Abstract
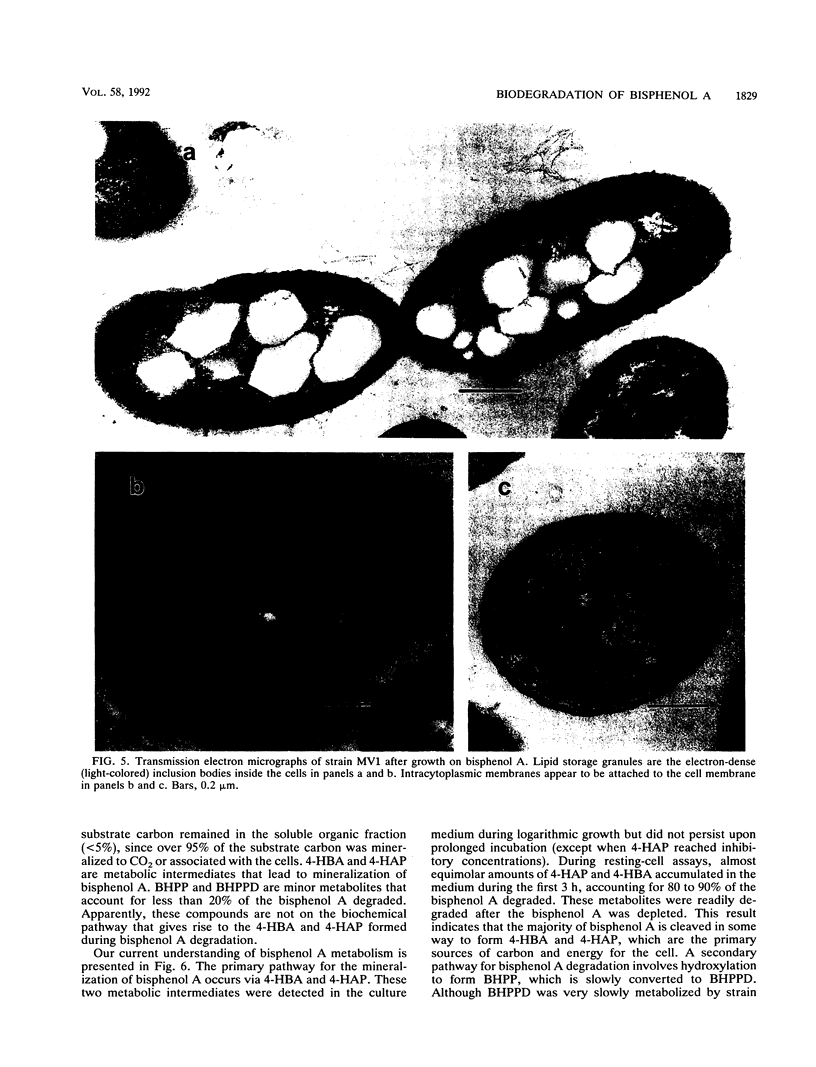
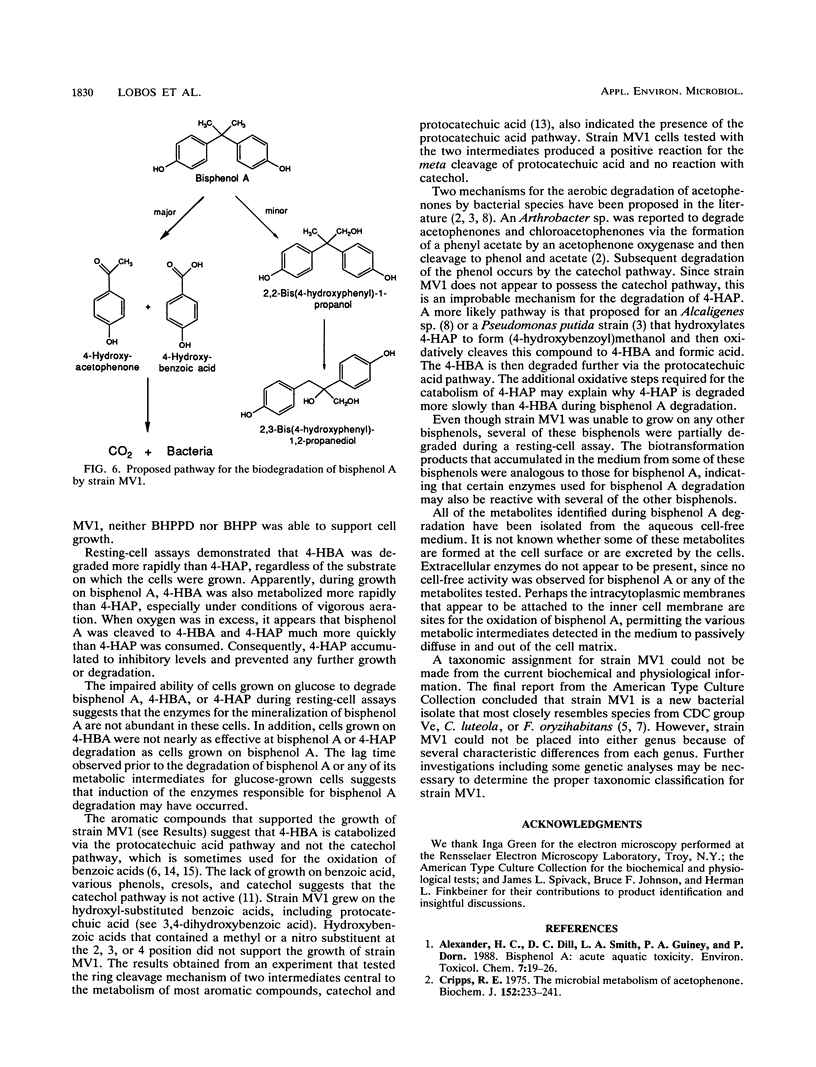
A novel bacterium designated strain MV1 was isolated from a sludge enrichment taken from the wastewater treatment plant at a plastics manufacturing facility and shown to degrade 2,2-bis(4-hydroxyphenyl)propane (4,4'-isopropylidenediphenol or bisphenol A). Strain MV1 is a gram-negative, aerobic bacillus that grows on bisphenol A as a sole source of carbon and energy. Total carbon analysis for bisphenol A degradation demonstrated that 60% of the carbon was mineralized to CO2, 20% was associated with the bacterial cells, and 20% was converted to soluble organic compounds. Metabolic intermediates detected in the culture medium during growth on bisphenol A were identified as 4-hydroxybenzoic acid, 4-hydroxyacetophenone, 2,2-bis(4-hydroxyphenyl)-1-propanol, and 2,3-bis(4-hydroxyphenyl)-1,2-propanediol. Most of the bisphenol A degraded by strain MV1 is cleaved in some way to form 4-hydroxybenzoic acid and 4-hydroxyacetophenone, which are subsequently mineralized or assimilated into cell carbon. In addition, about 20% of the bisphenol A is hydroxylated to form 2,2-bis(4-hydroxyphenyl)-1-propanol, which is slowly biotransformed to 2,3-bis(4-hydroxyphenyl)-1,2-propanediol. Cells that were grown on bisphenol A degraded a variety of bisphenol alkanes, hydroxylated benzoic acids, and hydroxylated acetophenones during resting-cell assays. Transmission electron microscopy of cells grown on bisphenol A revealed lipid storage granules and intracytoplasmic membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cripps R. E. The microbial metabolism of acetophenone. Metabolism of acetophenone and some chloroacetophenones by an Arthrobacter species. Biochem J. 1975 Nov;152(2):233–241. doi: 10.1042/bj1520233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi G. L., Hirschl S., Mandel M. Characteristics of yellow-pigmented nonfermentative bacilli (groups VE-1 and VE-2) encountered in clinical bacteriology. J Clin Microbiol. 1975 Apr;1(4):384–389. doi: 10.1128/jcm.1.4.384-389.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire H. C., Jr Experimental photoallergic contact dermatitis to bisphenol A. Acta Derm Venereol. 1988;68(5):408–412. [PubMed] [Google Scholar]
- Morrissey R. E., George J. D., Price C. J., Tyl R. W., Marr M. C., Kimmel C. A. The developmental toxicity of bisphenol A in rats and mice. Fundam Appl Toxicol. 1987 May;8(4):571–582. doi: 10.1016/0272-0590(87)90142-4. [DOI] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Mahaffey W. R., Pritchard P. H. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Appl Environ Microbiol. 1987 May;53(5):949–954. doi: 10.1128/aem.53.5.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Sutherland J. B., Crawford D. L., Pometto A. L. Catabolism of substituted benzoic acids by streptomyces species. Appl Environ Microbiol. 1981 Feb;41(2):442–448. doi: 10.1128/aem.41.2.442-448.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]