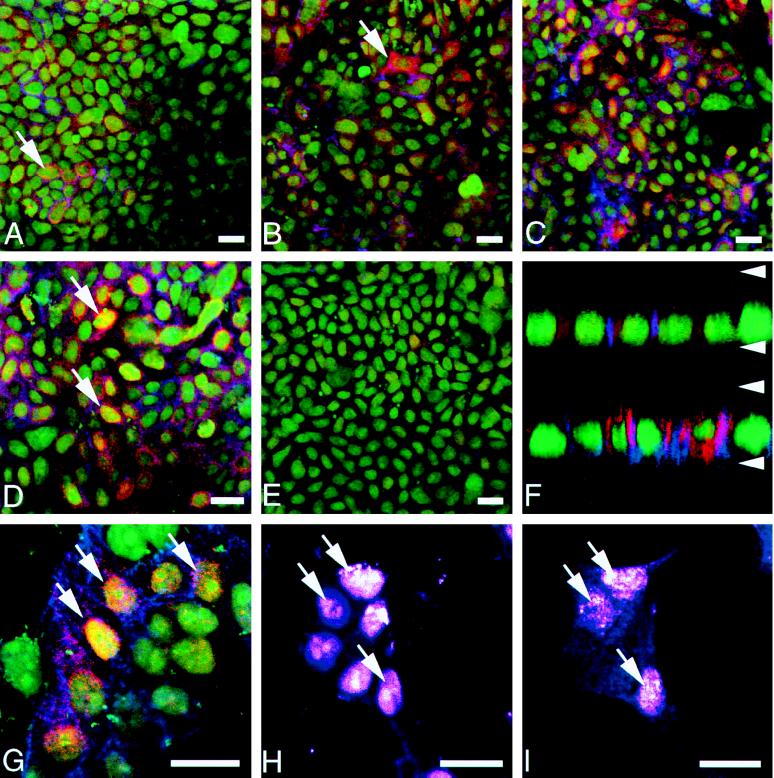
COX-2 immunoreactivity also was examined by immunofluorescence with laser scanning confocal microscopy. Heterogeneous, perinuclear COX-2 immunofluorescence was observed in polarized HCA-7 cells under serum-free conditions (Fig 2 A and top of F). Administration of TGFα resulted in a progressive increase in the level of COX-2 immunofluorescence (4, 8, and 24 h after TGFα in Fig. 2 B–D, respectively). Under serum-free conditions, 5–10% of cells exhibited COX-2 staining, whereas this percentage increased to 60–70% 24 h after TGFα treatment. The most intense COX-2 immunofluorescence remained perinuclear, but the staining extended into the basally oriented nuclei, as well as into the cytoplasm (Fig. 2 D and bottom of F). Increased COX-2 staining was verified by confocal analysis in the xz plane (top of Fig. 2F, serum-free; bottom, 24 h after TGFα administration). Nuclear COX-2 immunofluorescence was more distinct in sparsely growing cells and appeared to have a reticular pattern consistent with localization to the nuclear matrix (Fig. 2G). Sections through the same optical plane stained for the nuclear fluorochrome YO-PRO1 (Fig. 2H) and COX-2 (Fig. 2I), and provide additional support for nuclear COX-2 immunoreactivity. Perinuclear COX-1 staining was observed, but its pattern of expression did not change in these cells (data not shown).
Figure 2.
Distribution of COX-2 immunofluorescence in HCA-7 cells imaged with confocal microscopy. Both confluent (A–F) and sparsely plated cells (G–I) were fixed and labeled with rabbit anti-COX-2 (red), mAb to AR (blue), and a nuclear fluorochrome (green). (A) Overview of cells with serum withdrawn 24 h before fixation. Note that a few small clusters (arrow) exhibit constitutive COX-2 and AR fluorescent signals. (B) Four hours after the addition of TGFα to culture medium, COX-2 immunofluorescence has increased in some cells with a few cells with high levels (arrow). (C) After 8 h of TGFα treatment, HCA-7 cell culture exhibits many cells with significant levels of COX-2 immunofluorescence. The AR signal has also increased in this cell population, but not necessarily in the same cells with increased COX-2. (D) Twenty-four hours after the addition of TGFα, most of the cells display a bright cytoplasmic COX-2 signal, which is highest in the perinuclear region; however, a few cells exhibit both cytoplasmic and nuclear COX-2 immunofluorescence (arrows). (E) HCA-7 cells pretreated with 15 μg/ml mAb 528 for 24 h before immunostaining results in diminished COX-2 signal. (F) Confocal xz scan analysis of confluent HCA-7 cells either serum-deprived (upper) or TGFα-treated for 24 h (lower) shows significant enhancement of perinuclear COX-2 signal following treatment with TGFα. Arrowheads mark the approximate positions of base and apex of cells with a distance of 8.5 μm between them. (G) Higher magnification of sparsely plated cells 24 h after TGFα addition reveals that many cells display enhanced COX-2 and AR expression. Cells with strong nuclear COX-2 immunoreactivity (arrow) often exhibit striking perinuclear COX-2 signal as well. (H) Optical section through sparsely plated cells stained with nuclear fluorochrome YO-PRO1 (arrows) and (I) for COX-2 at same optical plane, demonstrating nuclear COX-2 immunoreactivity (arrows). (Bars = 10 μm.)
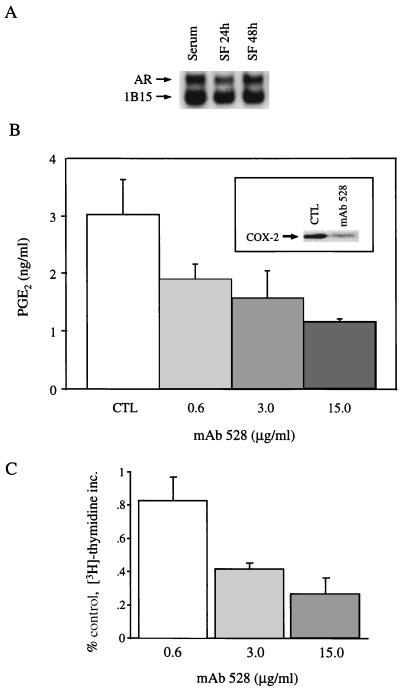
COX-2 immunofluorescence and PGE2 levels in the basolateral medium were detected in significant amounts in unstimulated, polarized HCA-7 cells under serum-free conditions. Since TGFα induced COX-2 protein and stimulated PGE2 release, we considered the possibility that an endogenous EGFR ligand mediates these baseline effects. AR transcripts were the most abundantly expressed message for EGFR ligands by northern blot (data not shown), and the 1.4-kb AR transcript intensity did not diminish significantly over time under serum-free conditions (Fig. 3A). Furthermore, AR immunoreactivity was identified at the basolateral surface (Fig. 2F), suggesting that AR binding to basolateral EGFRs contributes to baseline levels of PGE2 by an autocrine mechanism. Consistent with this hypothesis, administration of EGFR neutralizing mAb 528 (3 μg/ml) to serum-starved HCA-7 cells reduced COX-2 protein (Figs. 2E and 3B Inset) and resulted in a dose-dependent reduction in PGE2 release (Fig. 3B). Furthermore, the reduction in PGE2 levels achieved by EGFR blockade was correlated to a similar reduction in DNA replication (Fig. 3C), suggesting that there is a link between EGFR-mediated COX-2 production, basolateral release of PGs, and mitogenesis in these polarized HCA-7 cells.
Figure 3.
EGFR-regulated COX-2 production, basolateral release of PGs and mitogenesis in polarized HCA-7 cells. (A) Northern blot of HCA-7 cells grown on Transwell filters in 10% fetal calf serum and then kept in serum-free medium for 24 and 48 h. Two micrograms of poly(A) RNA was probed with cRNA probes for AR and constitutively expressed 1B15. (B) HCA-7 cells, grown on Transwell filters and maintained serum-free for 24 h, were exposed to increasing concentrations of mAb 528, and PGE2 levels in the basolateral medium were measured by mass spectrometry. (Inset) COX-2 immunoblot of HCA-7 cells under identical starting conditions as control cells that were then treated with mAb 528 (3 μg/ml) for 24 h. (C) Under identical experimental conditions as in B, a 3-h pulse of [3H]thymidine (1 μCi/ml) was administered 21 h after treatment with mAb 528 and trichloroacetic acid-precipitable counts were determined. All experiments were performed in triplicate and repeated at least twice. The mitogenic data were expressed as percent of control. Error bars represent standard deviation.
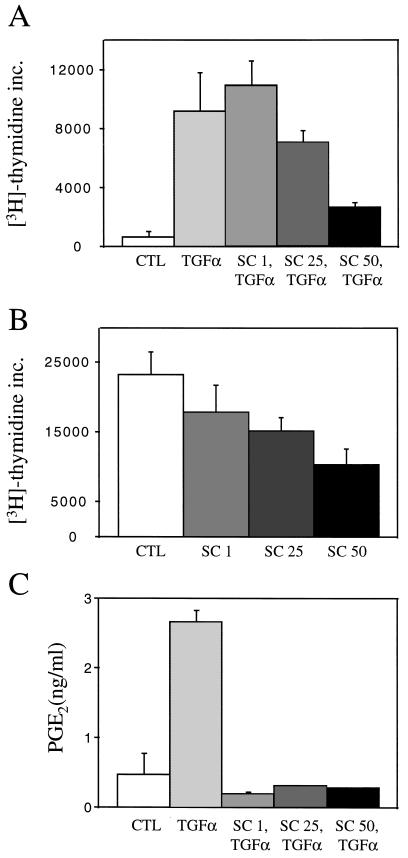
To investigate further whether COX-2-mediated events could be involved in proliferative responses to EGFR stimulation, the effects of COX-2 inhibition on TGFα-stimulated and baseline mitogenesis were examined. Pretreatment with a selective COX-2 inhibitor SC-58125 resulted in a dose-dependent decrease in TGFα-stimulated DNA replication (Fig. 4A). These doses of SC-58125 did not cause apoptosis, as determined by flow cytometry (lack of a subdiploid peak and annexin V staining). Similar results were found with a nonspecific COX inhibitor sulindac sulfide and a second COX-2 inhibitor NS 398. Furthermore, SC-58125 caused a dose-dependent decrease in mitogenesis in HCA-7 cells under serum-containing conditions (Fig. 4B), whereas the drug did not affect mitogenesis in polarizing Caco-2 cells, which do not express COX-2 or produce PGs (data not shown). Surprisingly, all doses of the COX-2 inhibitors resulted in significant reductions (>90%) in PGE2 levels in the basolateral medium of HCA-7 cells under TGFα-stimulated (Fig. 4C) and baseline conditions (data not shown). Moreover, administration of the two most abundant PGs produced by these cells, PGE2 and PGF2α, alone and in combination (10−9-10−5 M), failed to stimulate mitogenesis in HCA-7 cells that had been pretreated with SC-58125.
Figure 4.
COX-2 inhibition decreases TGFα-stimulated and baseline mitogenesis in polarized HCA-7 cells. (A) HCA-7 cells maintained 48 h serum-free on Transwell filters were pretreated for 1 h with increasing concentrations of SC-58125, and then TGFα (10 ng/ml) was administered. (B) HCA-7 cells cultured on Transwell filters in 10% serum were treated with increasing concentrations of SC-58125. In both experiments, a 3-h pulse of [3H]thymidine (1 μCi/ml) was performed 21 h after administration of the agents and trichloroacetic acid-precipitable counts were determined. (C) Under identical conditions as in A, a 30-min pulse of AA (20 μM) was administered 7.5 h after TGFα and PGE2 levels in the basolateral medium were determined by mass spectrometry. All experiments were performed in triplicate and repeated at least three times. Error bars represent standard deviation.
DISCUSSION
EGFRs are localized to the basolateral compartment of polarized epithelial cells. Administration of TGFα to the basolateral medium of polarized HCA-7 cells (but not to the apical medium or to cells cultured on plastic) resulted in increased COX-2 immunoreactivity and basolateral release of PGs. This observation led us to consider that baseline levels of PGE2 detected in HCA-7 cells might be regulated by an endogenous EGFR ligand. Five mammalian ligands bind to the EGFR: TGFα, epidermal growth factor (EGF), AR, heparin-binding EGF-like growth factor (HB-EGF), and betacellulin (24, 29). We have reported previously that AR mRNA expression is increased uniformly in human colon cancer specimens compared with adjacent normal mucosa, whereas intensity of TGFα transcripts vary between normal and malignant tissues (27). AR is the most abundantly expressed EGFR ligand in HCA-7 cells; a low-level transcript for HB-EGF was observed, but no other EGFR ligand transcripts were detected. The abundant and sustained expression for AR mRNA in serum-free conditions (Fig. 3A), the basolateral localization of AR immunoreactivity (Fig. 2F), and the ability of an EGFR neutralizing antibody to attenuate COX-2 immunoreactivity and PG levels (Figs. 2E and 3B) support the hypothesis that AR, acting in an autocrine manner through the EGFR, regulates baseline mitogenesis, as well as COX-2 expression and basolateral PG release. These findings also may be relevant to the recent report by Prescott and coworkers (12), who observed constitutive transcription of COX-2 in the colon cancer cell line HCT-116. Whether endogenous EGFR ligands are responsible for this constitutive COX-2 transcription in this cell line will require additional studies.
The ability of a specific COX-2 inhibitor, SC-58125, to attenuate baseline and TGFα-stimulated DNA replication (Fig. 4 A and B) suggests that signaling through the EGFR, COX-2 induction, and mitogenesis are interrelated. The ability of selective COX-2 inhibitors to decrease mitogenesis in HCA-7 cells implies that COX plays a role in modulating cellular proliferation. Furthermore, SC-58125 inhibits the soft agar and in vivo growth of HCA-7 cells, but not that of a colon line that does not express detectable levels of COX-2 (H. Sheng, J. Shao, R.J.C., R. D. Beauchamp, R.N.D., unpublished observations), nor, as we have found in the present studies, did it affect mitogenesis in Caco-2 cells, which do not express COX-2 or produce PGs when cultured as a polarizing monolayer on Transwell filters. On the other hand, our data suggest that the regulation of mitogenesis by COX-2 may not be mediated directly via PG production, since significant and equivalent reductions in eicosanoid synthesis occurred at every concentration of COX-2 inhibitor studied, whereas mitogenesis decreased proportionately with increasing concentrations of inhibitor. Moreover, addition of exogenous PGs to HCA-7 cells that had been pretreated with specific COX-2 inhibitors failed to stimulate mitogenesis. At present, mechanisms by which COX-2 might alter cellular proliferation independently of PG production are unknown. A recent report by Simmons and coworkers (30) has found that COX may modulate apotosis by interacting with the apotosis/autoimmunity-associated protein Nuc to retain it in the endoplasmic reticulum. Whether this interaction and/or other intracellular effects are involved in the ability of COX-2 to regulate cellular proliferation remains to be determined. These observations, however, do not discount potentially important paracrine effects of basolaterally released PGs in the pathogenesis of colorectal neoplasia.
A notable observation is the apparent translocation of the COX-2 immunoreactivity to the nucleus after TGFα treatment (Fig. 2). COX-2 immunoreactivity and histofluorescence previously have been detected in a perinuclear distribution (31). The precise role of nuclear COX-2 is uncertain. In addition to COX-2, Peters-Golden and coworkers (32) have reported nuclear localization for another AA metabolizing enzyme, 5-lipoxygenase.
PGs are lipid-soluble, and it has been assumed that traverse of cellular membranes occurs by passive diffusion. Carrier-mediated transport occurs in certain tissues. For example, F- and E-series PGs are rapidly cleared on passage through the circulation of various vascular beds (33–35). Recently, Schuster and coworkers (36) have identified a PG transporter encoded by rat matrin F/G DNA. This protein displays homology with other organic anion transporters. It is unlikely, however, that this transporter accounts for vectorial release of eicosanoids in HCA-7 cells, since the PG transporter is exclusively an importer and expression of this PG transporter was not detected in HCA-7 cells by Northern blot analysis (R.J.C., unpublished observation). It seems most likely that the basolateral release of PGs in HCA-7 cells reflects the basal localization of COX-2 or the presence of a novel basolateral outward transporter of PGs.
In summary, we demonstrate that EGFR activation leads to the nuclear localization of COX-2 and basolateral release of PGs in a polarizing human colon cancer cell line HCA-7. We submit that these studies of the spatial localization of biomolecules in the context of epithelial cell polarity and their movement by relevant experimental manipulation affords fresh insights into epithelial cell biology and neoplasia. In addition, we have made the intriguing observation that in HCA-7 cells there may be a dissociation between PG production and mitogenesis. Induction of COX-2 has been implicated in the pathogenesis of colon cancer, and it has been presupposed that the actions of COX-2 are mediated through production of PGs. The present data suggest that, whereas COX-2 protein is induced by EGFR activation in HCA-7 cells, the role that this enzyme and its PG products may play in cellular proliferation remains to be fully defined.
Acknowledgments
We thank Drs. John Barnard, Larry Marnett, Brigid Hogan, Alan Brash, and Dan Beauchamp for critical comments; V. Schuster for the rat matrin F/G DNA; and P. Isakson for SC-58125. This work was supported by National Institute of Health Grants CA46413 (R.J.C.) and DK48831 and GM42506 (J.D.M.) and the Joseph and Mary Keller Foundation. R.J.C. is a Veterans Affairs Clinical Investigator.
Footnotes
Abbreviations: PG, prostaglandin; COX, cyclooxygenase; AA, arachidonic acid; EGF, epidermal growth factor; EGFR, EGF receptor; TGFα, type α transforming growth factor; AR, amphiregulin.
References
- 1.Eberhart C, DuBois R. Gastroenterology. 1995;109:285–301. doi: 10.1016/0016-5085(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert A, Halushka P, Margolius H, Spayne J. Br J Pharmacol. 1984;82:597–607. doi: 10.1111/j.1476-5381.1984.tb10798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bito L, Wallenstein M, Baroody R. Adv Prostaglandin Thromboxane Res. 1976;1:297–303. [PubMed] [Google Scholar]
- 4.Garcia-Perez A, Smith W. J Clin Invest. 1984;74:63–74. doi: 10.1172/JCI111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortizo A, Besterman J, Leitner P, Chandrabose K. Prostaglandins. 1992;44:357–371. doi: 10.1016/0090-6980(92)90008-h. [DOI] [PubMed] [Google Scholar]
- 6.Simmons D, Levy D, Yannoni Y, Erickson R. Proc Natl Acad Sci USA. 1989;86:1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu J, Masferrer J, Seibert K, Raz A, Needleman P. J Biol Chem. 1990;265:16737–16740. [PubMed] [Google Scholar]
- 8.Kujubu D, Herschman H. J Biol Chem. 1992;267:7991–7994. [PubMed] [Google Scholar]
- 9.Williams C, DuBois R. Am J Physiol. 1996;270:G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 10.DuBois R N, Radhika A, Reddy B S, Entingh A J. Gastroenterology. 1996;110:1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- 11.Eberhart C E, Coffey R J, Radhika A, Giardiello F M, Ferrenbach S, DuBois R N. Gastroenterology. 1994;107:1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 12.Kutchera W, Jones D, Matsunami N, Groden J, McIntyre T, Zimmerman G, White R, Prescott S. Proc Natl Acad Sci USA. 1996;93:4816–4820. doi: 10.1073/pnas.93.10.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao C, Rivenson A, Simi B, Zang E, Kelloff G, Steele V, Reddy B. Cancer Res. 1995;55:1464–1472. [PubMed] [Google Scholar]
- 14.Reddy B S, Tokumo K, Kulkarni N, Aligia C, Kelloff G. Carcinogenesis. 1992;13:1019–1023. doi: 10.1093/carcin/13.6.1019. [DOI] [PubMed] [Google Scholar]
- 15.Reddy B S, Rao C V, Rivenson A, Kelloff G. Carcinogenesis. 1993;14:1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- 16.Thun M, Namboodiri M, Heath C. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 17.Thun M J, Namboodiri M M, Calle E, Flanders W, Heath C. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 18.Peleg, Maibach H T, Brown S H, Wilcox C M. Arch Intern Med (Moscow) 1994;154:394–399. [PubMed] [Google Scholar]
- 19.Giovannucci E, Egan K, Hunter D, Stampfer M, Colditz G, Willett W, Speizer F. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 20.Giardiello F M, Hamilton S R, Krush A J, Piantadosi S, Hylind L M, Celano P, Booker S V, Robinson C R, Offerhaus G J. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 21.DuBois R, Awad J, Morrow J, Roberts L J, Bishop P. J Clin Invest. 1994;93:493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsujii M, DuBois R N. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 23.Marsh K, Stamp G, Kirkland S. J Pathol. 1993;170:441–450. doi: 10.1002/path.1711700407. [DOI] [PubMed] [Google Scholar]
- 24.Barnard J, Graves-Deal R, Pittelkow M, DuBois R, Cook P, Ramsey G, Bishop P, Damstrup L, Coffey R. J Biol Chem. 1994;269:22817–22822. [PubMed] [Google Scholar]
- 25.Parsons W, Roberts L. J Immunol. 1988;141:2413–2419. [PubMed] [Google Scholar]
- 26.Thomas P. Proc Natl Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook P, Pittelkow M, Keeble W, Graves-Deal R, Coffey R J, Shipley G. Cancer Res. 1992;52:3224–3227. [PubMed] [Google Scholar]
- 28.Danielson P, Forss-Petter S, Brow M, Calavetta L, Douglass J, Milner R, Sutcliffe J. DNA. 1988;12:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- 29.Barnard J, Beauchamp R, Russell W, DuBois R, Coffey R. Gastroenterology. 1995;108:564–580. doi: 10.1016/0016-5085(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 30.Ballif B, Mincek N, Barratt J, Wilson M, Simmons D. Proc Natl Acad Sci USA. 1996;93:5544–5549. doi: 10.1073/pnas.93.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita I, Schindler M, Regier M, Otto J, Hori T, DeWitt D, Smith W. J Biol Chem. 1995;270:10902–10908. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- 32.Brock T, Paine R P, III, Peters-Golden M. J Biol Chem. 1994;269:22059–22066. [PubMed] [Google Scholar]
- 33.Piper P, Vane J, Wyllie J. Nature (London) 1970;225:600–604. doi: 10.1038/225600a0. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira S, Vane J. Nature (London) 1967;216:868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- 35.McGiff J, Terragno N, Strand J, Lee J, Lonigro A. Nature (London) 1969;223:742–745. doi: 10.1038/223742b0. [DOI] [PubMed] [Google Scholar]
- 36.Kanai N, Lu R, Satriano J, Bao Y, Wolkoff A, Schuster V. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]