Abstract
We have shown that coculture of bone marrow microvascular endothelial cells with hematopoietic progenitor cells results in proliferation and differentiation of megakaryocytes. In these long-term cultures, bone marrow microvascular endothelial cell monolayers maintain their cellular integrity in the absence of exogenous endothelial growth factors. Because this interaction may involve paracrine secretion of cytokines, we evaluated megakaryocytic cells for secretion of vascular endothelial growth factor (VEGF). Megakaryocytes (CD41a+) were generated by ex vivo expansion of hematopoietic progenitor cells with kit-ligand and thrombopoietin for 10 days and further purified with immunomagnetic microbeads. Using reverse transcription–PCR, we showed that megakaryocytic cell lines (Dami, HEL) and purified megakaryocytes expressed mRNA of the three VEGF isoforms (121, 165, and 189 amino acids). Large quantities of VEGF (>1 ng/106 cells/3 days) were detected in the supernatant of Dami cells, ex vivo-generated megakaryocytes, and CD41a+ cells isolated from bone marrow. The constitutive secretion of VEGF by CD41a+ cells was stimulated by growth factors of the megakaryocytic lineage (interleukin 3, thrombopoietin). Western blotting of heparin–Sepharose-enriched supernatant mainly detected the isoform VEGF165. In addition, immunohistochemistry showed intracytoplasmic VEGF in polyploid megakaryocytes. Thrombin stimulation of megakaryocytes and platelets resulted in rapid release of VEGF within 30 min. We conclude that human megakaryocytes produce and secrete VEGF in an inducible manner. Within the bone marrow microenvironment, VEGF secreted by megakaryocytes may contribute to the proliferation of endothelial cells. VEGF delivered to sites of vascular injury by activated platelets may initiate angiogenesis.
Recent studies implicate endothelial cells as a critical component of the bone marrow stroma in the regulation of hematopoiesis, particularly megakaryocytopoiesis. Bone marrow microvascular endothelial cells (BMEC) support the proliferation and differentiation of megakaryocytic progenitors in vitro by constitutive secretion of cytokines, including kit-ligand, interleukin 6 (IL-6), and thrombopoietin (TPO), and may also play a role for growth and maturation of megakaryocytes in vivo (1). In addition, platelet release from the bone marrow into the circulation involves either transendothelial migration of megakaryocytes, or protrusion of pseudopods through the endothelial layer (2). Although the physical juxtaposition of megakaryocytes and endothelium has long been observed (2), the reciprocal interactions of endothelial and megakaryocytic cells are only partially understood. It has been shown that megakaryocytic cells release a variety of cytokines such as IL-1, IL-3, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and basic fibroblast growth factor (bFGF) (3, 4). In coculture studies we have observed that the proliferation of megakaryocytic cells is associated with prolonged preservation of the endothelial monolayer in the absence of exogenous endothelial growth factors (1), suggesting that megakaryocytes produce cytokines that support survival and proliferation of endothelial cells.
Recently, vascular endothelial growth factor (VEGF) has been identified as a cytokine regulating proliferation, differentiation, and survival of microvascular endothelium. VEGF is a specific endothelial cell mitogen and a potent angiogenic factor (5, 6). In malignant tumors, overexpression of VEGF supports development of tumor vasculature and may be responsible for increased vascular permeability (7, 8). During embryogenesis, VEGF is required for structural development of the blood vessels and establishment of hematopoiesis (9). In vivo, VEGF increases endothelial fenestration and causes extravasation of plasma macromolecules (7, 8, 10). Furthermore, VEGF has been shown to support transendothelial migration of monocytes and to be chemotactic for mast cells, as well as monocytes (11–13). Given the capacity for VEGF to promote vasculogenesis and support functions such as transendothelial migration, we hypothesize that VEGF plays a role in hematopoiesis, particularly endothelium-dependent megakaryocytopoiesis.
Receptors for VEGF, fetal liver kinase 1 (FLK-1) and fms-like tyrosine kinase 1 (FLK-1), are expressed on endothelial cells and act as signal transducing molecules upon binding of VEGF (14–16). The mitogenic effect of VEGF is mainly related to signaling through FLK-1, while the role of FLK-1 remains to be fully determined (17). Alternative splicing results in four VEGF isoforms with 121, 165, 189, and 206 amino acids (18). VEGF206 is expressed only in the fetal liver, while the other three isoforms are produced by different malignant and nonmalignant cell types, including peripheral blood mononuclear cells and lymphocytes (18–21). VEGF121 and VEGF165 are secreted, while VEGF189 and VEGF206 remain cell-surface associated or are primarily deposited in the extracellular matrix (18, 22). VEGF165, VEGF189, and VEGF206 exhibit high affinity for heparin, which increases with peptide size. In contrast, VEGF121 is not efficiently bound by heparin (7, 22, 23).
In this study, we analyzed expression of VEGF mRNA and peptide in megakaryocytic cell lines and human megakaryocytes generated by ex vivo expansion of hematopoietic progenitor cells. The cell lines and the megakaryocytes expressed the three alternatively spliced isoforms of VEGF. Considerable amounts of VEGF were stored intracellularly by large, polyploid megakaryocytes and secreted into the medium constitutively and following stimulation. These findings support the concept that VEGF is an important cytokine in the bone marrow microenvironment, regulating the reciprocal interaction between megakaryocytes and endothelial cells.
MATERIALS AND METHODS
Ex Vivo Generation and Isolation of Megakaryocytes.
Granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood CD34+ hematopoietic progenitor cells were isolated with immunomagnetic beads (Dynal, Oslo) using mAb 11.1.6. and cultured for 10 days in serum-free medium (X-Vivo 20; BioWhittaker) supplemented with human kit-ligand (20 ng/ml; kindly provided by Immunex) and TPO (50 ng/ml; R & D Systems). For optimal recovery of the fragile megakaryocytic cells, an isolation technique was designed that required only one low-speed centrifugation step. The cells were incubated with fluorescein isothiocyanate (FITC)-conjugated CD41a mAb (P2; Immunotech, Marseille, France) for 20 min. After centrifuging for 15 min at 100 × g, the cells were resuspended in PBS/0.5% BSA supplemented with 5 mM EDTA and 10−5 M prostaglandin I2 to prevent activation of megakaryocytes, then incubated (20 min) with immunomagnetic microspheres conjugated with anti-FITC antibody (Miltenyi Biotec, Auburn, CA) and separated using a magnetic separation column designed for isolation of megakaryocytes (type WHM, MiniMACS; Miltenyi Biotec). The viability of the isolated cells was >90% by Trypan-blue dye exclusion. In some experiments, megakaryocytes were isolated from the mononuclear cell fraction of bone marrow aspirates from healthy individuals.
Cell Lines, Endothelial Cells, and Human Platelets.
Human BMEC were isolated and grown as described (1). The megakaryocytic cell lines Dami and HEL, and the myeloid cell line HL60 (American Type Culture Collection) were cultured in RPMI 1640 medium (GIBCO) supplemented with 10% fetal bovine serum. Platelets from healthy volunteers were isolated by differential centrifugation after venipuncture into acid/citrate/dextrose solution.
Flow Cytometry.
To characterize the ex vivo-expanded cells, the following FITC-conjugated and phycoerythrin (PE)-conjugated mAbs were used: CD14–FITC, CD15–FITC, glycophorin A–FITC (clone RMO52, 80H5, D2.10; Immunotech), CD34–PE, CD45–FITC (HPCA2, HLe-1; Becton Dickinson), CD41a–PE (clone HIP8; PharMingen), and IgG isotype control FITC/PE (Immunotech). For flow cytometry, 50,000 cells were incubated with the respective antibodies, washed, and analyzed using a Coulter Elite flow cytometer. To assess the purity of the positive and negative fraction after separation, an aliquot of 50,000 cells was analyzed which had already been stained with CD41a–FITC during the isolation procedure.
Stimulation of ex Vivo-Generated Megakaryocytes and Platelets with Thrombin.
Cells were centrifuged for 15 min at 100 × g, resuspended in fresh serum-free medium, and human thrombin was added at a final concentration of 1 unit/ml. Cells were incubated at 37°C with 5% CO2 for 30 min, and the supernatant was recovered after centrifugation.
Reverse Transcription (RT) and PCR.
Oligonucleotide primers spanning the insertion/deletion site of human VEGF165 were synthesized. The sense primer was 5′-CGAAGTGGTGAAGTTCATGGATG-3′ and the antisense primer was 5′-TTCTGTATCAGTCTTTCCTGGTGAG-3′. RT-PCR of mRNA encoding the 121-, 165-, and 189-amino acid-containing isoforms of VEGF results in PCR products 403, 535, and 607 bp long (18, 20). Primers were designed and synthesized for the VEGF receptor FLK-1 (sense, 5′-CTGGCATGGTCTTCTGTGAAGCA-3′; antisense, 5′-AATACCAGTGGATGTGATGCGG-3′; expected size of the PCR product, 790 bp). The PCR product obtained from Dami cells was sequenced to confirm the specificity of the chosen primers. For β-actin, the sense primer 5′-TCATGTTTGAGACCTTCAA-3′ and antisense primer 5′-GTCTTTGCGGATGTCCACG-3′ was used [expected size of the PCR product, 513 bp for cDNA and 607 bp for genomic DNA (24)].
First strand cDNA was synthesized by RT of 200 ng total RNA isolated from the cells using guanidine thiocyanate, and amplified by Taq DNA polymerase dissolved in PCR buffer (KlenTaq; CLONTECH) in a 50 μl reaction containing 0.2 mM dNTPs and 40 pmol of each primer. The PCR profile consisted of 1-min initial denaturation at 94°C, followed by 20–35 cycles of 1-min denaturation at 94°C, 1-min annealing at 62°C (VEGF, FLK-1) or 55°C (β-actin), 2-min polymerization at 72°C, and finally 10-min extension at 72°C. Twenty microliters of the PCR products was separated in 2% (wt/vol) agarose gels and stained with ethidium bromide.
Immunohistochemistry of VEGF Expression.
Cytospin preparations of Dami cells and ex vivo-generated megakaryocytes were fixed in 3% formalin and incubated for 1 h with rabbit polyclonal anti-VEGF antibody A-20 (diluted 1:100; Santa Cruz Biotechnology), which recognizes the three VEGF isoforms (121, 165, and 189 amino acids). Biotinylated anti-rabbit IgG diluted 1:200 was incubated with the cells for 30 min, and endogenous peroxidase was quenched with 0.3% hydrogen peroxide in PBS for 30 min. After 30-min incubation with avidin-labeled peroxidase, slides were rinsed and incubated with peroxide substrate aminoethyl carbazol for 15 min.
ELISA for VEGF.
The VEGF concentration in samples from conditioned medium was measured using a human VEGF immunoassay that recognizes the soluble isoforms VEGF121 and VEGF165 (R & D Systems). For cell culture supernatants, a sensitivity of 5 pg/ml could be achieved.
Western Blotting of VEGF Protein.
Conditioned medium was incubated with 10 μl/ml heparin-Sepharose CL-6B (Pharmacia) slurry for 24 h at 4°C. The beads were pelleted, washed 3–4 times, and boiled in 4× SDS sample buffer. Samples were resolved on 15% polyacrylamide and blotted. After incubation with the anti-VEGF antibody A-20 (diluted 1:500), detection of peroxidase secondary antibody employed chemiluminescence.
Coculture of BMEC with Megakaryocytes.
BMEC were grown to confluency in 24-well tissue culture plates. After 24-h culture in serum-free medium, 105 CD41a+ cells were added to the wells (day 0). After 1, 3, and 10 days the number of BMEC per well with and without megakaryocytic coculture was counted after trypsinization. To correct for adherent megakaryocytes, the cell count was multiplied by the proportion of CD41a− cells as assessed by flow cytometry.
RESULTS
Ex Vivo Expansion, Characterization, and Purification of Megakaryocytes.
Cultivation of CD34+ hematopoietic progenitor cells in serum-free medium with kit-ligand and thrombopoietin resulted in a 5- to 10-fold expansion of the total cell number. A total of 46.3 ± 8.3% cells (mean ± SEM, n = 6) expressed the specific megakaryocytic marker CD41a. The CD41a− cells consisted of CD45+CD34− hematopoietic precursors and mature cells, including a small proportion (<25%) of granulocytic (CD15+) and monocytic cells (CD14+). Erythroid cells (glycophorin A+) were not detected. Depending on the percentage of positive cells before separation (Fig. 1), the purity of the isolated CD41a+ cells ranged from 75 to 98% (86.9 ± 3.7%). The “negative” fraction contained only a few CD41+ cells (4.0 ± 2.3%).
Figure 1.
Isolation of CD41a+ megakaryocytic cells. Megakaryocytes were separated after labeling with anti-CD41a–FITC antibody and anti-FITC immunomagnetic microbeads. Cells before isolation, the positive and negative cell fraction, were analyzed by flow cytometry. While the positive fraction contained 90% CD41a+ cells, virtually no megakaryocytic cells were found in the negative population (0.5%).
VEGF and FLK-1 mRNA Expression by Megakaryocytic Cells.
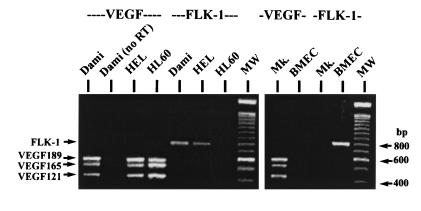
By RT-PCR, we showed that the cell lines Dami and HEL and ex vivo-generated megakaryocytes expressed the mRNA of the three isoforms VEGF121, VEGF165, and VEGF189 (Fig. 2). BMEC as a negative control did not transcribe VEGF mRNA. As expected, BMEC were positive for the VEGF receptor FLK-1, which was not expressed by the ex vivo-generated megakaryocytes and the myeloid cell line HL-60. The malignant megakaryocytic cell lines Dami and HEL, however, transcribed mRNA for both VEGF and the VEGF receptor FLK-1.
Figure 2.
RT-PCR of VEGF and FLK-1 mRNA expression. The megakaryocytic cell lines Dami and HEL, as well as ex vivo-generated megakaryocytes (Mk.), expressed mRNA of the three VEGF isoforms (VEGF189, VEGF165, and VEGF121). Only the malignant cell lines simultaneously expressed FLK-1 mRNA. RNA from Dami cells analyzed without reverse transcription (no RT) served as a negative control, and RNA from the cell line HL-60 as a positive control for VEGF expression. Endothelial cells (BMEC) as a positive control expressed only FLK-1.
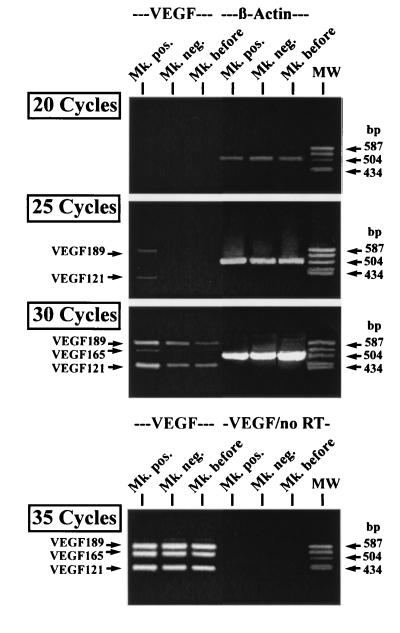
For semiquantitative analysis, total RNA was prepared from samples that contained >95% CD41a+ cells in the positive fraction and <5% CD41a+ cells in the negative fraction after separation. After 35 cycles of PCR amplification, bands corresponding to the three VEGF isoforms could be detected in both the positive and negative fraction (Fig. 3). However, after 25 cycles bands corresponding to VEGF121 and VEGF189 (and after 30 cycles also corresponding to VEGF165) were only found in the positive fraction, while bands of equal strength were obtained using primers for β-actin after 20 and 25 PCR cycles.
Figure 3.
Semiquantitative RT-PCR of VEGF mRNA expression. Equal amounts of cDNA from cells before isolation (Mk. before), CD41a+ (Mk. pos.), and CD41a− (Mk. neg.) cells were amplified by RT-PCR for VEGF and β-actin expression using different numbers of amplification cycles. VEGF121 and VEGF189 were detected after 25 PCR cycles only in CD41a+ cells. Furthermore, VEGF165 was detected after 30 cycles only in these cells. In contrast, no difference in the β-actin expression was observed. After 35 PCR cycles, PCR products corresponding to the three VEGF isoforms could also be amplified from CD41a− and unseparated cells. As a negative control, RNA without RT was also subjected to 35 cycles of PCR amplification (VEGF/no RT).
VEGF Antigen Expression by Megakaryocytic Cells.
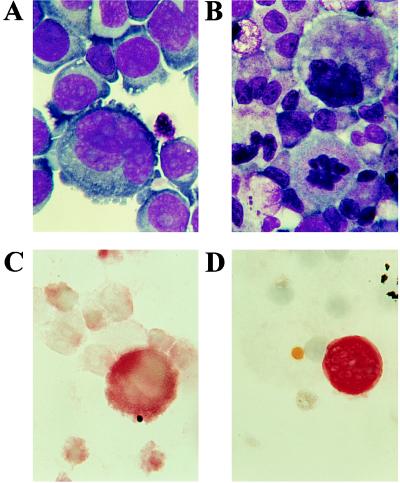
By immunohistochemistry, VEGF was detected in Dami cells and ex-vivo-generated megakaryocytes (Fig. 4). Of note, large polyploid Dami cells were more intensely stained compared with smaller cells. Among the ex vivo-generated megakaryocytes, only highly polyploid cells were positive. Due to the segmented nucleus, the staining could not accurately distinguish between cytoplasmic and nuclear VEGF expression. Staining with isotypic IgG was negative (data not shown).
Figure 4.
Immunohistochemistry of VEGF expression. Cytospin preparations of Dami cells and megakaryocytes were stained with Wright–Giemsa (A and B, respectively) and analyzed with immunohistochemistry for VEGF expression (C and D). The megakaryocytic cell line Dami was positive for VEGF (C), particularly with increasing cell size. Among the ex vivo-generated megakaryocytic cells, only highly polyploid, large megakaryocytes stained positive for VEGF (D). (×1000.)
Constitutive Secretion of VEGF by Megakaryocytes in Vitro.
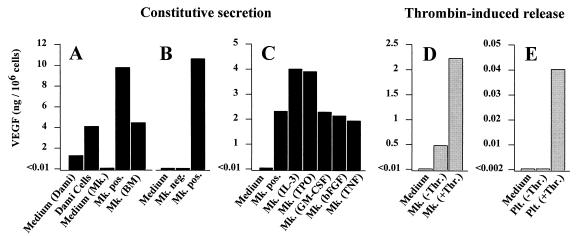
After incubation for 72 h, conditioned medium from Dami cells and megakaryocytes was analyzed by ELISA assay. Dami cells, ex vivo-generated, and bone marrow-derived megakaryocytes released 4.1, 10.2 and 4.6 ng VEGF/106 cells, respectively (Fig. 5A). In a second experiment, the constitutive secretion of VEGF by isolated CD41a+ megakaryocytes was compared with the CD41a− cell fraction that did not release measurable quantities of VEGF (Fig. 5B). To determine the effect of various recombinant human cytokines on VEGF secretion by megakaryocytes, the supernatant of CD41a+ cells was collected 24 h after addition of the respective cytokine (Fig. 5C). IL-3 (50 ng/ml) and TPO (100 ng/ml) increased the VEGF secretion nearly 2-fold. In contrast, GM-CSF (100 ng/ml) and bFGF (10 ng/ml) had no stimulatory effect, while tumor necrosis factor α (10 ng/ml) slightly inhibited VEGF secretion.
Figure 5.
(A and B) Constitutive secretion of VEGF. During 72 h of incubation (A), Dami-cells, ex vivo-generated (Mk. pos.) and bone marrow-derived megakaryocytes (Mk. BM) released VEGF into the supernatant. Medium not conditioned by cells served as control (Medium). Because the Dami cell line was cultured in serum-containing medium, VEGF was also detected in the respective control sample (Medium Dami). In a second experiment (B), secretion of VEGF by CD41a+ megakaryocytes was compared with CD41− cells. In contrast to CD41a+ cells (Mk. pos.), the amount of VEGF secreted by CD41a− cells during 72 h (Mk. neg.) was below detection limit. (C) Addition of IL-3 and TPO resulted in an increased secretion of VEGF by isolated CD41a+ cells during an incubation period of 24 h. GM-CSF, bFGF, or tumor necrosis factor α (TNF-α) had no effect or decreased VEGF secretion. (D and E) Thrombin-induced release of VEGF. Megakaryocytes and platelets were isolated, washed, and resuspended in serum-free medium. Thirty minutes after incubation with thrombin at a concentration of 1 unit/ml, VEGF was detected in the supernatant from megakaryocytes (Mk. +Thr.) (D) and platelets (Plt. +Thr). (E), compared with medium from unstimulated cells (Mk. −Thr., Plt. −Thr.).
Thrombin-Induced Secretion of VEGF by Megakaryocytes and Platelets.
Stimulation of ex vivo-generated megakaryocytes (Fig. 5D) and platelets (Fig. 5E) with thrombin at a concentration of 1 unit/ml resulted in the rapid release of 2.2 ng and 41 pg VEGF/106 cells, repectively, within 30 min. Medium without cells as a negative control did not contain measurable quantities of VEGF.
VEGF Isoforms Secreted by Megakaryocytic Cells.
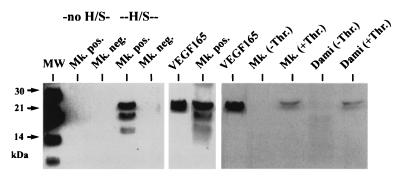
To determine which isoforms of VEGF were released constitutively and upon stimulation with thrombin, conditioned medium from CD41a+ megakaryocytes and CD41a− cells was analyzed by Western blot technique (Fig. 6). Enrichment of VEGF protein with heparin-Sepharose affinity resulted in the detection of bands at 19 and 22 kDa, corresponding to glycolysated and nonglycolysated VEGF165 (18). A faint lower band could correspond to VEGF121, which has only a low affinity for heparin. VEGF isoforms of higher molecular weight were not detected. No VEGF was found in the conditioned medium from CD41a− cells. After stimulation of megakaryocytes and Dami cells with thrombin, only expression of VEGF165 was found after enrichment with heparin-Sepharose.
Figure 6.
Western blotting of conditioned medium from megakaryocytic cells. Without heparin-Sepharose enrichment (no H/S), no VEGF could be detected in the conditioned medium (72-h incubation) of CD41a+ (Mk. pos.) and CD41a− (Mk. neg.) cells. Two bands at 21–22 and 17–18 kDa were visible after enrichment of supernatant from CD41a+ cells with heparin-Sepharose (H/S), corresponding to glycolysated and nonglycolysated VEGF165, while no detectable protein was found in the conditioned medium from CD41a− cells. The identity of the bands was confirmed by the positive control (recombinant VEGF165). Thirty minutes after stimulation with thrombin, VEGF165 was also detectable in the supernatant from ex vivo-generated megakaryocytes (Mk. +Thr.) and Dami-cells (Dami +Thr.) after enrichment with H/S, in contrast to unstimulated cells (−Thr.).
Maintenance of BMEC by Megakaryocytes in Vitro.
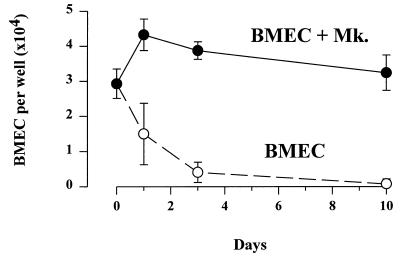
Coculture of BMEC with ex vivo-generated megakaryocytes resulted in the preservation of the endothelial layer in serum-free medium for longer than 1 week, while the number of endothelial cells dropped rapidly without coculture (Fig. 7).
Figure 7.
Coculture of BMEC and megakaryocytes. In coculture with megakaryocytes, BMEC monolayers could be maintained for longer than 1 week in serum-free medium (BMEC +Mk). In contrast, the number of BMEC per well (mean ± SD) rapidly dropped without coculture (BMEC).
DISCUSSION
In this study, we have demonstrated that mRNA of the three alternatively spliced isoforms of VEGF is expressed by ex vivo-generated human megakaryocytes. Intracellular VEGF was detected by immunohistochemistry, and secreted VEGF165 was found in the supernatant of megakaryocytes and megakaryocytic cell lines. Expression of VEGF mRNA by RT-PCR in CD41a+ cells separated from bone marrow using immunomagnetic beads has been reported previously (25). However, a positive PCR signal from this cell population without quantitation or demonstration of protein expression does not prove production of VEGF by megakaryocytes, because the separated fraction contains varying amounts of contaminating nonmegakaryocytic cells, which may give rise to a positive PCR signal. Even detection of VEGF in conditioned medium from megakaryocytes or platelets does not necessarily imply that VEGF is synthesized endogenously by these cells, because megakaryocytes can incorporate exogenous protein in their granules (26). However, our results using semiquantitative RT-PCR indicate that the positive signal in the CD41a+ fraction cannot be due to contaminating CD41a− cells. In contrast to megakaryocytes isolated from bone marrow, ex vivo-generated CD41a+ cells could not incorporate exogenous VEGF, because they were cultured in serum-free medium, and the CD41a− cells secreted no measurable quantities of VEGF.
In our study, large quantities of VEGF were secreted by purified megakaryocytes, while the level of VEGF in the conditioned medium from CD41a− cells was below the detection limit of the ELISA assay. By semiquantitative RT-PCR however, purified megakaryocytes transcribed only moderately more VEGF mRNA compared with CD41− cells. Because ex vivo-generated, mature megakaryocytes form proplatelets and platelet-like particles during in vitro culturing (27), these findings suggest that in addition to VEGF synthesis, intracellularly stored protein is released during the final stages of megakaryocytic maturation and platelet shedding. Given the large amount of VEGF released by mature polyploid megakaryocytes which are located in close juxtaposition to sinusoidal endothelium in vivo (2), megakaryocyte-derived VEGF could efficiently stimulate endothelial cell growth and motility in the bone marrow microenvironment. This concept is supported by the finding that the mitogenic VEGF-receptor FLK-1 is expressed by BMEC, and previous studies have shown that the dose of VEGF is important for its biological function as reflected by impaired vasculogenesis and hematopoiesis in mice even after disruption of only one VEGF allele (9). Furthermore, we could demonstrate that in coculture with megakaryocytes, BMEC can be maintained in vitro under serum-free conditions. Increased fenestration and permeability of endothelium caused by megakaryocyte-derived VEGF (10) may also play a role in platelet release through the sinusoidal endothelium.
Potent megakaryocytic growth factors, such as IL-3 and TPO, increased the constitutive production of VEGF by megakaryocytes, while moderate stimulators (GM-CSF and bFGF) did not affect VEGF secretion (27–29). Tumor necrosis factor, a cytokine with inhibitory effect on hematopoietic colony growth, decreased the amount of VEGF released into the supernatant. Megakaryocytic growth factors including IL-3 have also been shown to increase adhesion of megakaryocytes to endothelium (30), while TPO is important for final maturation of megakaryocytes and platelet release (27). Furthermore, megakaryocytic growth factors such as IL-3 stimulate secretion of hematopoietic cytokines (IL-3, IL-6, and GM-CSF) by megakaryocytes (3). These findings suggest that proliferating megakaryocytes secrete VEGF together with hematopoietic cytokines, resulting in autocrine stimulation of their own growth, increased endothelial proliferation, and adhesion to endothelium important for final maturation and platelet release. Because BMEC have been shown to support megakaryocyte growth by secretion of megakaryocytic growth factors (1), VEGF represents an important link in the cytokine network involved in the regulation of megakaryocytopoiesis.
We have demonstrated that stimulation of megakaryocytes with thrombin induced the rapid release of VEGF from megakaryocytic cells and platelets. While transcription and constitutive secretion of VEGF in different cell types has been shown to be inducible with cytokines (31, 32) and low oxygen tension (32, 33), a regulated release of intracellularly stored VEGF has not been reported previously. Rapid release of VEGF during tissue injury has been attributed to degradation of extracellular matrix proteins rather than stimulated cellular secretion (22). By RT-PCR, VEGF121 and VEGF189 were more prominent than VEGF165, which was the main isoform detected by Western blot. Therefore, VEGF121 might also be secreted constitutively and upon stimulation, since enrichment with heparin-Sepharose before Western blotting does not efficiently concentrate this isoform. However, thrombin did not release VEGF189, which is primarily cell-surface or extracellular matrix-associated.
While thrombin has been shown to play a major role in platelet physiology, its function in the regulation of megakaryocytopoiesis has remained elusive (34). Stimulation of megakaryocytes with thrombin results in rapid secretion of mitogens, such as bFGF, and induces megakaryocytic differentiation (4, 28). bFGF has also been shown to increase megakaryocyte colony formation in vitro (35). VEGF also stimulates hematopoietic colony growth in the presence of other cytokines (36). Circulating thrombin generated during vascular injury, or prothrombin cleaved into thrombin by megakaryocytes (34), might therefore support megakaryocytic development in the bone marrow involving the release of VEGF.
The fact that thrombin-activated platelets release VEGF implies a second biological function of megakaryocyte-derived VEGF. During megakaryocyte maturation and platelet shedding, intracytoplasmic VEGF is only partially released and is delivered by platelets to sites of vascular injury. After activation and clot formation, the cytokine is released and may initiate endothelial proliferation, resulting in wound healing and neovascularization. VEGF stimulates the migration of monocytes and mast cells to areas of tissue injury following clot formation (11–13). Release of VEGF by activated platelets could therefore play a role in recruiting these cells from the blood or surrounding tissue. In addition, for years the absence of the “nurturing” effect of platelets on endothelial cell integrity has been postulated as the basis for bleeding in thrombocytopenic states (37). VEGF released by platelets now provides a possible underlying physiological mechanism.
FLK-1, the receptor for VEGF that transduces the mitogenic signal, was not expressed in megakaryocytes. Conversely, endothelial cells were positive for FLK-1 and did not transcribe VEGF mRNA. These results suggest that in primary megakaryocytes, significant secretion of VEGF is incompatible with expression of FLK-1, which would result in sustained activation of the Ras/mitogen-activating protein kinase signaling pathway (38, 39). However, the malignant megakaryocytic cell lines Dami and HEL expressed both VEGF and FLK-1 mRNA. In tumor cells, binding of secreted VEGF to FLK-1 expressed on the same cell could result in an autocrine loop contributing to the transformed phenotype.
In summary, megakaryocytes produce VEGF in doses sufficient to induce angiogenesis, and release this cytokine in a stimulation-dependent manner. Because endothelial cells have emerged as a critical component of the bone marrow microenvironment in the regulation megakaryocytopoiesis, VEGF represents a cytokine involved in the reciprocal interaction between endothelium and cells of the megakaryocytic lineage. In addition, platelets may deliver VEGF to sites of vascular injury as a first step in vessel repair and wound healing, which may also be relevant to abnormalities of hemostasis in thrombocytopenic states.
Acknowledgments
We thank Barbara Ferris for technical assistance, and Roy Silverstein for critically reviewing this manuscript. This work was supported by the Gar Reichman Fund of the Cancer Research Institute (M.A.S.M.), by National Institutes of Health Grant KO8-HL-02926, by the Dorothy Rodbell Cohen Foundation for Sarcoma Research, and by the Rich Foundation (S.R.). R.M. is recipient of a fellowship from the Dr. Mildred Scheel Stiftung für Krebsforschung (Germany).
Footnotes
Abbreviations: VEGF, vascular endothelial growth factor; BMEC, bone marrow microvascular endothelial cells; TPO, thrombopoietin; IL, interleukin; GM-CSF, granulocyte-macrophage colony-stimulating factor; bFGF, basic fibroblast growth factor; FLK-1, fetal liver kinase 1; FITC, fluorescein isothiocyanate; RT, reverse transcription.
References
- 1.Rafii S, Shapiro F, Pettengell R, Ferris B, Nachman R L, Moore M A S, Asch A S. Blood. 1995;86:3353–3363. [PubMed] [Google Scholar]
- 2.Tavassoli M, Aoki M. Blood Cells. 1989;15:59–72. [PubMed] [Google Scholar]
- 3.Wickenhauser C, Lorenzen J, Thiele J, Hillienhof A, Jungheim K, Schmitz B, Hansmann M, Fischer R. Blood. 1995;85:685–691. [PubMed] [Google Scholar]
- 4.Jones C L A, Witte D P, Feller M J, Fugman D A, Dorn G W, Liebermann M A. Biochim Biophys Acta. 1992;1136:272–282. doi: 10.1016/0167-4889(92)90117-t. [DOI] [PubMed] [Google Scholar]
- 5.Connolly D T, Heuvelman D M, Nelson R, Olander J V, Eppley B L, Delfino J J, Siegel N R, Leimgruber R M, Feder J. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Henzel W J. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 7.Senger D R, Galli S J, Dvorak A M, Perruzzi C A, Harvey V S, Dvorak H F. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 8.Senger D R, Connolly D T, VanDeWater L, Feder J, Dvorak H F. Cancer Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- 9.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 10.Roberts W G, Palade G E. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 11.Clauss M, Gerlach M, Brett J, Wang F, Familletti P C, Pan Y C E, Olander J V, Connolly D T, Stern D. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber B L, Marchese M J, Kew R. Blood. 1995;86:2488–2493. [PubMed] [Google Scholar]
- 13.Barleon B, Sozzani S, Zhou D, Weich H A, Mantovani A, Marme D. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 14.Terman B I, Dougher-Vermazen M, Carrion M E, Dimitrov D, Armelino D C, Gospodarowicz D, Bohlen P. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 15.de Vries C, Escobedo J A, Ueno H, Houck K, Ferrara N, Williams L T. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 16.Millauer B, Wizigmann-Voos S, Schurch H, Martines R, Moller N P H, Risau W, Ullrich A. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 17.Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. Oncogene. 1995;10:135–147. [PubMed] [Google Scholar]
- 18.Houck K A, Ferrara N, Winer J, Cachianes G, Li B, Leung D W. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak H F, Brown L F, Detmar M, Dvorak A M. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 20.Iijima K, Yoshikawa N, Connolly D T, Nakamura H. Kidney Int. 1993;44:959–966. doi: 10.1038/ki.1993.337. [DOI] [PubMed] [Google Scholar]
- 21.Freeman M R, Schneck F X, Gagnon M L, Corless C, Soker S, Niknejad K, Peoples G E, Klagsbrun M. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- 22.Park J E, Keller G A, Ferrara N. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houck K A, Leung D W, Rowland A M, Winer J, Ferrara N. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 24.Thoma S J, Lamping C P, Ziegler B L. Blood. 1994;83:2103–2114. [PubMed] [Google Scholar]
- 25.Katoh O, Tauchi H, Kawaishi K, Kimura A, Satow Y. Cancer Res. 1995;55:5687–5692. [PubMed] [Google Scholar]
- 26.Handagama P J, Shuman M A, Bainton D F. J Clin Invest. 1989;84:73–82. doi: 10.1172/JCI114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debili N, Wendling F, Katz A, Guichard J, Breton-Gorius J, Hunt P, Vainchenker W. Blood. 1995;86:2516–2525. [PubMed] [Google Scholar]
- 28.Dorn G W, II, Davis M G. J Pharmacol Exp Ther. 1992;262:1242–1247. [PubMed] [Google Scholar]
- 29.Debili N, Hegyi E, Navarro S, Katz A, Mouthon M A, Breton-Gorius J, Vainchenker W. Blood. 1991;77:2326–2338. [PubMed] [Google Scholar]
- 30.Avraham H, Cowley S, Chi S Y, Jiang S, Groopman E. J Clin Invest. 1993;91:2378–2384. doi: 10.1172/JCI116470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolecki G J, Connolly D T. Biochem Biophys Res Commun. 1991;180:572–578. doi: 10.1016/s0006-291x(05)81103-9. [DOI] [PubMed] [Google Scholar]
- 32.Brogi E, Wu T, Namiki A, Isner J M. Circulation. 1994;90:649–652. doi: 10.1161/01.cir.90.2.649. [DOI] [PubMed] [Google Scholar]
- 33.Shweiki D, Itin A, Soffer D, Keshet E. Nature (London) 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 34.Vittet D, Chevillard C. Blood Coagulation Fibrinolysis. 1993;4:759–768. [PubMed] [Google Scholar]
- 35.Avraham H, Banu N, Scadden D T, Abraham J, Groopman J E. Blood. 1994;83:2126–2132. [PubMed] [Google Scholar]
- 36.Broxmeyer H E, Cooper S, Li Z H, Lu L, Song H Y, Kwon B S, Warren R E, Donner D B. Int J Hematol. 1995;62:203–215. doi: 10.1016/0925-5710(95)00412-2. [DOI] [PubMed] [Google Scholar]
- 37.Gimbrone M A, Jr, Aster R H, Cotran R S, Corkery J, Jandl J H, Folkman J. Nature (London) 1969;221:33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- 38.Guo D, Jia Q, Song H Y, Warren P R, Donner D B. J Biol Chem. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- 39.D’Angelo G, Struman I, Martial J, Weiner R I. Proc Natl Acad Sci USA. 1995;92:6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]